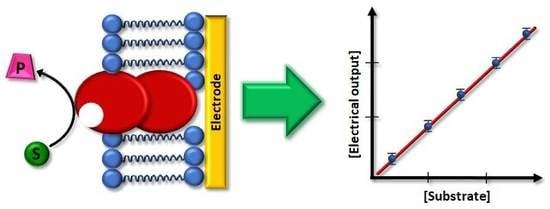
Electrochemical Biosensors Based on Membrane-Bound Enzymes in Biomimetic Configurations
Abstract
:1. Introduction
2. Biomimetic Membranes on Electrodes
3. Reconstitution of Membrane-Bound Enzyme on Electrodes
4. Applications as Biosensors
5. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst 2005, 130, 421–426. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [Green Version]
- Jeuken, L.J.C. Electrodes for integral membrane enzymes. Nat. Prod. Rep. 2009, 26, 1234–1240. [Google Scholar] [CrossRef]
- Laftsoglou, T.; Jeuken, L.J.C. Supramolecular electrode assemblies for bioelectrochemistry. Chem. Commun. 2017, 53, 3801–3809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saboe, P.O.; Conte, E.; Farell, M.; Bazan, G.C.; Kumar, M. Biomimetic and bioinspired approaches for wiring enzymes to electrode interfaces. Energy Environ. Sci. 2017, 10, 14–42. [Google Scholar] [CrossRef]
- Marchal, D.; Pantigny, M.; Laval, J.M.; Moiroux, J.; Bourdillon, C. Rate constants in two dimensions of electron transfer between pyruvate oxidase, a membrane enzyme, and ubiquinone (coezyme Q8), its water-insoluble electron carrier. Biochemistry 2001, 40, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Infossi, P.; Lojou, E.; Chauvin, J.P.; Herbette, G.; Brugna, M.; Giudici-Orticoni, M.T. Aquifex aeolicus membrane hydrogenase of hydrogen biooxidation: Role of lipids and physiological partners in enzyme stability and activity. Int. J. Hydrogen Energy 2010, 35, 10778–10789. [Google Scholar] [CrossRef]
- Lipkowski, J. Biomimetics: A new research opportunity for surface electrochemistry. J. Solid State Electrochem. 2020. [Google Scholar] [CrossRef]
- Junghans, A.; Köper, I. Structural analysis of tethered bilayer lipid membranes. Langmuir 2010, 26, 11035–11040. [Google Scholar] [CrossRef]
- Kycia, A.H.; Su, Z.; Brosseau, C.L.; Lipkowski, J. In situ PM–IRRAS studies of biomimetic membranes supported at gold electrode surfaces. In Vibrational Spectroscopy at Electrified Interfaces; Wieckowski, A., Korzeniewski, C., Braunschweig, B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 345–417. [Google Scholar]
- Zawisza, I.; Bin, X.; Lipkowski, J. Potential-driven structural changes in Langmuir−Blodgett DMPC bilayers determined by in situ spectroelectrochemical PM IRRAS. Langmuir 2007, 23, 5180–5194. [Google Scholar] [CrossRef]
- Sackmann, E. Supported membranes: Scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Reviakine, I.; Brisson, A. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir 2000, 16, 1806–1815. [Google Scholar] [CrossRef]
- Leonenko, Z.V.; Carnini, A.; Cramb, D.T. Supported planar bilayer formation by vesicle fusion: The interaction of phospholipid vesicles with surfaces and the effect of gramicidin on bilayer properties using atomic force microscopy. BBA Biomembr. 2000, 1509, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Lipowsky, R.; Seifert, U. Adhesion of vesicles and membranes. Mol. Cryst. Liq. Cryst. 1991, 202, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Pawłowski, J.; Juhaniewicz, J.; Güzeloğlu, A.; Sek, S. Mechanism of lipid vesicles spreading and bilayer formation on a Au(111) Surface. Langmuir 2015, 31, 11012–11019. [Google Scholar] [CrossRef] [PubMed]
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model cell membranes: Techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Curr. Opin. Colloid Interface Sci. 2013, 18, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Cornell, B.A.; Braach-Maksvytis, V.L.B.; King, L.G.; Osman, P.D.J.; Raguse, B.; Wieczorek, L.; Pace, R.J. A biosensor that uses ion-channel switches. Nature 1997, 387, 580–583. [Google Scholar] [CrossRef]
- Su, Z.; Leitch, J.J.; Lipkowski, J. Electrode-supported biomimetic membranes: An electrochemical and surface science approach for characterizing biological cell membranes. Curr. Opin. Electrochem. 2018, 12, 60–72. [Google Scholar] [CrossRef]
- Juhaniewicz, J.; Sek, S. Atomic force microscopy and electrochemical studies of melittin action on lipid bilayers supported on gold electrodes. Electrochim. Acta 2015, 162, 53–61. [Google Scholar] [CrossRef]
- Bin, X.; Zawisza, I.; Goddard, J.D.; Lipkowski, J. Electrochemical and PM-IRRAS studies of the effect of the static electric field on the structure of the DMPC bilayer supported at a Au(111) electrode surface. Langmuir 2005, 21, 330–347. [Google Scholar] [CrossRef]
- Zawisza, I.; Lachenwitzer, A.; Zamlynny, V.; Horswell, S.L.; Goddard, J.D.; Lipkowski, J. Electrochemical and photon polarization modulation infrared reflection absorption spectroscopy study of the electric field driven transformations of a phospholipid bilayer supported at a gold electrode surface. Biophys. J. 2003, 85, 4055–4075. [Google Scholar] [CrossRef] [Green Version]
- Horswell, S.L.; Zamlynny, V.; Li, H.Q.; Merrill, A.R.; Lipkowski, J. Electrochemical and PM-IRRAS studies of potential controlled transformations of phospholipid layers on Au (111) electrodes. Faraday Discuss. 2002, 121, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Araez, N.; Brosseau, C.L.; Rodriguez, P.; Lipkowski, J. Layer-by-layer PMIRRAS characterization of DMPC bilayers deposited on a Au(111) electrode surface. Langmuir 2006, 22, 10365–10371. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.L.; Leitch, J.; Bin, X.; Chen, M.; Roscoe, S.G.; Lipkowski, J. Electrochemical and PM-IRRAS a glycolipid-containing biomimetic membrane prepared using Langmuir−Blodgett/Langmuir−Schaefer deposition. Langmuir 2008, 24, 13058–13067. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Matsunaga, S.; Yamada, T.; Kobayashi, T.; Kawai, M. Formation of ordered phospholipid monolayer on a hydrophilically modified Au (111) substrate. ACS Nano 2016, 10, 7811–7820. [Google Scholar] [CrossRef] [PubMed]
- Sek, S.; Xu, S.; Chen, M.; Szymanski, G.; Lipkowski, J. STM studies of fusion of cholesterol suspensions and mixed 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC)/cholesterol vesicles onto a Au (111) electrode surface. J. Am. Chem. Soc. 2008, 130, 5736–5743. [Google Scholar] [CrossRef]
- Xu, S.; Szymanski, G.; Lipkowski, J. Self-assembly of phospholipid molecules at a Au (111) electrode surface. J. Am. Chem. Soc. 2004, 126, 12276–12277. [Google Scholar] [CrossRef]
- Prieto, F.; Rueda, M.; Naitlho, N.; Vázquez-González, M.; González-Rodríguez, M.L.; Rabasco, A.M. Electrochemical characterization of a mixed lipid monolayer supported on Au (111) electrodes with implications for doxorubicin delivery. J. Electroanal. Chem. 2018, 815, 246–254. [Google Scholar] [CrossRef]
- Alvarez-Malmagro, J.; Jablonowska, E.; Nazaruk, E.; Szwedziak, P.; Bilewicz, R. How do lipid nanocarriers – cubosomes affect electrochemical properties of DMPC bilayers deposited on gold (111) electrodes? Bioelectrochemistry 2020, 134, 107516. [Google Scholar] [CrossRef]
- Zamlynny, V.; Zawisza, I.; Lipkowski, J. PM FTIRRAS studies of potential-controlled transformations of a monolayer and a bilayer of 4-pentadecylpyridine, a model surfactant, adsorbed on a Au(111) electrode surface. Langmuir 2003, 19, 132–145. [Google Scholar] [CrossRef]
- Groves, J.T.; Ulman, N.; Boxer, S.G. Micropatterning fluid lipid bilayers on solid supports. Science 1997, 275, 651–653. [Google Scholar] [CrossRef]
- Groves, J.T.; Boxer, S.G. Micropattern formation in supported lipid membranes. Acc. Chem. Res. 2002, 35, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.F.; Shodiev, M.; Jay Leitch, J.; Abbasi, F.; Lipkowski, J. In situ electrochemical and PM-IRRAS studies of alamethicin ion channel formation in model phospholipid bilayers. J. Electroanal. Chem. 2018, 819, 251–259. [Google Scholar] [CrossRef]
- Yang, T.; Baryshnikova, O.K.; Mao, H.; Holden, M.A.; Cremer, P.S. Investigations of bivalent antibody binding on fluid-supported phospholipid membranes: The effect of hapten density. J. Am. Chem. Soc. 2003, 125, 4779–4784. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Malmagro, J.; Matyszewska, D.; Nazaruk, E.; Szwedziak, P.; Bilewicz, R. PM-IRRAS study on the effect of phytantriol-based cubosomes on DMPC bilayers as model lipid membranes. Langmuir 2019, 35, 16650–16660. [Google Scholar] [CrossRef]
- Kycia, A.H.; Wang, J.; Merrill, A.R.; Lipkowski, J. Atomic force microscopy studies of a floating-bilayer lipid membrane on a Au(111) surface modified with a hydrophilic monolayer. Langmuir 2011, 27, 10867–10877. [Google Scholar] [CrossRef]
- Knoll, W.; Köper, I.; Naumann, R.; Sinner, E.K. Tethered bimolecular lipid membranes. A novel model membrane platform. Electrochim. Acta 2008, 53, 6680–6689. [Google Scholar] [CrossRef]
- Vockenroth, I.K.; Rossi, C.; Shah, M.R.; Köper, I. Formation of tethered bilayer lipid membranes probed by various surface sensitive techniques. Biointerphases 2009, 4, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Ragaliauskas, T.; Mickevicius, M.; Rakovska, B.; Penkauskas, T.; Vanderah, D.J.; Heinrich, F.; Valincius, G. Fast formation of low-defect-density tethered bilayers by fusion of multilamellar vesicles. Biochim. Biophys. Acta Biomembr. 2017, 1859, 669–678. [Google Scholar] [CrossRef]
- McGillivray, D.J.; Valincius, G.; Vanderah, D.J.; Febo-Ayala, W.; Woodward, J.T.; Heinrich, F.; Kasianowicz, J.J.; Lösche, M. Molecular-scale structural and functional characterization of sparsely tethered bilayer lipid membranes. Biointerphases 2007, 2, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Budvytyte, R.; Mickevicius, M.; Vanderah, D.J.; Heinrich, F.; Valincius, G. Modification of tethered bilayers by phospholipid exchange with vesicles. Langmuir 2013, 29, 4320–4327. [Google Scholar] [CrossRef]
- Leitch, J.; Kunze, J.; Goddard, J.D.; Schwan, A.L.; Faragher, R.J.; Naumann, R.; Knol, W.; Dutcher, J.R.; Lipkowski, J. In situ PM-IRRAS studies of an archaea analogue thiolipid assembled on a Au(111) electrode surface. Langmuir 2009, 25, 10354–10363. [Google Scholar] [CrossRef] [PubMed]
- Vockenroth, I.K.; Ohm, C.; Robertson, J.W.; McGillivray, D.J.; Lösche, M.; Köper, I. Stable insulating tethered bilayer lipid membranes. Biointerphases 2008, 3, FA68–FA73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbrig, E.; Staffa, J.K.; Salewski, J.; Mroginski, M.A.; Hildebrandt, P.; Kozuch, J. Monitoring the orientational changes of alamethicin during incorporation into bilayer lipid membranes. Langmuir 2018, 34, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Schiller, S.M.; Naumann, R.; Lovejoy, K.; Kunz, H.; Knoll, W. Archaea analogue thiolipids for tethered bilayer lipid membranes on ultrasmooth gold surfaces. Ang. Chem. Int. Ed. 2003, 42, 208–211. [Google Scholar] [CrossRef]
- Valincius, G.; Meškauskas, T.; Ivanauskas, F. Electrochemical impedance spectroscopy of tethered bilayer membranes. Langmuir 2012, 28, 977–990. [Google Scholar] [CrossRef]
- Valincius, G.; Mickevicius, M.; Penkauskas, T.; Jankunec, M. Electrochemical impedance spectroscopy of tethered bilayer membranes: An effect of heterogeneous distribution of defects in membranes. Electrochim. Acta 2016, 222, 904–913. [Google Scholar] [CrossRef]
- Girard-Egrot, A.P.; Blum, L. Langmuir-Blodgett technique for synthesis of biomimetic lipid membranes. In Nanobiotechnology of Biomimetic Membranes; Ferrari, M., Martin, D.K., Eds.; Springer: Boston, MA, USA, 2007; pp. 23–74. [Google Scholar]
- Györvary, E.; Wetzer, B.; Sleytr, U.B.; Sinner, A.; Offenhäusser, A.; Knoll, W. Lateral diffusion of lipids in silane-, dextran-, and s-layer-supported mono-and bilayers. Langmuir 1999, 15, 1337–1347. [Google Scholar] [CrossRef]
- Purrucker, O.; Förtig, A.; Jordan, R.; Tanaka, M. Supported membranes with well-defined polymer tethers-Incorporation of cell receptors. Chem. Phys. Chem. 2004, 5, 327–335. [Google Scholar] [CrossRef]
- Tanaka, M.; Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 2005, 437, 656–663. [Google Scholar] [CrossRef]
- Hertrich, S.; Stetter, F.; Rühm, A.; Hugel, T.; Nickel, B. Highly hydrated deformable polyethylene glycol-tethered lipid bilayers. Langmuir 2014, 30, 9442–9447. [Google Scholar] [CrossRef] [PubMed]
- Kibrom, A.; Roskamp, R.F.; Jonas, U.; Menges, B.; Knoll, W.; Paulsen, H.; Naumann, R.L. Hydrogel-supported protein-tethered bilayer lipid membranes: A new approach toward polymer-supported lipid membranes. Soft Matter 2011, 7, 237–246. [Google Scholar] [CrossRef]
- Su, Z.; Jiang, Y.; Velázquez-Manzanares, M.; Leitch, J.J.; Kycia, A.; Lipkowski, J. Electrochemical and PM-IRRAS studies of floating lipid bilayers assembled at the Au(111) electrode pre-modified with a hydrophilic monolayer. J. Electroanal. Chem. 2013, 688, 76–85. [Google Scholar] [CrossRef]
- Su, Z.; Juhaniewicz-Debinska, J.; Sek, S.; Lipkowski, J. Water structure in the submembrane region of a floating lipid bilayer: The effect of an ion channel formation and the channel blocker. Langmuir 2020, 36, 409–418. [Google Scholar] [CrossRef]
- Ryu, H.; Fuwad, A.; Yoon, S.; Jang, H.; Lee, J.C.; Kim, S.M.; Jeon, T.J. Biomimetic membranes with transmembrane proteins: State-of-the-art in transmembrane protein applications. Int. J. Mol. Sci. 2019, 20, 1437. [Google Scholar] [CrossRef] [Green Version]
- Casero, E.; Darder, M.; Pariente, F.; Lorenzo, E.; Martín-Benito, J.; Vázquez, L. Thiol-functionalized gold surfaces as a strategy to induce order in membrane-bound enzyme immobilization. Nano Lett. 2002, 2, 577–582. [Google Scholar] [CrossRef]
- Ciaccafava, A.; Infossi, P.; Ilbert, M.; Guiral, M.; Lecomte, S.; Giudici-Orticoni, M.T.; Lojou, E. Electrochemistry, AFM, and PM-IRRA spectroscopy of immobilized hydrogenase: Role of a hydrophobic helix in enzyme orientation for efficient H2 oxidation. Angew. Chem. Int. Ed. 2012, 51, 953–956. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, C.; Olea, D.; Marques, M.; Fernández, V.M.; Pereira, I.A.C.; Vélez, M.; De Lacey, A.L. Oriented immobilization of a membrane-bound hydrogenase onto an electrode for direct electron transfer. Langmuir 2011, 27, 6449–6457. [Google Scholar] [CrossRef] [Green Version]
- Kawai, S.; Yakushi, T.; Matsushita, K.; Kitazumi, Y.; Shirai, O.; Kano, K. Role of a non-ionic surfactant in direct electron transfer-type bioelectrocatalysis by fructose dehydrogenase. Electrochim. Acta 2015, 152, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Sultana, N.; Schenkman, J.B.; Rusling, J.F. Protein film electrochemistry of microsomes genetically enriched in human cytochrome P450 monooxygenases. J. Am. Chem. Soc. 2005, 127, 13460–13461. [Google Scholar] [CrossRef]
- Mie, Y.; Suzuki, M.; Komatsu, Y. Electrochemically driven drug metabolism by membranes containing human cytochrome P450. J. Am. Chem. Soc. 2009, 131, 6646–6647. [Google Scholar] [CrossRef] [PubMed]
- Cashman, J.R. The implications of polymorphisms in mammalian flavin-containing monooxygenases in drug discovery and development. Drug Discov. Today 2004, 9, 574–581. [Google Scholar] [CrossRef]
- Cashman, J.R. Role of flavin-containing monooxgenase in drug development. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Castrignano, S.; Gilardi, G.; Sadeghi, S.J. Human flavin-containing monooxygenase 3 on graphene oxide for drug metabolism screening. Anal. Chem. 2015, 87, 2974–2980. [Google Scholar] [CrossRef]
- Weiss, S.A.; Jeuken, L.J.C. Lipid-membrane modified electrodes to study quinone oxidoreductases. Biochem. Soc. Trans. 2009, 37, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Heath, G.R.; Li, M.; Rong, H.; Radu, V.; Frielingsdorf, S.; Lenz, O.; Butt, J.N.; Jeuken, L.J.C. Multilayered lipid membrane stacks for biocatalysis using membrane enzymes. Adv. Funct. Mater. 2017, 27, 1606265. [Google Scholar] [CrossRef] [Green Version]
- Sezer, M.; Frielingsdorf, S.; Millo, D.; Heidary, N.; Utesch, T.; Mroginski, M.-A.; Friedrich, B.; Hildebrandt, P.; Zebger, I.; Weidinger, I.M. Role of the HoxZ subunit in the electron transfer pathway of the membrane-bound [NiFe]-hydrogenase from Ralstonia eutropha immobilized on electrodes. J. Phys. Chem. B 2011, 115, 10368–10374. [Google Scholar] [CrossRef]
- Valente, F.M.A.; Pereira, P.M.; Venceslau, S.S.; Regalla, M.; Coelho, A.V.; Pereira, I.A.C. The [NiFeSe] hydrogenase from Desulfovibrio vulgaris Hildenborough is a bacterial lipoprotein lacking a typical lipoprotein signal peptide. FEBS Lett. 2007, 581, 3341–3344. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Sanz, O.; Tapia, C.; Marques, M.C.; Zacarias, S.; Vélez, M.; Pereira, I.A.C.; De Lacey, A.L. Induction of a proton gradient across a gold-supported biomimetic membrane by electroenzymatic H2 oxidation. Angew. Chem. Int. Ed. 2015, 54, 2684–2687. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Sanz, O.; Marques, M.; Pereira, I.A.C.; De Lacey, A.L.; Lubitz, W.; Rüdiger, O. Orientation and function of a membrane-bound enzyme monitored by electrochemical surface-enhanced infrared absorption spectroscopy. J. Phys. Chem. Lett. 2013, 4, 2794–2798. [Google Scholar] [CrossRef]
- Shi, Y. Common folds and transport mechanisms of secondary active transporters. Annu. Rev. Biophys. 2013, 42, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Junge, W.; Nelson, N. ATP synthase. Annu. Rev. Biochem. 2015, 84, 631–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, S. Dynamism and regulation of the stator, the energy conversion complex of the bacterial flagellar motor. Curr. Opin. Microbiol 2015, 28, 66–71. [Google Scholar] [CrossRef] [PubMed]
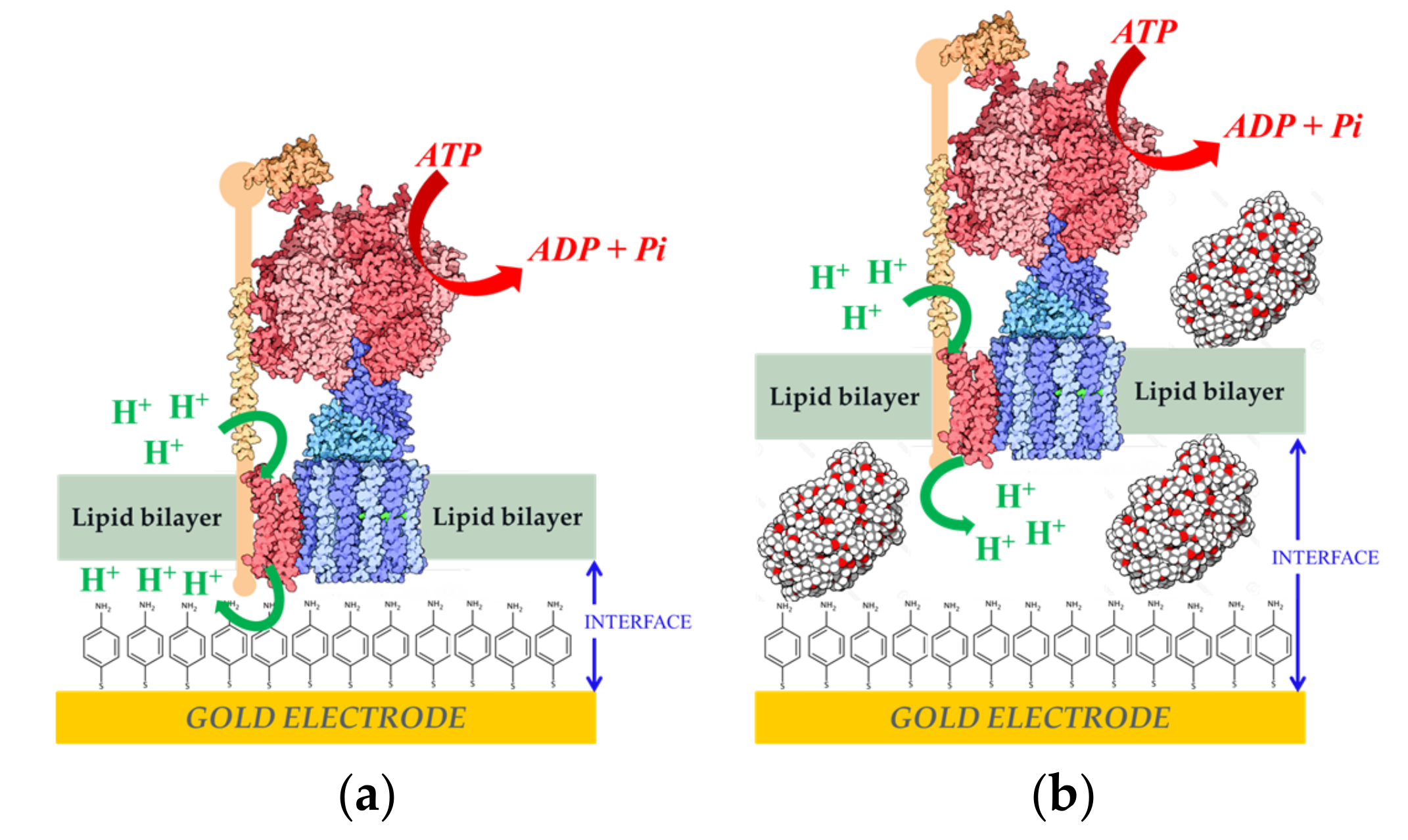
- Gutiérrez-Sanz, O.; Natale, P.; Márquez, I.; Marques, M.C.; Zacarias, S.; Pita, M.; Pereira, I.A.C.; López-Montero, I.; De Lacey, A.L.; Vélez, M. H2-fueled ATP synthesis on an electrode: Mimicking cellular respiration. Angew. Chem. Int. Ed. 2016, 55, 6216–6220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Molina, G.; Natale, P.; Valenzuela, L.; Alvarez-Malmagro, J.; Gutiérrez-Sánchez, C.; Iglesias-Juez, A.; López-Montero, I.; Vélez, M.; Pita, M.; De Lacey, A.L. Potentiometric detection of ATP based on the transmembrane proton gradient generated by ATPase reconstituted on a gold electrode. Bioelectrochemistry 2020, 133, 107490. [Google Scholar] [CrossRef]
- Gutiérrez-Sanz, O.; Olea, D.; Pita, M.; Batista, A.P.; Alonso, A.; Pereira, M.M.; Vélez, M.; De Lacey, A.L. Reconstitution of respiratory complex I on a biomimetic membrane supported on gold electrodes. Langmuir 2014, 30, 9007–9015. [Google Scholar] [CrossRef] [Green Version]
- Schapira, A. Human complex I defects in neurodegenerative diseases. Biochim. Biophys. Acta-Bioenerg. 1998, 1364, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Hunte, C.; Zickermann, V.; Brandt, U. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science 2010, 329, 448–451. [Google Scholar] [CrossRef]
- Baradaran, R.; Berrisford, J.M.; Minhas, G.S.; Sazanov, L.A. Crystal structure of the entire respiratory complex I. Nature 2013, 494, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Sanz, O.; Forbrig, E.; Batista, A.P.; Pereira, M.M.; Salewski, J.; Mroginski, M.A.; Götz, R.; De Lacey, A.L.; Kozuch, J.; Zebger, I. Catalytic activity and proton translocation of reconstituted respiratory complex I monitored by surface-enhanced infrared absorption spectroscopy. Langmuir 2018, 34, 5703–5711. [Google Scholar] [CrossRef]
- Kinnear, K.T.; Monbouquette, H.G. An amperometric fructose biosensor based on fructose dehydrogenase immobilized in a membrane layer on gold. Anal. Chem. 1997, 69, 1771–1775. [Google Scholar] [CrossRef]
- Darder, M.; Casero, E.; Pariente, F.; Lorenzo, E. Biosensors based on membrane-bound enzymes immobilized in 5-(octyldithio)-2-nitrobenzoic acid layer on gold electrodes. Anal. Chem. 2000, 72, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Landau, E.M.; Bilewicz, R. Membranne bound enzyme hosted in liquid crystalline cubic phase for sensing and fuel cells. Electrochim. Acta 2014, 140, 96–100. [Google Scholar] [CrossRef]
- Kreit, J.; Sampson, N.S. Cholesterol oxidase: Physiological functions. FEBS J. 2009, 276, 6844–6856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.; Marz, W.; Wieland, H. Is lipoprotein(a) cholesterol a significant indicator of cardiovascular risk? Clin. Chem. 2000, 46, 436–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicklein, B.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Phospholipid-sepiolite biomimetic interfaces for the immobilization of enzymes. ACS Appl. Mater. Interfaces 2011, 3, 4339–4348. [Google Scholar] [CrossRef]
- Psychoyios, V.N.; Nikoleli, G.P.; Tzamtzis, N.; Nikolelis, D.P.; Psaroudakis, N.; Danielsson, B.; Qadir Israr, M.; Willander, M. Potentiometric cholesterol biosensor based on ZnO nanowalls and stabilized polymerized lipid film. Electroanalysis 2013, 25, 367–372. [Google Scholar] [CrossRef]
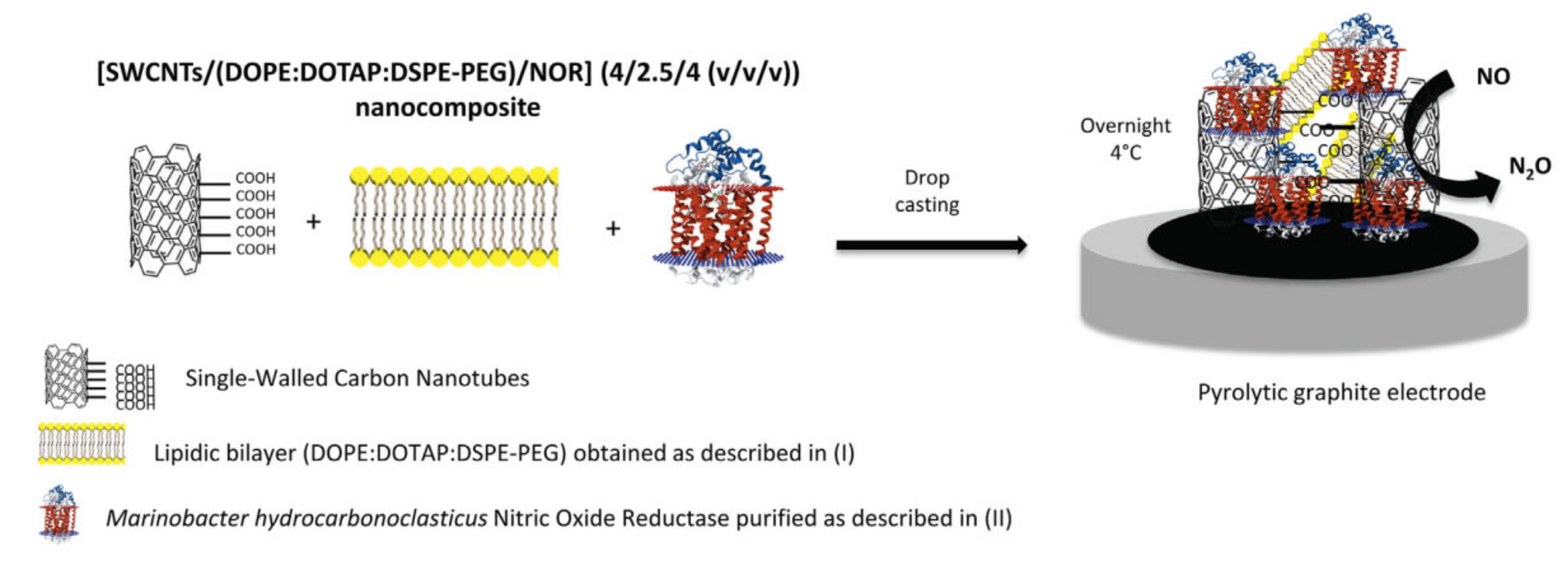
- Gomes, F.O.; Maia, L.B.; Loureiro, J.A.; Pereira, M.C.; Delerue-Matos, C.; Moura, I.; Moura, J.J.G.; Morais, S. Biosensor for direct bioelectrocatalysis detection of nitric oxide using nitric oxide reductase incorporated in carboxylated single-walled carbon nanotubes/lipidic 3 bilayer nanocomposite. Bioelectrochemistry 2019, 127, 76–86. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Rizarelli, E.; Owen, J.B.; Dinkova-Kostova, A.T.; Butterfield, D.A. Nitric oxide in in cell survival: A janus molecule. Antioxid. Redox Signal 2009, 11, 2717–2739. [Google Scholar] [CrossRef] [Green Version]
- Ziller, C.; Lin, J.; Knittel, P.; Friedrich, L.; Andronescu, C.; Pöller, S.; Schuhmann, W.; Kranz, C. Poly(benzooxacine) as an immobilization matrix for miniaturized ATP and glucose biosensors. ChemElectroChem 2017, 4, 864–871. [Google Scholar] [CrossRef]
- Moretro, T.; Normann, M.A.; Saebo, H.R.; Langsrud, S. Evaluation of ATP bioluminiscence-based methods for hygienic assessment in fish industry. J. Appl. Microbiol. 2019, 127, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Chen, T.; Feng, C.; Li, G. Electrochemical analysis of enzyme based on the self-assembly of lipid bilayer on an electrode surface mediated by hydrazone chemistry. Anal. Chem. 2017, 89, 13245–13251. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhang, F.; Yu, J.; Chi, D. The novel acetylcholinesterase biosensor based on liposome bioreactors-chitosan nanocomposite film for detection of organophosphates pesticides. Food Res. Int. 2012, 49, 15–21. [Google Scholar] [CrossRef]
- Yan, J.; Guan, H.; Yu, J.; Chi, D. Acetylcholinesterase biosensor based on assembly of multiwall carbon natubes onto liposome bioreactors for detection of organophosphates pesticides. Pesticide Biochem. Physiol. 2013, 105, 197–202. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Malmagro, J.; García-Molina, G.; López De Lacey, A. Electrochemical Biosensors Based on Membrane-Bound Enzymes in Biomimetic Configurations. Sensors 2020, 20, 3393. https://doi.org/10.3390/s20123393
Alvarez-Malmagro J, García-Molina G, López De Lacey A. Electrochemical Biosensors Based on Membrane-Bound Enzymes in Biomimetic Configurations. Sensors. 2020; 20(12):3393. https://doi.org/10.3390/s20123393
Chicago/Turabian StyleAlvarez-Malmagro, Julia, Gabriel García-Molina, and Antonio López De Lacey. 2020. "Electrochemical Biosensors Based on Membrane-Bound Enzymes in Biomimetic Configurations" Sensors 20, no. 12: 3393. https://doi.org/10.3390/s20123393
APA StyleAlvarez-Malmagro, J., García-Molina, G., & López De Lacey, A. (2020). Electrochemical Biosensors Based on Membrane-Bound Enzymes in Biomimetic Configurations. Sensors, 20(12), 3393. https://doi.org/10.3390/s20123393