Enhanced Electrochemical Characteristics of the Glucose Oxidase Bioelectrode Constructed by Carboxyl-Functionalized Mesoporous Carbon
Abstract
1. Introduction
2. Materials and Methods
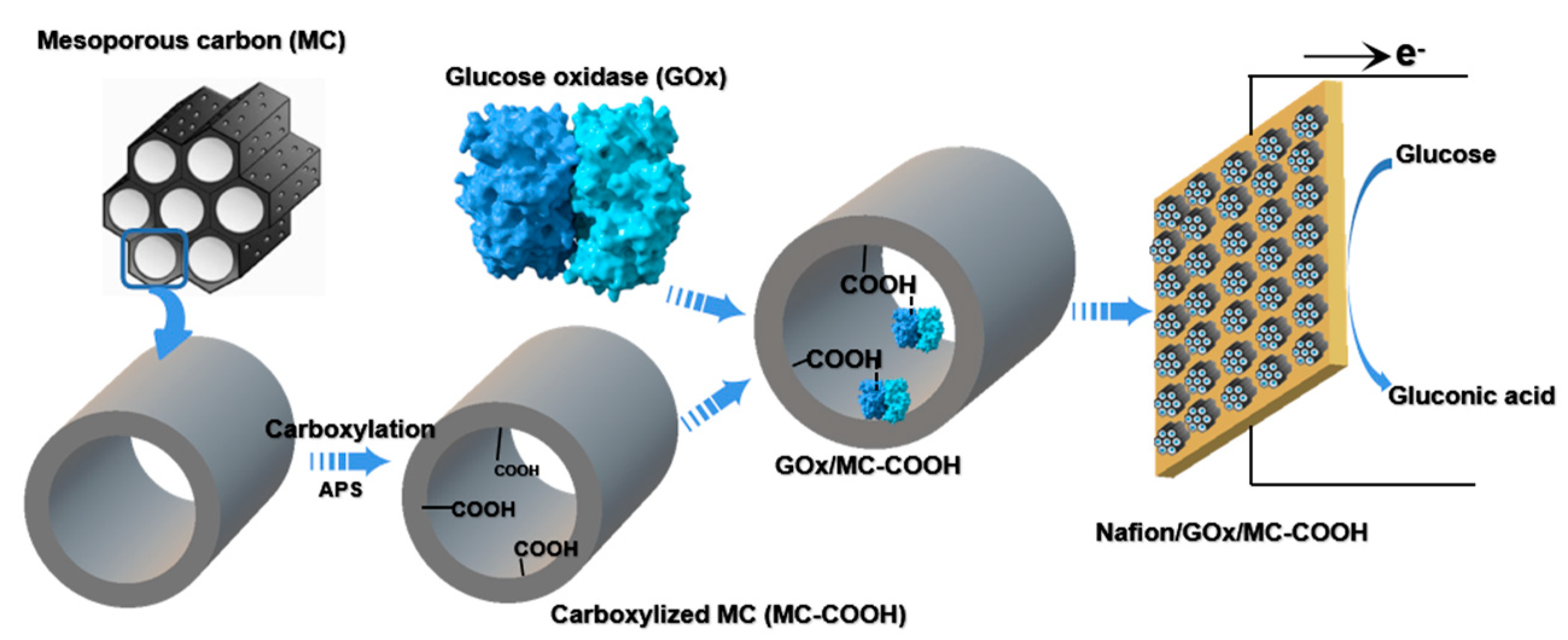
2.1. MC Carboxyl Functionalization
2.2. GOx Immobilization on MC-COOH
2.3. Fabrication of GOx/MC and GOx/MC-COOH Bioelectrodes
2.4. Characterization of MC-COOH
2.5. Electrochemical Measurements of Glucose Bioelectrodes
3. Results
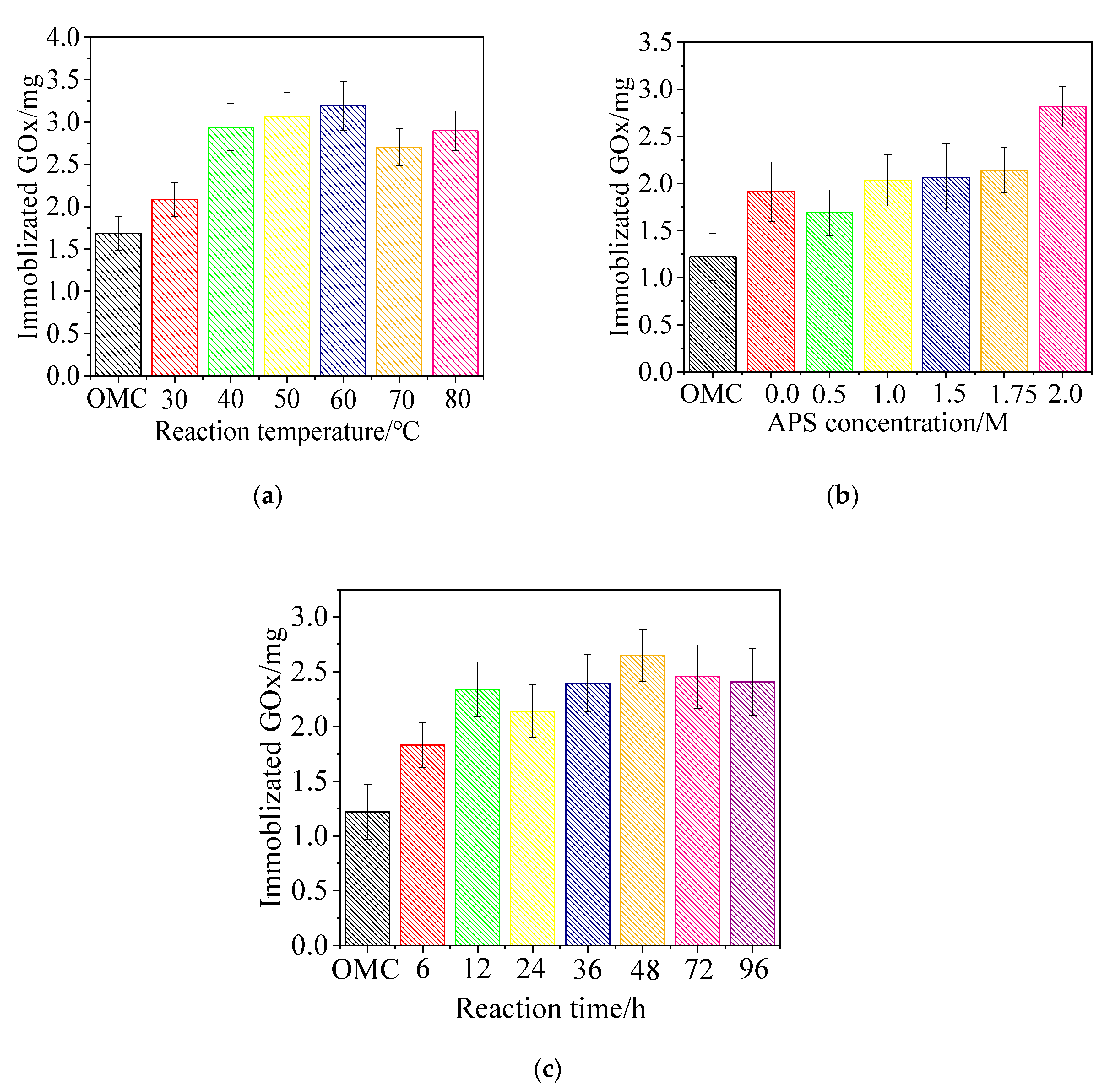
3.1. Effect of Carboxylation Conditions on GOx Adsorption
3.1.1. Temperature
3.1.2. APS Concentration
3.1.3. Time
3.2. Characterization of MC-COOH
3.2.1. Fourier Transform Infrared (FTIR) Spectrum Analysis
3.2.2. Wide-Angle X-ray Diffraction (XRD) Analysis
3.2.3. Nitrogen Adsorption Specific Surface Area (BET) Analysis
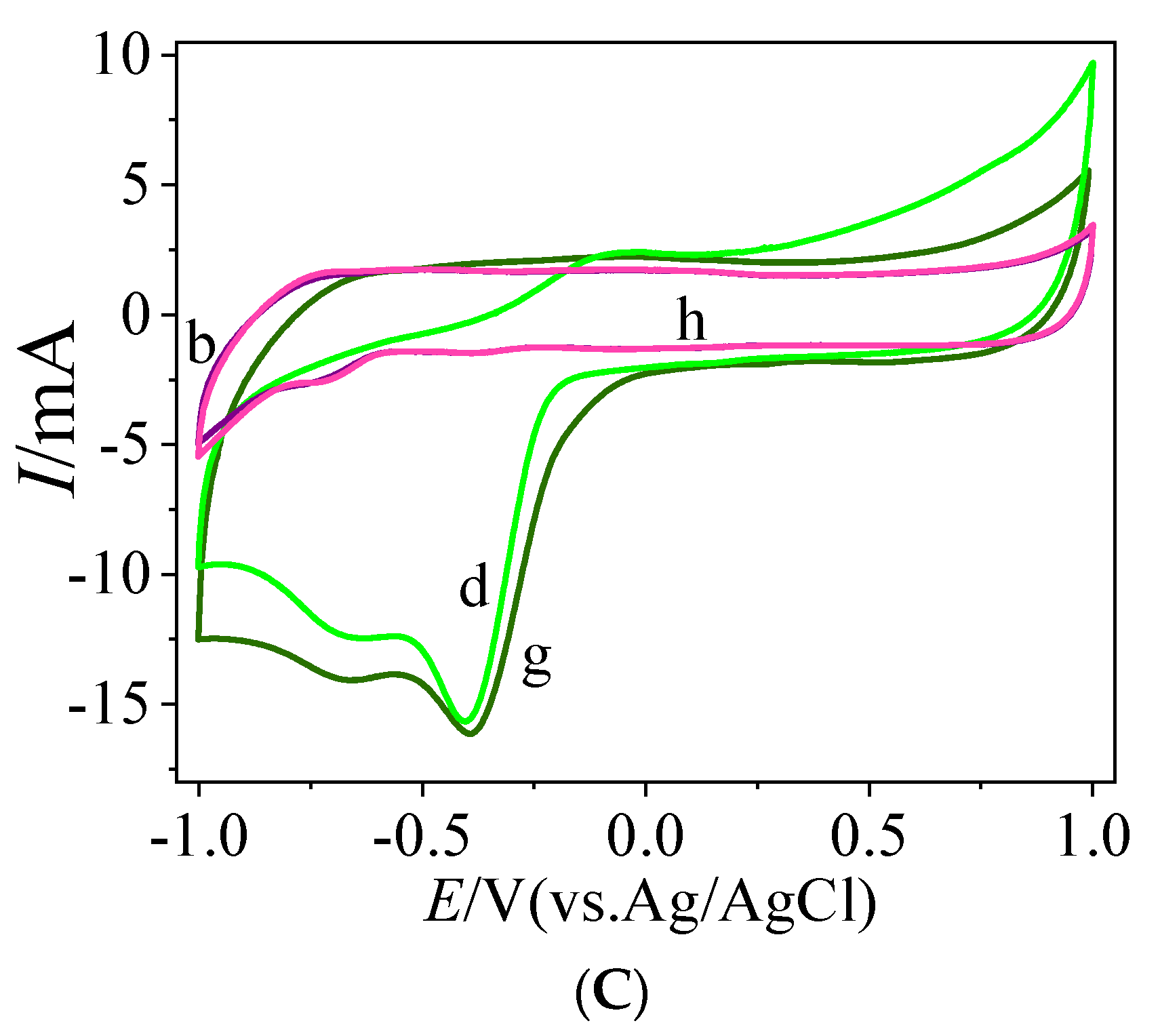
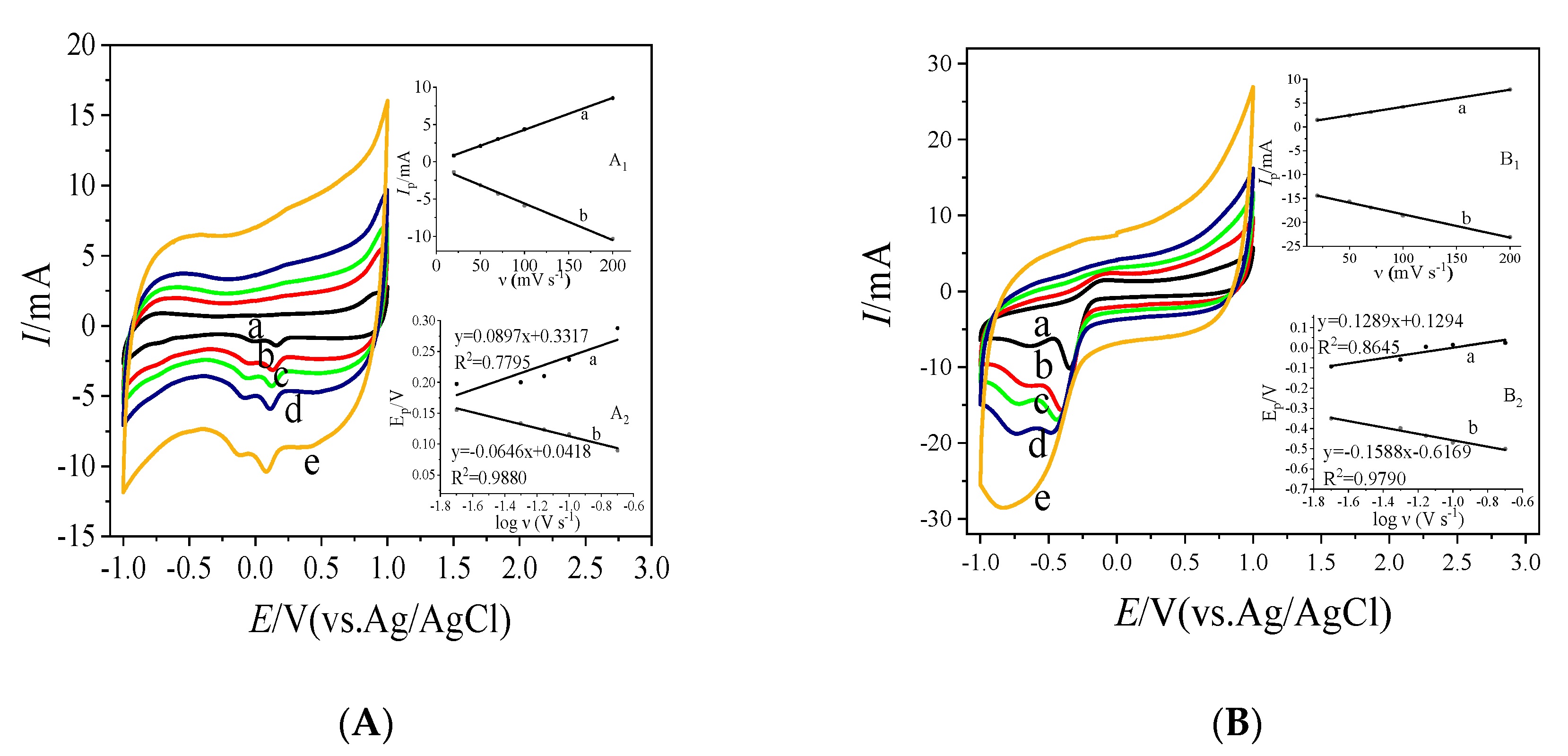
3.3. Direct Electrochemistry of Bioelectrodes Nafion/GOx/MC and Nafion/GOx/MC-COOH
4. Discussion
4.1. Effect of Oxidation Reaction Conditions on the GOx Adsorption
4.2. Effect of MC Carboxylation on GOx Immobilization
4.3. Improved Characteristics of the Bioelectrode
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du Toit, H.; Di Lorenzo, M. Glucose Oxidase Directly Immobilized onto Highly Porous Gold Electrodes for Sensing and Fuel Cell Applications. Electrochim. Acta 2014, 138, 86–92. [Google Scholar] [CrossRef]
- Chen, Y.; Gai, P.; Zhang, J.; Zhu, J.-J. Design of an Enzymatic Biofuel Cell with Large Power Output. J. Mater. Chem. A 2015, 3, 11511–11516. [Google Scholar] [CrossRef]
- Karimi, A.; Othman, A.; Uzunoglu, A.; Stanciu, L.; Andreescu, S. Graphene Based Enzymatic Bioelectrodes and Biofuel Cells. Nanoscale 2015, 7, 6909–6923. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Carbon Nanotube/Enzyme Biofuel Cells. Electrochim. Acta 2012, 82, 179–190. [Google Scholar] [CrossRef]
- Yang, J.; Hu, D.; Zhang, X.; Wang, K.; Wang, B.; Sun, B.; Qiu, Z. Enzyme-Catalyzed Biocathode in a Photoelectrochemical Biofuel Cell. J. Power Sources 2014, 267, 617–621. [Google Scholar] [CrossRef]
- Cosnier, S.; Le Goff, A.; Holzinger, M. Towards Glucose Biofuel Cells Implanted in Human Body for Powering Artificial Organs: Review. Electrochem. Commun. 2014, 38, 19–23. [Google Scholar] [CrossRef]
- Cooney, M.; Svoboda, V.; Lau, C.; Martin, G.; Minteer, S. Enzyme Catalysed Biofuel Cells. Energy Environ. Sci. 2008, 1, 320–337. [Google Scholar] [CrossRef]
- Ndamanisha, J.C.; Guo, L.-P. Ordered Mesoporous Carbon for Electrochemical Sensing: A Review. Anal. Chim. Acta 2012, 747, 19–28. [Google Scholar] [CrossRef]
- Wu, P.; Shao, Q.; Hu, Y.; Jin, J.; Yin, Y.; Zhang, H.; Cai, C. Direct Electrochemistry of Glucose Oxidase Assembled on Graphene and Application to Glucose Detection. Electrochim. Acta 2010, 55, 8606–8614. [Google Scholar] [CrossRef]
- Yu, J.; Yu, D.; Zhao, T.; Zeng, B. Development of Amperometric Glucose Biosensor through Immobilizing Enzyme in a Pt Nanoparticles/Mesoporous Carbon Matrix. Talanta 2008, 74, 1586–1591. [Google Scholar] [CrossRef]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic Biofuel Cells: 30 Years of Critical Advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Grate, J.W.; Wang, P. Nanobiocatalysis and Its Potential Applications. Trends Biotechnol. 2008, 26, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Alwarappan, S.; Chen, Z.; Kong, X.; Li, C.-Z. Membraneless Enzymatic Biofuel Cells Based on Graphene Nanosheets. Biosens. Bioelectron. 2010, 25, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Ravenna, Y.; Xia, L.; Gun, J.; Mikhaylov, A.A.; Medvedev, A.G.; Lev, O.; Alfonta, L. Biocomposite Based on Reduced Graphene Oxide Film Modified with Phenothiazone and Flavin Adenine Dinucleotide-Dependent Glucose Dehydrogenase for Glucose Sensing and Biofuel Cell Applications. Anal. Chem. 2015, 87, 9567–9571. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-M.; Yehezkeli, O.; Willner, I. Integrated, Electrically Contacted Nad(P)+-Dependent Enzyme–Carbon Nanotube Electrodes for Biosensors and Biofuel Cell Applications. Chem. Eur. J. 2007, 13, 10168–10175. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.H.; Lee, S.-H.; Choi, Y.-B.; Lee, J.A.; Kim, S.H.; Kim, H.-H.; Spinks, G.M.; Wallace, G.G.; Lima, M.D.; Kozlov, M.E.; et al. High-Power Biofuel Cell Textiles from Woven Biscrolled Carbon Nanotube Yarns. Nat. Commun. 2014, 5, 3928. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Yu, P.; Su, L.; Ohsaka, T.; Mao, L. A Miniature Glucose/O2 Biofuel Cell with Single-Walled Carbon Nanotubes-Modified Carbon Fiber Microelectrodes as the Substrate. Electrochem. Commun. 2008, 10, 851–854. [Google Scholar] [CrossRef]
- Guo, C.X.; Hu, F.P.; Lou, X.W.; Li, C.M. High-Performance Biofuel Cell Made with Hydrophilic Ordered Mesoporous Carbon as Electrode Material. J. Power Sources 2010, 195, 4090–4097. [Google Scholar] [CrossRef]
- Trifonov, A.; Herkendell, K.; Tel-Vered, R.; Yehezkeli, O.; Woerner, M.; Willner, I. Enzyme-Capped Relay-Functionalized Mesoporous Carbon Nanoparticles: Effective Bioelectrocatalytic Matrices for Sensing and Biofuel Cell Applications. ACS Nano 2013, 7, 11358–11368. [Google Scholar] [CrossRef]
- Walcarius, A. Recent Trends on Electrochemical Sensors Based on Ordered Mesoporous Carbon. Sensors 2017, 17, 8. [Google Scholar] [CrossRef]
- Walcarius, A. Mesoporous Materials-Based Electrochemical Sensors. Electroanalysis 2015, 27, 1303–1340. [Google Scholar] [CrossRef]
- Catalano, P.N.; Wolosiuk, A.; Soler-Illia, G.J.A.A.; Bellino, M.G. Wired Enzymes In Mesoporous Materials: A Benchmark for Fabricating Biofuel Cells. Bioelectrochemistry 2015, 106, 14–21. [Google Scholar] [CrossRef]
- Wang, H.; Qi, B.; Lu, B.; Bo, X.; Guo, L. Comparative Study on the Electrocatalytic Activities of Ordered Mesoporous Carbons and Graphene. Electrochim. Acta 2011, 56, 3042–3048. [Google Scholar] [CrossRef]
- Jia, N.; Wang, Z.; Yang, G.; Shen, H.; Zhu, L. Electrochemical Properties of Ordered Mesoporous Carbon and Its Electroanalytical Application for Selective Determination of Dopamine. Electrochem. Commun. 2007, 9, 233–238. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.-M.; Thomas, A.; Antonietti, M. Aminated Hydrophilic Ordered Mesoporous Carbons. J. Mater. Chem. 2007, 17, 3412–3418. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Shin, Y.; Fryxell, G.E.; Um, W.; Parker, K.; Mattigod, S.V.; Skaggs, R. Sulfur-Functionalized Mesoporous Carbon. Adv. Funct. Mater. 2007, 17, 2897–2901. [Google Scholar] [CrossRef]
- Yang, G.; Tang, L.; Zeng, G.; Cai, Y.; Tang, J.; Pang, Y.; Zhou, Y.; Liu, Y.; Wang, J.; Zhang, S.; et al. Simultaneous Removal of Lead and Phenol Contamination from Water by Nitrogen-Functionalized Magnetic Ordered Mesoporous Carbon. Chem. Eng. J. 2015, 259, 854–864. [Google Scholar] [CrossRef]
- Vinu, A.; Hossian, K.Z.; Srinivasu, P.; Miyahara, M.; Anandan, S.; Gokulakrishnan, N.; Mori, T.; Ariga, K.; Balasubramanian, V.V. Carboxy-Mesoporous Carbon and Its Excellent Adsorption Capability for Proteins. J. Mater. Chem. 2007, 17, 1819–1825. [Google Scholar] [CrossRef]
- Chi, Y.; Geng, W.; Zhao, L.; Yan, X.; Yuan, Q.; Li, N.; Li, X. Comprehensive Study of Mesoporous Carbon Functionalized with Carboxylate Groups and Magnetic Nanoparticles as a Promising Adsorbent. J. Colloid Interface Sci. 2012, 369, 366–372. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, K.; Jin, J.; Dong, Z.; Wang, J.; Li, R. Preparation and Characterization of Octyl-Modified Ordered Mesoporous Carbon Cmk-3 for Phenol Adsorption. Microporous Mesoporous Mater. 2009, 121, 173–177. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.-B.; Qiu, J.-D.; Sun, D.-C.; Xia, X.-H. Biocomposites of Covalently Linked Glucose Oxidase on Carbon Nanotubes for Glucose Biosensor. Anal. Bioanal. Chem. 2005, 383, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Mangun, C.L.; Benak, K.R.; Daley, M.A.; Economy, J. Oxidation of Activated Carbon Fibers: Effect on Pore Size, Surface Chemistry, and Adsorption Properties. Chem. Mater. 1999, 11, 3476–3483. [Google Scholar] [CrossRef]
- Li, N.; Ma, X.; Zha, Q.; Kim, K.; Chen, Y.; Song, C. Maximizing the Number of Oxygen-Containing Functional Groups on Activated Carbon by Using Ammonium Persulfate and Improving the Temperature-Programmed Desorption Characterization of Carbon Surface Chemistry. Carbon 2011, 49, 5002–5013. [Google Scholar] [CrossRef]
- Wu, Z.; Webley, P.A.; Zhao, D. Comprehensive Study of Pore Evolution, Mesostructural Stability, and Simultaneous Surface Functionalization of Ordered Mesoporous Carbon (Fdu-15) by Wet Oxidation as a Promising Adsorbent. Langmuir 2010, 26, 10277–10286. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; Carrasco-Marín, F.; Mueden, A. the Creation of Acid Carbon Surfaces by Treatment with (Nh4)2s2o8. Carbon 1997, 35, 1619–1626. [Google Scholar] [CrossRef]
- Simaioforidou, A.; Kostas, V.; Karakassides, M.A.; Louloudi, M. Surface Chemical Modification of Macroporous and Mesoporous Carbon Materials: Effect on their Textural and Catalytic Properties. Microporous Mesoporous Mater. 2019, 279, 334–344. [Google Scholar] [CrossRef]
- Deb Nath, N.C.; Jeon, I.-Y.; Ju, M.J.; Ansari, S.A.; Baek, J.-B.; Lee, J.-J. Edge-Carboxylated Graphene Nanoplatelets as Efficient Electrode Materials for Electrochemical Supercapacitors. Carbon 2019, 142, 89–98. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, S.; Zeng, G.-M.; Zhang, Y.; Yang, G.-D.; Chen, J.; Wang, J.-J.; Wang, J.-J.; Zhou, Y.-Y.; Deng, Y.-C. Rapid Adsorption of 2,4-Dichlorophenoxyacetic Acid by Iron Oxide Nanoparticles-Doped Carboxylic Ordered Mesoporous Carbon. J. Colloid Interface Sci. 2015, 445, 1–8. [Google Scholar] [CrossRef]
- Bazuła, P.A.; Lu, A.-H.; Nitz, J.-J.; Schüth, F. Surface and Pore Structure Modification of Ordered Mesoporous Carbons via a Chemical Oxidation Approach. Microporous Mesoporous Mater. 2008, 108, 266–275. [Google Scholar] [CrossRef]
- Zheng, N.; Sun, W.; Liu, H.-Y.; Huang, Y.; Gao, J.; Mai, Y.-W. Effects of Carboxylated Carbon Nanotubes on the Phase Separation Behaviour and Fracture-Mechanical Properties of an Epoxy/Polysulfone Blend. Compos. Sci. Technol. 2018, 159, 180–188. [Google Scholar] [CrossRef]
- Bo, X.; Ndamanisha, J.C.; Bai, J.; Guo, L. Nonenzymatic Amperometric Sensor of Hydrogen Peroxide and Glucose Based on Pt Nanoparticles/Ordered Mesoporous Carbon Nanocomposite. Talanta 2010, 82, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Khan, A.; Adewole, J.K.; Naim, M.; Basha, S.I.; Aziz, M.A. Biomass Derived Carboxylated Carbon Nanosheets Blended Polyetherimide Membranes for Enhanced Co2/Ch4 Separation. J. Nat. Gas Sci. Eng. 2020, 75, 103156. [Google Scholar] [CrossRef]
- Eba, H.; Kitakubo, Y.; Awaji, S.; Takahashi, M. Observation of Crystalline Phase Distribution with Confocal Angle-Dispersive X-Ray Diffractometer. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 456, 42–48. [Google Scholar] [CrossRef]
- Sierra, U.; Mercado, A.; Cuara, E.; Barriga-Castro, E.D.; Cortés, A.; Gallardo-Vega, C.; Fernández, S. Coke-Derived Few Layer Graphene-Like Materials by Mild Planetary Milling Exfoliation. Fuel 2020, 262, 116455. [Google Scholar] [CrossRef]
- Jo, Y.; Cheon, J.Y.; Yu, J.; Jeong, H.Y.; Han, C.-H.; Jun, Y.; Joo, S.H. Highly Interconnected Ordered Mesoporous Carbon–Carbon Nanotube Nanocomposites: Pt-Free, Highly Efficient, and Durable Counter Electrodes for Dye-Sensitized Solar Cells. Chem. Commun. 2012, 48, 8057–8059. [Google Scholar] [CrossRef]
- Pazoki, H.; Anbia, M. Synthesis of a Microporous Copper Carboxylate Metal Organic Framework as a New High Capacity Methane Adsorbent. Polyhedron 2019, 171, 108–111. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of Pore Structure and Temperature on Voc Adsorption on Activated Carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. Development of Activated Carbon Pore Structure via Physical and Chemical Activation of Biomass Fibre Waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, H.; Li, Y.; Qi, D.; Wang, S.; Shen, K. Hierarchical Porous Carbon Derived from Carboxylated Coal-Tar Pitch for Electrical Double-Layer Capacitors. RSC Adv. 2019, 9, 29131–29140. [Google Scholar] [CrossRef]
- Ania, C.O.; Gomis-Berenguer, A.; Dentzer, J.; Vix-Guterl, C. Nanoconfinement of Glucose Oxidase on Mesoporous Carbon Electrodes with Tunable Pore Sizes. J. Electroanal. Chem. 2018, 808, 372–379. [Google Scholar] [CrossRef]
- Wang, K.; Yang, H.; Zhu, L.; Ma, Z.; Xing, S.; Lv, Q.; Liao, J.; Liu, C.; Xing, W. Direct Electron Transfer and Electrocatalysis of Glucose Oxidase Immobilized on Glassy Carbon Electrode Modified with Nafion and Mesoporous Carbon Fdu-15. Electrochim. Acta 2009, 54, 4626–4630. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Gu, S.-X.; Li, W.-W.; Chen, T.-T.; Xu, Q.; Wang, W. A Paper Disk Equipped with Graphene/Polyaniline/Au Nanoparticles/Glucose Oxidase Biocomposite Modified Screen-Printed Electrode: Toward Whole Blood Glucose Determination. Biosens. Bioelectron. 2014, 56, 77–82. [Google Scholar] [CrossRef]
- Cui, M.; Xu, B.; Hu, C.; Shao, H.B.; Qu, L. Direct Electrochemistry and Electrocatalysis of Glucose Oxidase on Three-Dimensional Interpenetrating, Porous Graphene Modified Electrode. Electrochim. Acta 2013, 98, 48–53. [Google Scholar] [CrossRef]
- Hui, J.; Cui, J.; Xu, G.; Adeloju, S.B.; Wu, Y. Direct Electrochemistry of Glucose Oxidase Based on Nafion-Graphene-God Modified Gold Electrode and Application To Glucose Detection. Mater. Lett. 2013, 108, 88–91. [Google Scholar] [CrossRef]
- Bagyalakshmi, S.; Sivakami, A.; Balamurugan, K.S. A Zno Nanorods Based Enzymatic Glucose Biosensor by Immobilization of Glucose Oxidase on a Chitosan Film. Obes. Med. 2020, 18, 100229. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, X.; Li, J.; Yao, X.; Li, J. Direct Electrochemistry of Glucose Oxidase and Electrochemical Biosensing of Glucose on Quantum Dots/Carbon Nanotubes Electrodes. Biosens. Bioelectron. 2007, 22, 3203–3209. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhao, F.; Xu, Z.; Dong, S. the Direct Electron Transfer of Glucose Oxidase and Glucose Biosensor Based on Carbon Nanotubes/Chitosan Matrix. Biosens. Bioelectron. 2005, 21, 984–988. [Google Scholar] [CrossRef]
- Fu, C.; Yang, W.; Chen, X.; Evans, D.G. Direct Electrochemistry of Glucose Oxidase on A Graphite Nanosheet–Nafion Composite Film Modified Electrode. Electrochem. Commun. 2009, 11, 997–1000. [Google Scholar] [CrossRef]
- Xu, Q.; Gu, S.-X.; Jin, L.; Zhou, Y.-E.; Yang, Z.; Wang, W.; Hu, X. Graphene/Polyaniline/Gold Nanoparticles Nanocomposite for the Direct Electron Transfer of Glucose Oxidase and Glucose Biosensing. Sens. Actuators B Chem. 2014, 190, 562–569. [Google Scholar] [CrossRef]
- Garcia-Perez, T.; Hong, S.-G.; Kim, J.; Ha, S. Entrapping Cross-Linked Glucose Oxidase Aggregates within A Graphitized Mesoporous Carbon Network for Enzymatic Biofuel Cells. Enzym. Microb. Technol. 2016, 90, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Barathi, P.; Thirumalraj, B.; Chen, S.-M.; Angaiah, S. A Simple and Flexible Enzymatic Glucose Biosensor Using Chitosan Entrapped Mesoporous Carbon Nanocomposite. Microchem. J. 2019, 147, 848–856. [Google Scholar] [CrossRef]
- Deng, S.; Jian, G.; Lei, J.; Hu, Z.; Ju, H. A Glucose Biosensor Based on Direct Electrochemistry of Glucose Oxidase Immobilized on Nitrogen-Doped Carbon Nanotubes. Biosens. Bioelectron. 2009, 25, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Woi, P.M.; Alias, Y. the Selective Electrochemical Detection of Dopamine in the Presence of ascorbic Acid and Uric Acid Using Electro-Polymerised-Β-Cyclodextrin Incorporated F-Mwcnts/Polyaniline Modified Glassy Carbon Electrode. Microchem. J. 2019, 148, 322–330. [Google Scholar] [CrossRef]
- Rębiś, T.; Sobczak, A.; Wierzchowski, M.; Frankiewicz, A.; Teżyk, A.; Milczarek, G. An Approach for Electrochemical Functionalization of Carbon Nanotubes/1-Amino-9,10-Anthraquinone Electrode with Catechol Derivatives for the Development of Nadh Sensors. Electrochim. Acta 2018, 260, 703–715. [Google Scholar] [CrossRef]
- Jędrzak, A.; Rębiś, T.; Klapiszewski, Ł.; Zdarta, J.; Milczarek, G.; Jesionowski, T. Carbon Paste Electrode Based on Functional Gox/Silica-Lignin System To Prepare an Amperometric Glucose Biosensor. Sens. Actuators B Chem. 2018, 256, 176–185. [Google Scholar] [CrossRef]
- Mani, V.; Devadas, B.; Chen, S.-M. Direct Electrochemistry of Glucose Oxidase at Electrochemically Reduced Graphene Oxide-Multiwalled Carbon Nanotubes Hybrid Material Modified Electrode for Glucose Biosensor. Biosens. Bioelectron. 2013, 41, 309–315. [Google Scholar] [CrossRef]
- Xu, X.; Guo, M.; Lu, P.; Wang, R. Development of Amperometric Laccase Biosensor Through Immobilizing Enzyme in Copper-Containing Ordered Mesoporous Carbon (Cu-Omc)/Chitosan Matrix. Mater. Sci. Eng. C 2010, 30, 722–729. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, X.; Huang, J.; Wang, S.; Chen, J. Porous Hollow Tubular Carbon Materials Based on Zeolitic Imidazolate Framework-8 Derived from Zno Nanorods as New Enzyme Immobilizing Matrix for High-Performance Bioanode of Glucose/O2 Biofuel Cells. J. Electroanal. Chem. 2017, 796, 88–95. [Google Scholar] [CrossRef]
- Baker, R.; Wilkinson, D.P.; Zhang, J. Electrocatalytic Activity and Stability of Substituted Iron Phthalocyanines towards Oxygen Reduction Evaluated at Different Temperatures. Electrochim. Acta 2008, 53, 6906–6919. [Google Scholar] [CrossRef]
- Mazar, F.M.; Alijanianzadeh, M.; Molaeirad, A.; Heydari, P. Development of Novel Glucose Oxidase Immobilization on Graphene/Gold Nanoparticles/Poly Neutral Red Modified Electrode. Process. Biochem. 2017, 56, 71–80. [Google Scholar] [CrossRef]
- Gonçales, V.R.; Colombo, R.N.P.; Minadeo, M.A.O.S.; Matsubara, E.Y.; Rosolen, J.M.; Córdoba De Torresi, S.I. Three-Dimensional Graphene/Carbon Nanotubes Hybrid Composites for Exploring Interaction Between Glucose Oxidase and Carbon Based Electrodes. J. Electroanal. Chem. 2016, 775, 235–242. [Google Scholar] [CrossRef]
- Zuo, S.; Teng, Y.; Yuan, H.; Lan, M. Direct Electrochemistry of Glucose Oxidase on Screen-Printed Electrodes Through one-Step Enzyme Immobilization Process with Silica Sol–Gel/Polyvinyl Alcohol Hybrid Film. Sens. Actuators B Chem. 2008, 133, 555–560. [Google Scholar] [CrossRef]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram In the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Hess, A.; Roode-Gutzmer, Q.; Heubner, C.; Schneider, M.; Michaelis, A.; Bobeth, M.; Cuniberti, G. Determination of State of Charge-Dependent Asymmetric Butler–Volmer Kinetics for Lixcoo2 Electrode Using Gitt Measurements. J. Power Sources 2015, 299, 156–161. [Google Scholar] [CrossRef]
- Wang, L.; Bai, J.; Bo, X.; Zhang, X.; Guo, L. A Novel Glucose Sensor Based on Ordered Mesoporous Carbon–Au Nanoparticles Nanocomposites. Talanta 2011, 83, 1386–1391. [Google Scholar] [CrossRef]
- Jin-Zhong, X.; Jun-Jie, Z.; Qiang, W.; Zheng, H.; Hong-Yuan, C. Direct Electron Transfer Between Glucose Oxidase and Multi-Walled Carbon Nanotubes. Chin. J. Chem. 2003, 21, 1088–1091. [Google Scholar] [CrossRef]
- Lata, S.; Batra, B.; Karwasra, N.; Pundir, C.S. an Amperometric H2o2 Biosensor Based on Cytochrome C Immobilized onto Nickel Oxide Nanoparticles/Carboxylated Multiwalled Carbon Nanotubes/Polyaniline Modified Gold Electrode. Process Biochem. 2012, 47, 992–998. [Google Scholar] [CrossRef]
- Wang, G.; Thai, N.M.; Yau, S.-T. Preserved Enzymatic Activity of Glucose Oxidase Immobilized on an Unmodified Electrode. Electrochem. Commun. 2006, 8, 987–992. [Google Scholar] [CrossRef]
- Razmi, H.; Mohammad-Rezaei, R. Graphene Quantum Dots as a New Substrate for Immobilization and Direct Electrochemistry of Glucose Oxidase: Application to Sensitive Glucose Determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, B.; Jakubow, K. The Impact of Immobilization Process on the Electrochemical Performance, Bioactivity and Conformation of Glucose Oxidase Enzyme. Sens. Actuators B Chem. 2017, 238, 852–861. [Google Scholar] [CrossRef]

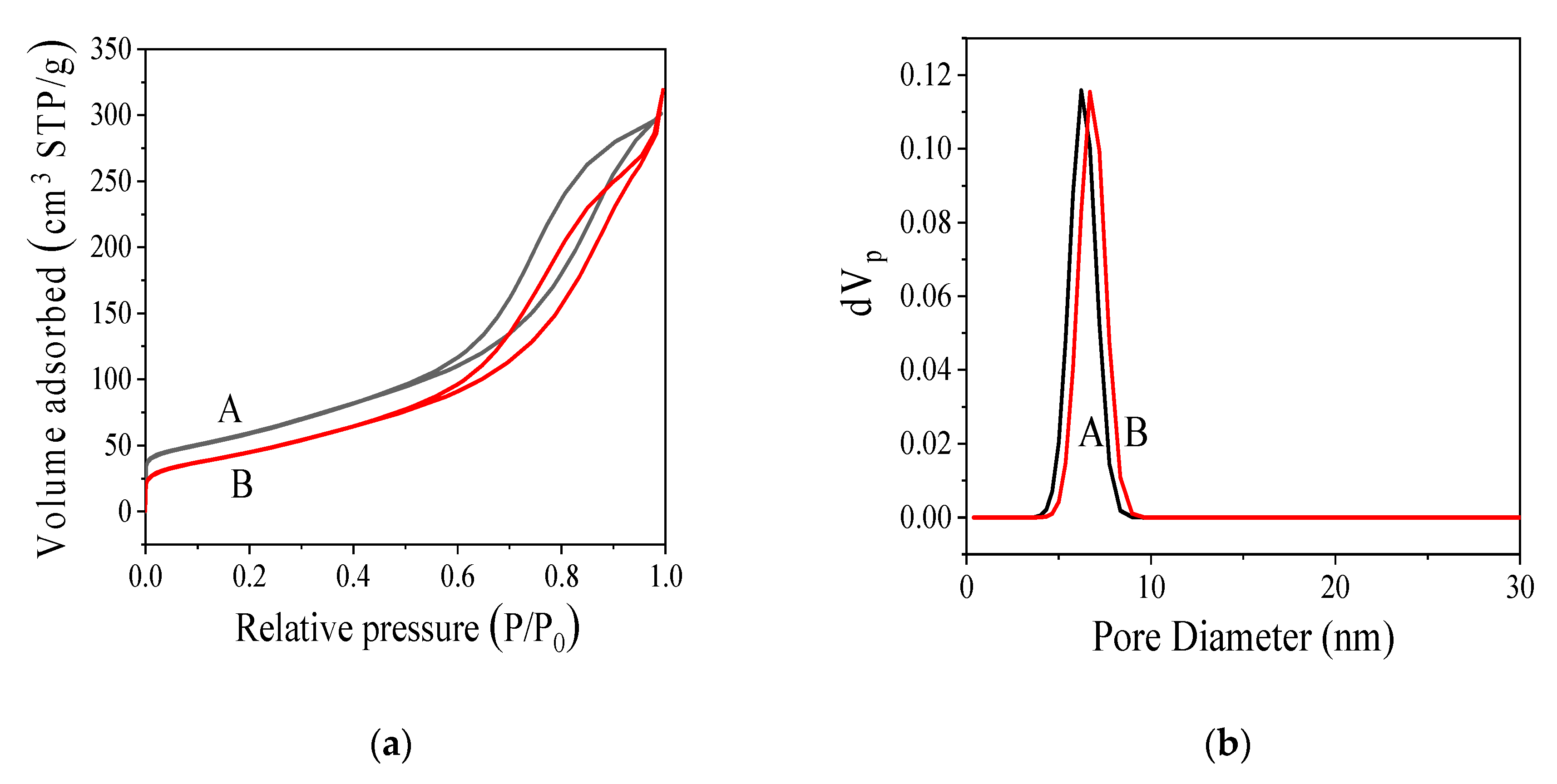

| SBET (m2 g−1) | V (cm3 g−1) | WBJH (nm) | Wd (nm) | SBJH (m2 g−1) | |
|---|---|---|---|---|---|
| MC | 226 | 0.4515 | 4.42 | 7.99 | 202.79 |
| MC-COOH | 181 | 0.4184 | 4.42 | 9.24 | 181.55 |
| Carbon Type | Quality of Immobilized Enzyme (GOx mg/MC g) | Immobilized Enzyme Activity (U/g) | Unit Enzyme Activity U/mg |
|---|---|---|---|
| GOx/MC | 61.06 | 16220.60 | 265.64 |
| GOx/MC-COOH | 140.72 | 57216.50 | 406.60 |
| Electrode | Substrate | Anodic Peak Current | Reduction Peak Current | Quality of Immobilized Enzyme (GOx mg/g) | GOx Surface Coverage | References |
|---|---|---|---|---|---|---|
| Nafion/GOx/MC-COOH | C6H12O6 | 15.66 mA (50 mv/s) | 2.39 mA | 140.72 | 1.29 × 10−8 mol cm−2 | This study |
| GC/CB/GOx | C6H12O6 | 0.045 mA | 0.036 mA | 42 | [52] | |
| RGO-AuNPs/PNR/GOx | C6H12O6 | 0.012 mA | 0.006 mA | 3.06 × 10−11 mol cm−2 | [72] | |
| GOD/PGR2/GCE | C6H12O6 | 0.009 mA | 0.007 mA | 2.3 × 10−10 mol cm−2 | [73] | |
| CPE/GOx-SiO2/Lig/Fc | C6H12O6 | 1.10 mA | 1.25 mA | 25.28 | [67] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Li, S.; Liu, L.; Zhu, X.; Yang, X. Enhanced Electrochemical Characteristics of the Glucose Oxidase Bioelectrode Constructed by Carboxyl-Functionalized Mesoporous Carbon. Sensors 2020, 20, 3365. https://doi.org/10.3390/s20123365
Lv C, Li S, Liu L, Zhu X, Yang X. Enhanced Electrochemical Characteristics of the Glucose Oxidase Bioelectrode Constructed by Carboxyl-Functionalized Mesoporous Carbon. Sensors. 2020; 20(12):3365. https://doi.org/10.3390/s20123365
Chicago/Turabian StyleLv, Chuhan, Shuangfei Li, Liangxu Liu, Xingyu Zhu, and Xuewei Yang. 2020. "Enhanced Electrochemical Characteristics of the Glucose Oxidase Bioelectrode Constructed by Carboxyl-Functionalized Mesoporous Carbon" Sensors 20, no. 12: 3365. https://doi.org/10.3390/s20123365
APA StyleLv, C., Li, S., Liu, L., Zhu, X., & Yang, X. (2020). Enhanced Electrochemical Characteristics of the Glucose Oxidase Bioelectrode Constructed by Carboxyl-Functionalized Mesoporous Carbon. Sensors, 20(12), 3365. https://doi.org/10.3390/s20123365





