Optical Methods Based on Ultraviolet, Visible, and Near-Infrared Spectra to Estimate Fat and Protein in Raw Milk: A Review
Abstract
:1. Introduction
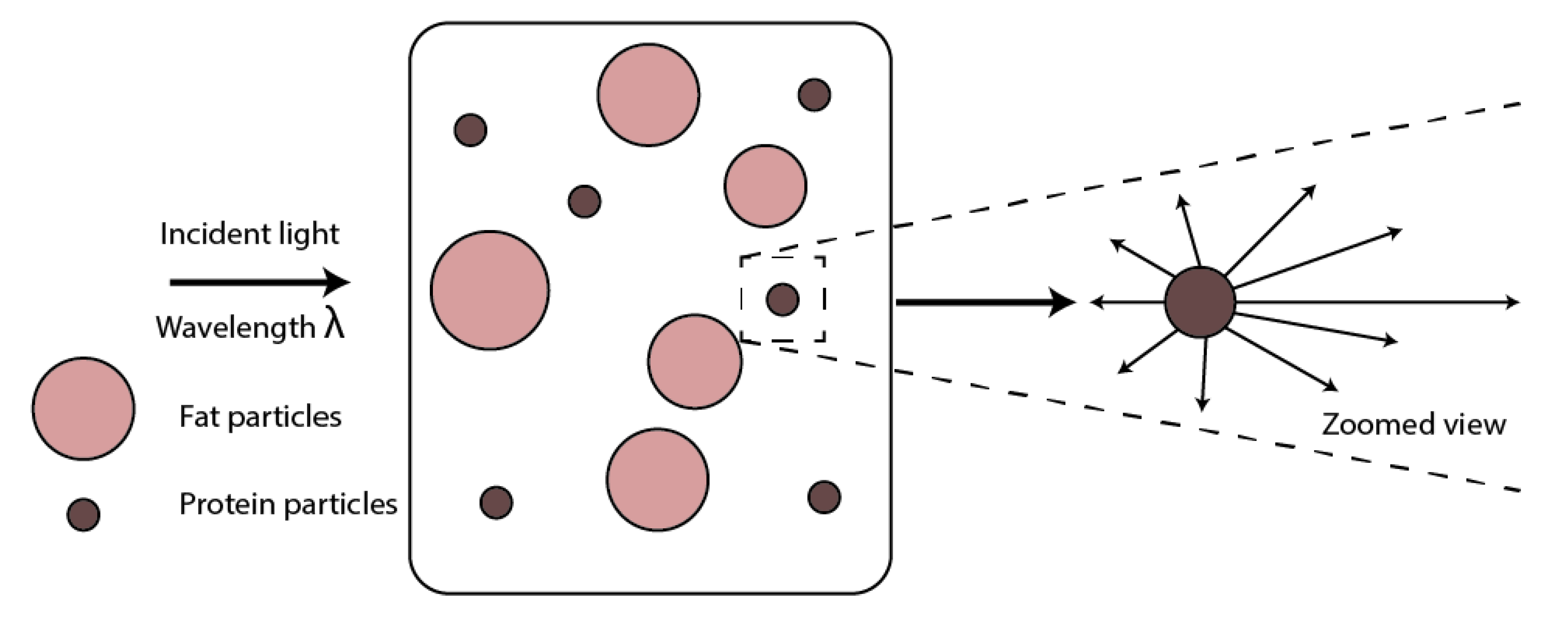
2. Optical Properties of Milk
3. Spectroscopy Techniques
3.1. UV Techniques
3.2. VIS Techniques
3.3. IR Techniques
4. Challenges and Prospects
4.1. Costs
4.2. Embedded and Integration
4.3. Self-Calibrated Mathematical Models
4.4. Communication
5. Trends
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragni, L.; Iaccheri, E.; Cevoli, C.; Berardinelli, A. Spectral-sensitive Pulsed Photometry to predict the fat content of commercialized milk. J. Food Eng. 2016, 171, 95–101. [Google Scholar] [CrossRef]
- Aernouts, B.; Polshin, E.; Lammertyn, J.; Saeys, W. Visible and near-infrared spectroscopic analysis of raw milk for cow health monitoring: Reflectance or transmittance? J. Dairy Sci. 2011, 94, 5315–5329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aernouts, B.; Van Beers, R.; Watté, R.; Huybrechts, T.; Lammertyn, J.; Saeys, W. Visible and near-infrared bulk optical properties of raw milk. J. Dairy Sci. 2015, 98, 6727–6738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, B.J.S.C.; Markwell, J. Assays for Determination of Protein Concentration. In Current Protocols in Protein Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 3.4.1–3.4.29. [Google Scholar]
- Fox, P.F.; Uniacke-Lowe, T.; Mcsweeney, P.L.H.; O’Mahony, J.A. Dairy Chemistry and Biochemistry, 2nd ed.; Springer: Cham, Switzerland, 2015; Volume 1542, ISBN 0412720000. [Google Scholar]
- Yin, J.; Zhang, S.; Yang, H.; Zhou, Z. Theoretical Study of the Effect of Multi-Diameter Distribution on the Mie Scattering Characteristics of Milk Fat. J. Harbin Inst. Technol. 2015, 22, 115–120. [Google Scholar] [CrossRef]
- Crofcheck, C.L.; Payne, F.A.; Mengüç, M.P. Characterization of milk properties with a radiative transfer model. Appl. Opt. 2002, 41, 2028. [Google Scholar] [CrossRef] [PubMed]
- Crofcheck, C.; Wade, J.; Swamy, J.N.; Aslan, M.M.; Mengüç, M.P. Effect of fat and casein particles in milk on the scattering of elliptically polarized light. Trans. Am. Soc. Agric. Eng. 2005, 48, 1147–1155. [Google Scholar] [CrossRef]
- Stocker, S.; Foschum, F.; Krauter, P.; Bergmann, F.; Hohmann, A.; Scalfi Happ, C.; Kienle, A. Broadband Optical Properties of Milk. Appl. Spectrosc. 2016, 0, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Regnima, G.-O.; Koffi, T.; Bagui, O.; Kouacou, A.; Kristensson, E.; Zoueu, J.; Berrocal, E. Quantitative measurements of turbid liquids via structured laser illumination planar imaging where absorption spectrophotometry fails. Appl. Opt. 2017, 56, 3929–3938. [Google Scholar] [CrossRef] [PubMed]
- Hulst, H.C.; van de Hulst, H.C. Light Scattering by Small Particles; Courier Corporation: North Chelmsford, MA, USA, 1981. [Google Scholar]
- Mäntele, W.; Deniz, E. UV–VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 965–968. [Google Scholar]
- Xin, Q.; Ling, H.Z.; Long, T.J.; Zhu, Y. The rapid determination of fat and protein content in fresh raw milk using the laser light scattering technology. Opt. Lasers Eng. 2006, 44, 858–869. [Google Scholar] [CrossRef]
- Jenkins, F.A.; White, H.E. Fundamentals of Optics; Tata McGraw-Hill Education: New York, NY, USA, 1937. [Google Scholar]
- Swinehart, D.F. The Beer-Lambert law. J. Chem. Educ. 1962, 39, 333–335. [Google Scholar] [CrossRef]
- McCarthy, O.J. Physical and Physico-Chemical Properties of Milk. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 467–477. ISBN 9780123744029. [Google Scholar]
- Penner, M.H. Basic Principles of Spectroscopy. In Food Analysis; Food Science Text Series; Springer: Berlin/Heidelberg, Germany, 2017; pp. 79–88. ISBN 9783319457765. [Google Scholar]
- Nakai, S.; Le, A.C. Spectrophotometric Determination of Protein and Fat in Milk Simultaneously. J. Dairy Sci. 1969, 53, 276–278. [Google Scholar] [CrossRef]
- Kuaye, A.Y. An ultraviolet spectrophotometric method to determine milk protein content in alkaline medium. Food Chem. 1994, 49, 207–211. [Google Scholar] [CrossRef]
- Lüthi-Peng, Q.; Puhan, Z. Determination of protein and casein in milk by fourth derivative UV spectrophotometry. Anal. Chim. Acta. 1999, 393, 227–234. [Google Scholar] [CrossRef]
- IDF Standard No 20-1. Determination of Nitrogen Content—Part 1: Kjeldahl Method; International Dairy Federation: Brussels, Belgium, 2001. [Google Scholar]
- Forcato, D.O.; Carmine, M.P.; Echeverría, G.E.; Pécora, R.P.; Kivatinitz, S.C. Milk Fat Content Measurement by a Simple UV Spectrophotometric Method: An Alternative Screening Method. J. Dairy Sci. 2005, 88, 478–481. [Google Scholar] [CrossRef] [Green Version]
- Haugaard, G.; Pettinati, J.D. Photometric Milk Fat Determination. J. Dairy Sci. 1959, 42, 1255–1275. [Google Scholar] [CrossRef]
- Muniz, R.; Perez, M.A.; de la Torre, C.; Carleos, C.E.; Corral, N.; Baro, J. A Comparison of Principal Component Regression (Pcr) and Partial Least Square (Pls) Methods in Prediction of Raw Milk Composition by Vis-Nir Spectrometry. Application to Development of on-Line Sensors for Fat, Protein and Lactose Contents. In Proceedings of the XIX IMEKO World Congress Fundamental and Applied Metrology, Lisbon, Portugal, 6–11 September 2009; pp. 2564–2638. [Google Scholar]
- Bogomolov, A.; Dietrich, S.; Boldrini, B.; Kessler, R.W. Quantitative determination of fat and total protein in milk based on visible light scatter. Food Chem. 2012, 134, 412–418. [Google Scholar] [CrossRef]
- Kucheryavskiy, S.; Melenteva, A.; Bogomolov, A. Determination of fat and total protein content in milk using conventional digital imaging. Talanta 2014, 121, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Bogomolov, A.; Melenteva, A. Scatter-based quantitative spectroscopic analysis of milk fat and total protein in the region 400–1100nm in the presence of fat globule size variability. Chemom. Intell. Lab. Syst. 2013, 126, 129–139. [Google Scholar] [CrossRef]
- Gowri, A.; Rajamani, A.S.; Ramakrishna, B.; Sai, V.V.R. U-bent plastic optical fiber probes as refractive index based fat sensor for milk quality monitoring. Opt. Fiber Technol. 2019, 47, 15–20. [Google Scholar] [CrossRef]
- Goulden, J.D.S. Analysis of milk by infra-red absorption. J. Dairy Res. 1964, 31, 273–284. [Google Scholar] [CrossRef]
- Luinge, H.J.; Hop, E.; Lutz, E.T.G.; van Hemert, J.A.; de Jong, E.A.M. Determination of the fat, protein and lactose content of milk using Fourier transform infrared spectrometry. Anal. Chim. Acta 1993, 284, 419–433. [Google Scholar] [CrossRef]
- Šašić, S.; Ozaki, Y. Short-wave near-infrared spectroscopy of biological fluids. 1. Quantitative analysis of fat, protein, and lactose in raw milk by partial least-squares regression and band assignment. Anal. Chem. 2001, 73, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.A.; Terazawa, Y.; Chen, J.Y.; Iyo, C.; Terada, F.; Kawano, S. Development of a new measurement unit (MilkSpec-1) for rapid determination of fat, lactose, and protein in raw milk using near-infrared transmittance spectroscopy. Appl. Spectrosc. 2002, 56, 599–604. [Google Scholar] [CrossRef]
- Etzion, Y.; Linker, R.; Cogan, U.; Shmulevich, I. Determination of protein concentration in raw milk by mid-infrared fourier transform infrared/attenuated total reflectance spectroscopy. J. Dairy Sci. 2004, 87, 2779–2788. [Google Scholar] [CrossRef] [Green Version]
- Rahmelow, K.; Hübner, W. Infrared spectroscopy in aqueous solution: Difficulties and accuracy of water subtraction. Appl. Spectrosc. 1997, 51, 160–170. [Google Scholar] [CrossRef]
- Kawamura, S.; Kawasaki, M.; Nakatsuji, H.; Natsuga, M. Near-infrared spectroscopic sensing system for online monitoring of milk quality during milking. Sens. Instrum. Food Qual. Saf. 2007, 1, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, M.; Kawamura, S.; Tsukahara, M.; Morita, S.; Komiya, M.; Natsuga, M. Near-infrared spectroscopic sensing system for on-line milk quality assessment in a milking robot. Comput. Electron. Agric. 2008, 63, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Muñoz Ossa, H.; Reyes Vera, E.E.; Causado Buelvas, J.D.; Lora Jaramillo, G.J.; Dominguez Gomez, D.M.; Torres Trujillo, P. Medición Del Porcentaje De Grasa En Leche Líquida Usando “Tapers” De Fibra Óptica. Revista Colombiana de Física 2011, 43, 873. [Google Scholar]
- Melfsen, A.; Hartung, E.; Haeussermann, A. Accuracy of in-line milk composition analysis with diffuse reflectance near-infrared spectroscopy. J. Dairy Sci. 2012, 95, 6465–6476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Su, R.; Xu, N.; Wang, X.; Yu, A.; Zhang, H.; Cao, Y. Portable analyzer for rapid analysis of total protein, fat and lactose contents in raw milk measured by non-dispersive short-wave near-infrared spectrometry. Chem. Res. Chin. Univ. 2013, 29, 15–19. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Z.; Qian, K.; Wang, L.; Lan, X. A rapid method for measuring fat content in milk based on W-type optical fibre sensor system. Trans. Inst. Meas. Control 2016, 38, 1471–1479. [Google Scholar] [CrossRef]
- Niero, G.; Penasa, M.; Gottardo, P.; Cassandro, M.; De Marchi, M. Short communication: Selecting the most informative mid-infrared spectra wavenumbers to improve the accuracy of prediction models for detailed milk protein content. J. Dairy Sci. 2016, 99, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huo, G.; Wang, Y.; Sun, H.; Kong, Q. Research on Rapid Detection Method of Protein and Fat in Raw Milk Based on Mid-infrared Spectrum. Int. J. Multimed. Ubiquitous Eng. 2016, 11, 131–142. [Google Scholar] [CrossRef]
- Tremblay, L.; Laporte, M.F.; Léonil, J.; Dupont, D.; Paquin, P. Quantitation of Proteins in Milk and Milk Products. In Advanced Dairy Chemistry—1 Proteins; Springer US: Boston, MA, USA, 2003; pp. 49–138. [Google Scholar]
- Dave, A.; Banwari, D.; Mansinghani, S.; Srivastava, S.; Sadistap, S. Ultrasonic Sensing System for Detecting Water Adulteration in Milk. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; pp. 2228–2231. [Google Scholar]
- Serra, P.A.; Rocchitta, G.; Bazzu, G.; Manca, A.; Puggioni, G.M.; Lowry, J.P.; O’Neill, R.D. Design and construction of a low cost single-supply embedded telemetry system for amperometric biosensor applications. Sensors Actuators B Chem. 2007, 122, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheng, X.; Yan, K.; Gong, P. A monitoring system for vegetable greenhouses based on a wireless sensor network. Sensors 2010, 10, 8963–8980. [Google Scholar] [CrossRef] [PubMed]
- Kalomiros, J.; Lygouras, J. Design and evaluation of a hardware/software FPGA-based system for fast image processing. Microprocess. Microsyst. 2008, 32, 95–106. [Google Scholar] [CrossRef]
- Andonovic, I.; Michie, C.; Cousin, P.; Janati, A.; Pham, C.; Diop, M. Precision Livestock Farming Technologies. In Proceedings of the 2018 Global Internet of Things Summit (GIoTS), Bilbao, Spain, 4–7 June 2018; IEEE: New York, NY, USA, 2018; pp. 1–6. [Google Scholar]
- Valle-Atilano, F.J.; Estudillo-Ayala, J.M.; Filoteo-Razo, J.D.; Hernández-García, J.C.; Jáuregui-Vázquez, D.; Sierra-Hernández, J.M.; Rojas-Laguna, R.; Mata-Chavez, R.I.; Samano-Aguilar, L.F. Generation of supercontinuum light in micro-structured fiber and polarization study at different wavelengths. In Photonic Fiber and Crystal Devices: Advances in Materials and Innovations in Device Applications X; International Society for Optics and Photonics: San Francisco, CA, USA, 2016; Volume 9958, p. 995817. [Google Scholar]
- Lee, C.E.; Taylor, H.F. Fiber-optic Fabry-Perot temperature sensor using a low-coherence light source. J. lightwave Technol. 1991, 9, 129–134. [Google Scholar] [CrossRef]
- Wang, S.-N.; Lv, R.-Q.; Zhao, Y.; Qian, J. A Mach-Zehnder interferometer-based High Sensitivity Temperature sensor for human body monitoring. Opt. Fiber Technol. 2018, 45, 93–97. [Google Scholar] [CrossRef]
- Tian, Z.; Yam, S.S.H.; Loock, H.P. Single-mode fiber refractive index sensor based on core-offset attenuators. IEEE Photonics Technol. Lett. 2008, 20, 1387–1389. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Kim, Y.H.; Park, K.S.; Eom, J.B.; Kim, M.J.; Rho, B.S.; Choi, H.Y. Interferometric fiber optic sensors. Sensors 2012, 12, 2467–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastélum-Barrios, A.; Soto-Zarazúa, G.M.; García-Trejo, J.F.; Sierra-Hernandez, J.M.; Jauregui-Vazquez, D. A new method for total fat detection in raw milk based on dual low-coherence interferometer. Sensors 2019, 19, 4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora-Izquierdo, M.A.; Santa, J.; Martínez, J.A.; Martínez, V.; Skarmeta, A.F. Smart farming IoT platform based on edge and cloud computing. Biosyst. Eng. 2019, 177, 4–17. [Google Scholar] [CrossRef]
- Tao, F.; Ngadi, M. Applications of spectroscopic techniques for fat and fatty acids analysis of dairy foods. Curr. Opin. Food Sci. 2017, 17, 100–112. [Google Scholar] [CrossRef]
- Bernuy, B.; Meurens, M.; Mignolet, E.; Larondelle, Y. Performance comparison of UV and FT-raman spectroscopy in the determination of conjugated linoleic acids in cow milk fat. J. Agric. Food Chem. 2008, 56, 1159–1163. [Google Scholar] [CrossRef] [PubMed]



| Year | Acquisition Method | Component Determination | Spectral Range (nm) | Data Processing | Validation | References | ||
|---|---|---|---|---|---|---|---|---|
| R2 | Sensitivity | RMSEP (%) | ||||||
| 2019 | Reflection | Fat | 500 | 0.9763 | 616 pm/% fat | [54] | ||
| Protein | 0.878 | |||||||
| 2019 | Absorbance | Fat | 530 | 0.15 ∆A/∆% fat | [28] | |||
| 2016 | Transmission | Fat | 2500–25,000 | PLS | 0.91007989 | 0.045329 | [42] | |
| Protein | 0.8010929 | 0.0207505 | ||||||
| 2016 | Scatter | Fat | 400–1000 | PLS | 0.05 | [41] | ||
| Protein | 0.03 | |||||||
| 2015 | Scatter | Fat | 1300–1400 | 0.975 | [3] | |||
| 2014 | Transmission | Fat | 400–700 | PLS | 0.973 | [26] | ||
| Protein | 0.974 | |||||||
| 2013 | Scatter | Fat | 400–1100 | PLS | 0.952 | 0.13 | [27] | |
| Protein | 0.959 | 0.04 | ||||||
| 2013 | Transmission | Protein | 600–1100 | PLS | 0.932 | 0.201 | [39] | |
| Fat | 0.981 | 0.172 | ||||||
| Lactose | 0.933 | 0.247 | ||||||
| 2012 | Transmission | Fat | 400–1000 | PLS | 0.915 | 0.05 | [25] | |
| Protein | 0.964 | 0.03 | ||||||
| 2011 | Reflectance | Fat | 1000–1700 | PLS | 0.997 | 0.047 | [2] | |
| Transmission | Protein | 400–1700 | 0.90 | 0.162 | ||||
| Transmission | Lactose | 400–1700 | 0.883 | 0.115 | ||||
| 2008 | Transmission | Fat | 600–1050 | PLS | 0.95 | 0.25 | [36] | |
| Lactose | 0.83 | 0.26 | ||||||
| Protein | 0.72 | 0.15 | ||||||
| 2007 | Transmission | Fat | 600–1050 | 0.95 | 0.42 | [35] | ||
| Protein | 0.91 | 0.09 | ||||||
| Lactose | 0.94 | 0.05 | ||||||
| 2004 | Reflectance | Protein | 5800–9400 | PLS | 0.22 | [33] | ||
| 2002 | Transmission | Fat | 700–1100 | PLS | 0.999 | 0.06 | [32] | |
| Lactose | 0.964 | 0.10 | ||||||
| Protein | 0.97 | 0.10 | ||||||
| 2001 | Transmission | Protein | 800–1100 | PLS | 0.996 | 0.087 | [31] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastélum-Barrios, A.; Soto-Zarazúa, G.M.; Escamilla-García, A.; Toledano-Ayala, M.; Macías-Bobadilla, G.; Jauregui-Vazquez, D. Optical Methods Based on Ultraviolet, Visible, and Near-Infrared Spectra to Estimate Fat and Protein in Raw Milk: A Review. Sensors 2020, 20, 3356. https://doi.org/10.3390/s20123356
Gastélum-Barrios A, Soto-Zarazúa GM, Escamilla-García A, Toledano-Ayala M, Macías-Bobadilla G, Jauregui-Vazquez D. Optical Methods Based on Ultraviolet, Visible, and Near-Infrared Spectra to Estimate Fat and Protein in Raw Milk: A Review. Sensors. 2020; 20(12):3356. https://doi.org/10.3390/s20123356
Chicago/Turabian StyleGastélum-Barrios, Abraham, Genaro M. Soto-Zarazúa, Axel Escamilla-García, Manuel Toledano-Ayala, Gonzalo Macías-Bobadilla, and Daniel Jauregui-Vazquez. 2020. "Optical Methods Based on Ultraviolet, Visible, and Near-Infrared Spectra to Estimate Fat and Protein in Raw Milk: A Review" Sensors 20, no. 12: 3356. https://doi.org/10.3390/s20123356
APA StyleGastélum-Barrios, A., Soto-Zarazúa, G. M., Escamilla-García, A., Toledano-Ayala, M., Macías-Bobadilla, G., & Jauregui-Vazquez, D. (2020). Optical Methods Based on Ultraviolet, Visible, and Near-Infrared Spectra to Estimate Fat and Protein in Raw Milk: A Review. Sensors, 20(12), 3356. https://doi.org/10.3390/s20123356





