Impact of Trunk Resistance and Stretching Exercise on Fall-Related Factors in Patients with Parkinson’s Disease: A Randomized Controlled Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Functional Fitness Test
2.2.2. Trunk Mobility Scale Test
2.2.3. Standing Balance Test
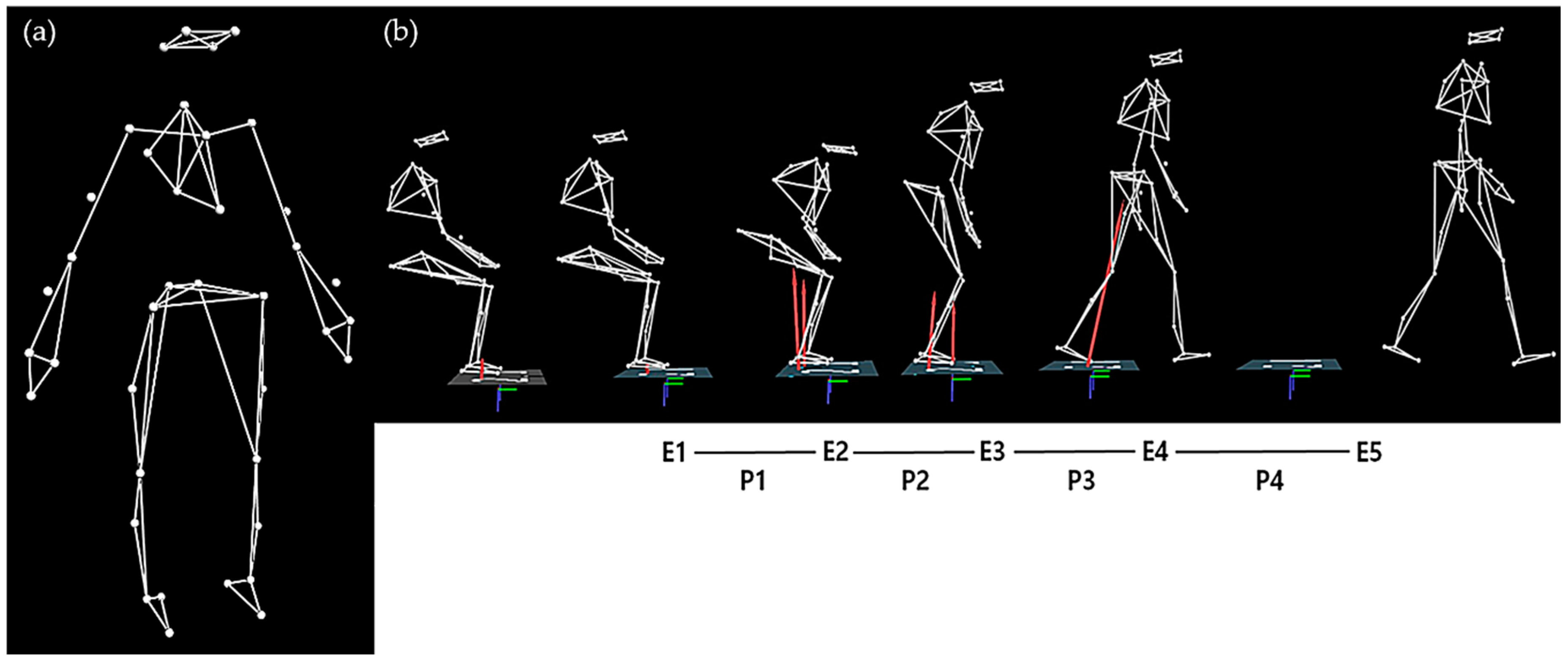
2.2.4. Sit-to-Walk Test
2.2.5. Progressive Trunk Resistance and Stretching Exercise Program
2.3. Data Processing
2.3.1. Standing Balance Test
2.3.2. Sit-to-Walk Test
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeMaagd, G.; Philip, A. Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm. Ther. 2015, 40, 504. [Google Scholar]
- Doherty, K.M.; van de Warrenburg, B.P.; Peralta, M.C.; Silveira-Moriyama, L.; Azulay, J.P.; Gershanik, O.S.; Bloem, B.R. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011, 10, 538–549. [Google Scholar] [CrossRef]
- Ashour, R.; Jankovic, J. Joint and skeletal deformities in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Mov. Disord. 2006, 21, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Van Emmerik, R.E.A.; Wagenaar, R.C.; Winogrodzka, A.; Wolters, E.C. Identification of axial rigidity during locomotion in Parkinson disease. Arch. Phys. Med. Rehabil. 1999, 80, 186–191. [Google Scholar] [CrossRef]
- Benatru, I.; Vaugoyeau, M.; Azulay, J.P. Postural disorders in Parkinson’s disease. Neurophysiol. Clin. 2008, 38, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.H.; Silburn, P.A.; Wood, J.M.; Worringham, C.J.; Kerr, G.K. Falls in Parkinson’s disease: Kinematic evidence for impaired head and trunk control. Mov. Disord. 2010, 25, 2369–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolo, M.; Serrao, M.; Tassorelli, C.; Don, R.; Ranavolo, A.; Draicchio, F.; Pacchetti, C.; Buscone, S.; Perrotta, A.; Furnari, A.; et al. Four-week trunk-specific rehabilitation treatment improves lateral trunk flexion in Parkinson’s disease. Mov. Disord. 2010, 25, 325–331. [Google Scholar] [CrossRef]
- Gandolfi, M.; Tinazzi, M.; Magrinelli, F.; Busselli, G.; Dimitrova, E.; Polo, N.; Manganotti, P.; Fasano, A.; Smania, N.; Geroin, C. Four-week trunk-specific exercise program decreases forward trunk flexion in Parkinson’s disease: A single-blinded, randomized controlled trial. Parkinsonism Relat. Disord. 2019, 64, 268–274. [Google Scholar] [CrossRef]
- Hubble, R.P.; Naughton, G.; Silburn, P.A.; Cole, M.H. Trunk exercises improve gait symmetry in Parkinson disease: A blind phase II randomized controlled trial. Am. J. Phys. Med. Rehabil. 2018, 97, 151–159. [Google Scholar] [CrossRef]
- Hubble, R.P.; Silburn, P.A.; Naughton, G.A.; Cole, M.H. Trunk exercises improve balance in Parkinson disease: A phase II randomized controlled trial. J. Neurol. Phys. 2019, 43, 96–105. [Google Scholar] [CrossRef]
- Paul, S.S.; Sherrington, C.; Canning, C.G.; Fung, V.S.; Close, J.C.; Lord, S.R. The relative contribution of physical and cognitive fall risk factors in people with Parkinson’s disease: A large prospective cohort study. Neurorehabil. Neural Repair 2014, 28, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Latt, M.D.; Lord, S.R.; Morris, J.G.; Fung, V.S. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov. Disord. 2009, 24, 1280–1289. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cancela, J.M.; Ayán, C.; Gutiérrez-Santiago, A.; Prieto, I.; Varela, S. The senior fitness test as a functional measure in Parkinson’s disease: A pilot study. Parkinsonism Relat. Disord. 2012, 18, 170–173. [Google Scholar] [CrossRef]
- Franco, C.R.; Leão, P.; Townsend, R.; Rieder, C.R. Reliability and validity of a scale for measurement of trunk mobility in Parkinson’s disease: Trunk Mobility Scale. Arq. Neuropsiquiatr. 2011, 69, 636–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlenstedt, C.; Muthuraman, M.; Witt, K.; Weisser, B.; Fasano, A.; Deuschl, G. Postural control and freezing of gait in Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 24, 107–112. [Google Scholar] [CrossRef]
- Buckley, T.A.; Pitsikoulis, C.; Hass, C.J. Dynamic postural stability during sit-to-walk transitions in Parkinson disease patients. Mov. Disord. 2008, 23, 1274–1280. [Google Scholar] [CrossRef]
- Akuthota, V.; Ferreiro, A.; Moore, T.; Fredericson, M. Core stability exercise principles. Curr. Sports Med. Rep. 2008, 7, 39–43. [Google Scholar] [CrossRef]
- Granacher, U.; Lacroix, A.; Muehlbauer, T.; Roettger, K.; Gollhofer, A. Effects of core instability strength training on trunk muscle strength, spinal mobility, dynamic balance and functional mobility in older adults. Gerontology 2013, 59, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Doyle, T.L.; Dugan, E.L.; Humphries, B.; Newton, R.U. Discriminating between elderly and young using a fractal dimension analysis of centre of pressure. Int. J. Med. Sci 2004, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Son, M.J.; Kim, Y.K.; Lee, M.G.; Kim, J.H.; Youm, C.H. Effects of freezing of gait and visual information on the static postural control ability in patients with Parkinson’s disease. Korean J. Sport Biomech. 2016, 26, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Toole, T.; Hirsch, M.A.; Forkink, A.; Lehman, D.A.; Maitland, C.G. The effects of a balance and strength training program on equilibrium in Parkinsonism: A preliminary study. NeuroRehabilitation 2000, 14, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.A.; Toole, T.; Maitland, C.G.; Rider, R.A. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch. Phys. Med. Rehabil. 2003, 84, 1109–1117. [Google Scholar] [CrossRef]
- Chung, C.L.H.; Thilarajah, S.; Tan, D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 11–23. [Google Scholar] [CrossRef] [PubMed]
- David, F.J.; Rafferty, M.R.; Robichaud, J.A.; Prodoehl, J.; Kohrt, W.M.; Vaillancourt, D.E.; Corcos, D.M. Progressive resistance exercise and Parkinson’s disease: A review of potential mechanisms. Parkinsons Dis. 2012, 2012, 124527. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.O.; Scianni, A.A.; Rodrigues-de-Paula, F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson’s disease: A systematic review. J. Physiother. 2013, 59, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Lang, A.E.; Lozano, A.M. Parkinson’s disease. N. Engl. J. Med. 1998, 339, 130–143. [Google Scholar] [CrossRef]
- McHugh, M.P.; Cosgrave, C.H. To stretch or not to stretch: The role of stretching in injury prevention and performance. Scand. J. Med. Sci. Sports 2010, 20, 169–181. [Google Scholar] [CrossRef]
- Behm, D.G.; Blazevich, A.J.; Kay, A.D.; McHugh, M. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: A systematic review. Appl. Physiol. Nutr. Metab. 2016, 41, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Masugi, Y.; Obata, H.; Inoue, D.; Kawashima, N.; Nakazawa, K. Neural effects of muscle stretching on the spinal reflexes in multiple lower-limb muscles. PLos ONE 2017, 12, e0180275. [Google Scholar] [CrossRef]
- Oliveri, M.; Caltagirone, C.; Loriga, R.; Pompa, M.N.; Versace, V.; Souchard, P. Fast increase of motor cortical inhibition following postural changes in healthy subjects. Neurosci. Lett. 2012, 530, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cabré, A.; Oliveri, M.; Gangitano, M.; Pascual-Leone, A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. NeuroReport 2001, 12, 3845–3848. [Google Scholar] [CrossRef] [PubMed]

| Exercise Group (n = 10) | Control Group (n = 7) | t-Value | p-Value | |
|---|---|---|---|---|
| Sex (M/F) | 6/4 | 4/3 | ||
| Age (years) | 68.0 ± 6.8 | 72.1 ± 6.0 | 1.298 | 0.214 |
| Height (cm) | 160.1 ± 8.6 | 155.8 ± 9.7 | 0.943 | 0.360 |
| Weight (kg) | 60.2 ± 8.8 | 62.1 ± 6.3 | 0.511 | 0.617 |
| Body mass index (kg/m2) | 23.5 ± 3.2 | 25.7 ± 2.6 | 1.495 | 0.156 |
| Symptom duration (years) | 6.4 ± 3.6 | 8.0 ± 4.0 | 1.402 | 0.181 |
| Treatment duration (years) | 3.9 ± 3.1 | 6.9 ± 3.0 | 1.971 | 0.067 |
| L-Dopa equivalent dose (mg/day) | 561.0 ± 274.6 | 852.9 ± 564.4 | 1.425 | 0.175 |
| Exercise Group | Control Group | F-Value | p-Value | |||
|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | |||
| MMSE (score) | 26.60 ± 2.84 | 26.90 ± 3.73 | 27.60 ± 1.27 | 27.29 ± 1.70 | 0.090 | 0.914 |
| UPDRS total (score) | 64.55 ± 18.33 | 68.05 ± 18.40 | 66.00 ± 10.17 | 62.14 ± 12.51 | 1.132 | 0.350 |
| UPDRS III (score) | 40.35 ± 10.85 | 40.05 ± 11.03 | 44.43 ± 8.80 | 43.86 ± 7.36 | 1.677 | 0.222 |
| H&Y stage | 2.40 ± 0.32 | 2.20 ± 0.82 | 2.29 ± 0.39 | 2.29 ± 0.39 | 0.930 | 0.418 |
| Exercise Group | Control Group | F-Value | p-Value | ES | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | |||||
| Functional Fitness Test | ||||||||
| 30 s chair sit to stand (reps.) | 15.3 ± 6.3 | 22.0 ± 7.7 | 13.9 ± 1.7 | 16.3 ± 4.9 | 4.460 | 0.053 | 0.242 | |
| 2 min step (steps) | 93.9 ± 18.5 | 113.9 ± 12.0 | 83.9 ± 15.2 | 86.7 ± 23.8 | 7.232 | 0.018 | 0.341 | |
| 2.44 m TUG (s) | 8.5 ± 1.8 | 6.5 ± 1.1 | 7.5 ± 1.1 | 7.7 ± 1.1 | 5.985 | 0.028 | 0.299 | |
| Arm curl (reps.) | R | 28.6 ± 6.2 | 31.6 ± 4.3 | 29.4 ± 4.9 | 29.6 ± 8.2 | 0.889 | 0.362 | 0.060 |
| L | 28.4 ± 5.6 | 31.3 ± 5.3 | 27.7 ± 3.2 | 30.1 ± 6.1 | 0.097 | 0.759 | 0.007 | |
| Chair sit & reach (cm) | R | 6.0 ± 10.0 | 8.9 ± 14.5 | 6.2 ± 16.8 | 6.1 ± 16.6 | 0.714 | 0.412 | 0.049 |
| L | 5.7 ± 9.8 | 9.4 ± 16.7 | 5.9 ± 16.1 | 5.2 ± 17.7 | 1.392 | 0.258 | 0.090 | |
| Back scratch (cm) | R-up | −20.6 ± 9.5 | −16.8 ± 11.4 | −26.4 ± 16.4 | −27.2 ± 15.9 | 2.443 | 0.140 | 0.149 |
| L-up | −25.0 ± 10.9 | −20.9 ± 11.1 | −27.8 ± 11.2 | −28.3 ± 10.0 | 3.996 | 0.065 | 0.222 | |
| TMS | ||||||||
| Total score | 5.8 ± 2.8 | 4.2 ± 2.6 | 6.3 ± 2.6 | 6.5 ± 2.3 | 6.520 | 0.023 | 0.318 | |
| Standing Balance Test | ||||||||
| AP velocity (cm/s) | 1.37 ± 0.73 | 0.99 ± 0.32 | 1.31 ± 0.45 | 1.35 ± 0.52 | 5.792 | 0.030 | 0.293 | |
| AP RMS (cm) | 0.64 ± 0.19 | 0.66 ± 0.21 | 0.51 ± 0.16 | 0.52 ± 0.15 | 0.040 | 0.844 | 0.003 | |
| ML velocity (cm/s) | 1.42 ± 0.59 | 1.08 ± 0.34 | 1.35 ± 0.42 | 1.28 ± 0.34 | 5.977 | 0.028 | 0.299 | |
| ML RMS (cm) | 0.65 ± 0.17 | 0.67 ± 0.21 | 0.56 ± 0.24 | 0.60 ± 0.26 | 0.037 | 0.850 | 0.003 | |
| First Step Phase for Sit-to-Walk Test | ||||||||
| Step time (s) | 0.40 ± 0.04 | 0.40 ± 0.03 | 0.39 ± 0.04 | 0.41 ± 0.04 | 1.585 | 0.229 | 0.102 | |
| Step length (m) | 0.51 ± 0.11 | 0.62 ± 0.04 | 0.53 ± 0.07 | 0.54 ± 0.07 | 13.311 | 0.003 | 0.487 | |
| Step speed (m/s) | 1.31 ± 0.29 | 1.57 ± 0.21 | 1.36 ± 0.22 | 1.32 ± 0.17 | 10.519 | 0.006 | 0.429 | |
| Toe clearance height (cm) | 5.71 ± 1.10 | 6.72 ± 0.60 | 6.11 ± 0.46 | 5.80 ± 1.81 | 2.396 | 0.144 | 0.146 | |
| Second Step Phase for Sit-to-Walk Test | ||||||||
| Step time (s) | 0.49 ± 0.07 | 0.46 ± 0.05 | 0.48 ± 0.05 | 0.48 ± 0.06 | 1.520 | 0.238 | 0.098 | |
| Step length (m) | 0.52 ± 0.11 | 0.59 ± 0.07 | 0.52 ± 0.10 | 0.54 ± 0.08 | 6.953 | 0.020 | 0.332 | |
| Step speed (m/s) | 1.10 ± 0.30 | 1.29 ± 0.19 | 1.08 ± 0.19 | 1.14 ± 0.12 | 6.027 | 0.028 | 0.301 | |
| Toe clearance height (cm) | 6.89 ± 0.91 | 7.83 ± 1.10 | 7.75 ± 1.39 | 7.36 ± 2.19 | 5.587 | 0.033 | 0.285 | |
| Variables | Estimate | SE | Odds Ratio | 95% CI | p-Value | RN2 |
|---|---|---|---|---|---|---|
| Functional fitness test | ||||||
| 2.44 m TUG | −2.078 | 0.981 | 0.125 | 0.018–0.856 | 0.034 | 0.496 |
| 2 min step test | 2.359 | 0.850 | 10.584 | 1.065–105.167 | 0.044 | 0.451 |
| Sit-to-walk test | ||||||
| First step length | 1.269 | 0.584 | 3.558 | 1.133–11.170 | 0.030 | 0.383 |
| First toe clearance height | 1.564 | 0.780 | 4.777 | 1.037–22.015 | 0.045 | 0.349 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youm, C.; Kim, Y.; Noh, B.; Lee, M.; Kim, J.; Cheon, S.-M. Impact of Trunk Resistance and Stretching Exercise on Fall-Related Factors in Patients with Parkinson’s Disease: A Randomized Controlled Pilot Study. Sensors 2020, 20, 4106. https://doi.org/10.3390/s20154106
Youm C, Kim Y, Noh B, Lee M, Kim J, Cheon S-M. Impact of Trunk Resistance and Stretching Exercise on Fall-Related Factors in Patients with Parkinson’s Disease: A Randomized Controlled Pilot Study. Sensors. 2020; 20(15):4106. https://doi.org/10.3390/s20154106
Chicago/Turabian StyleYoum, Changhong, Youkyung Kim, Byungjoo Noh, Myeounggon Lee, Jinhee Kim, and Sang-Myung Cheon. 2020. "Impact of Trunk Resistance and Stretching Exercise on Fall-Related Factors in Patients with Parkinson’s Disease: A Randomized Controlled Pilot Study" Sensors 20, no. 15: 4106. https://doi.org/10.3390/s20154106
APA StyleYoum, C., Kim, Y., Noh, B., Lee, M., Kim, J., & Cheon, S.-M. (2020). Impact of Trunk Resistance and Stretching Exercise on Fall-Related Factors in Patients with Parkinson’s Disease: A Randomized Controlled Pilot Study. Sensors, 20(15), 4106. https://doi.org/10.3390/s20154106








