A Microfluidic Paper-Based Analytical Device for Type-II Pyrethroid Targets in an Environmental Water Sample
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
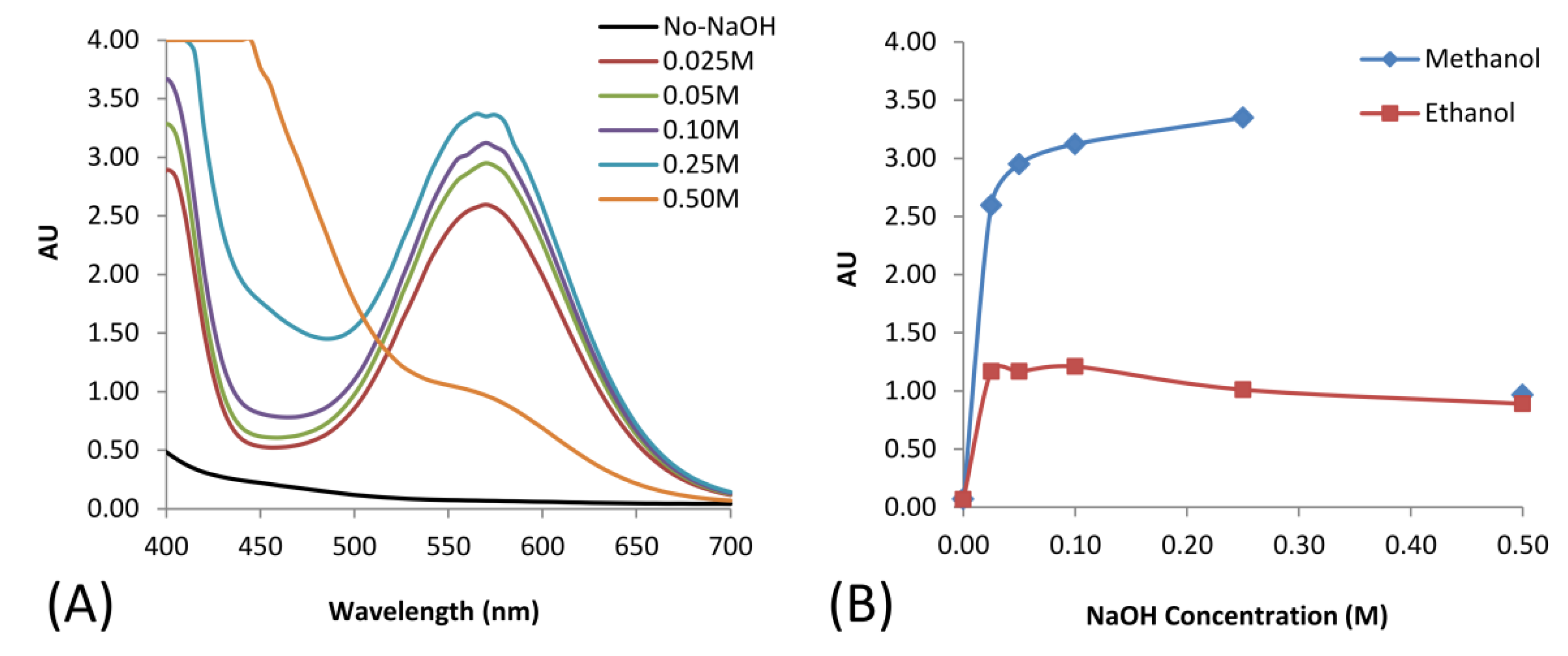
2.2. Optimization of a Hydrolysis Solution for Type-II Pyrethroids
2.3. Reagent Preparation for the µPAD
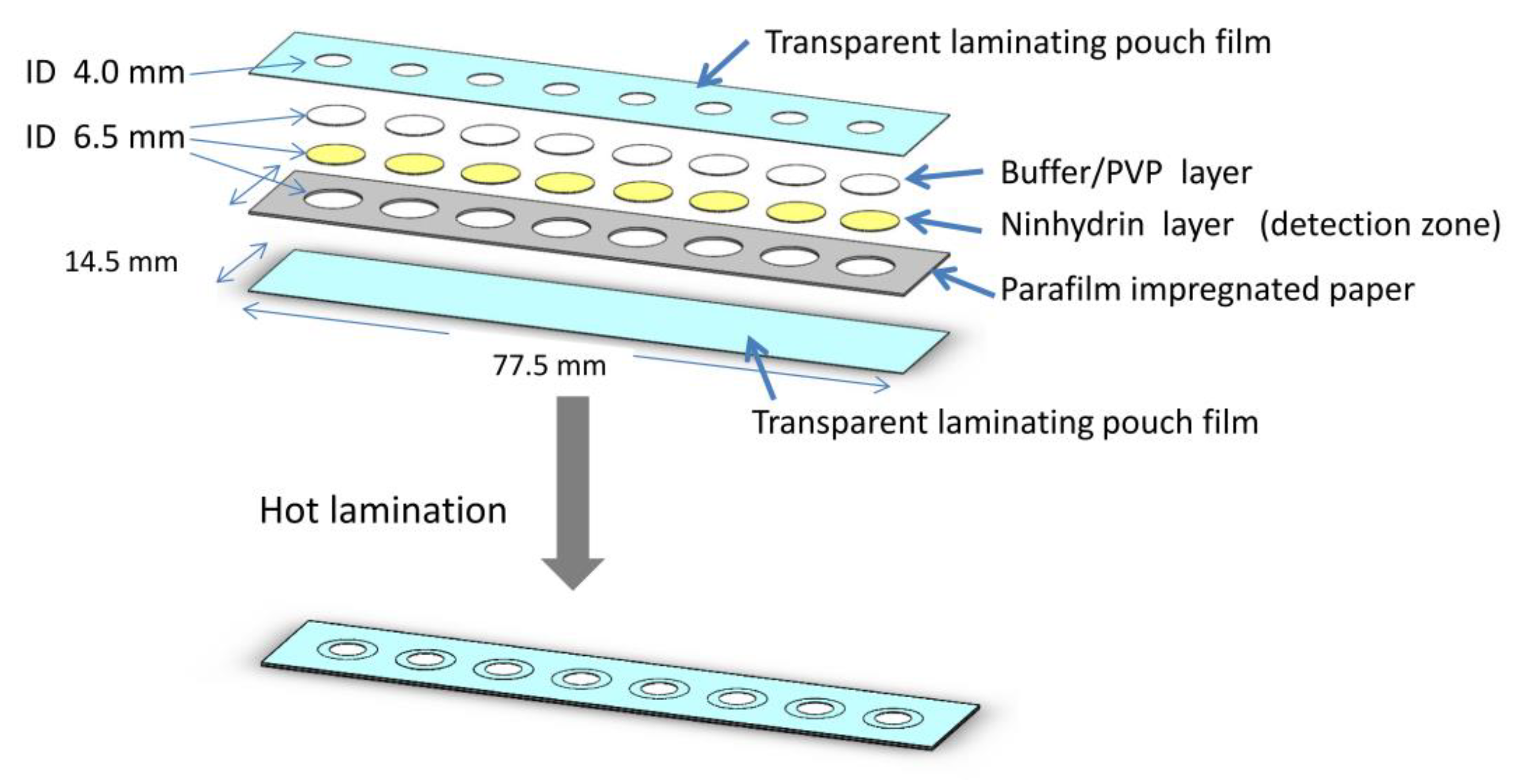
2.4. Design of the µPAD
2.5. Assay Procedure for Type-II Pyrethroids
2.6. Environmental Water Sample with Preconcentration Step
2.7. Digital Image Analysis and Data Processing
3. Results and Discussion
3.1. Hydrolysis of Cypermethrin
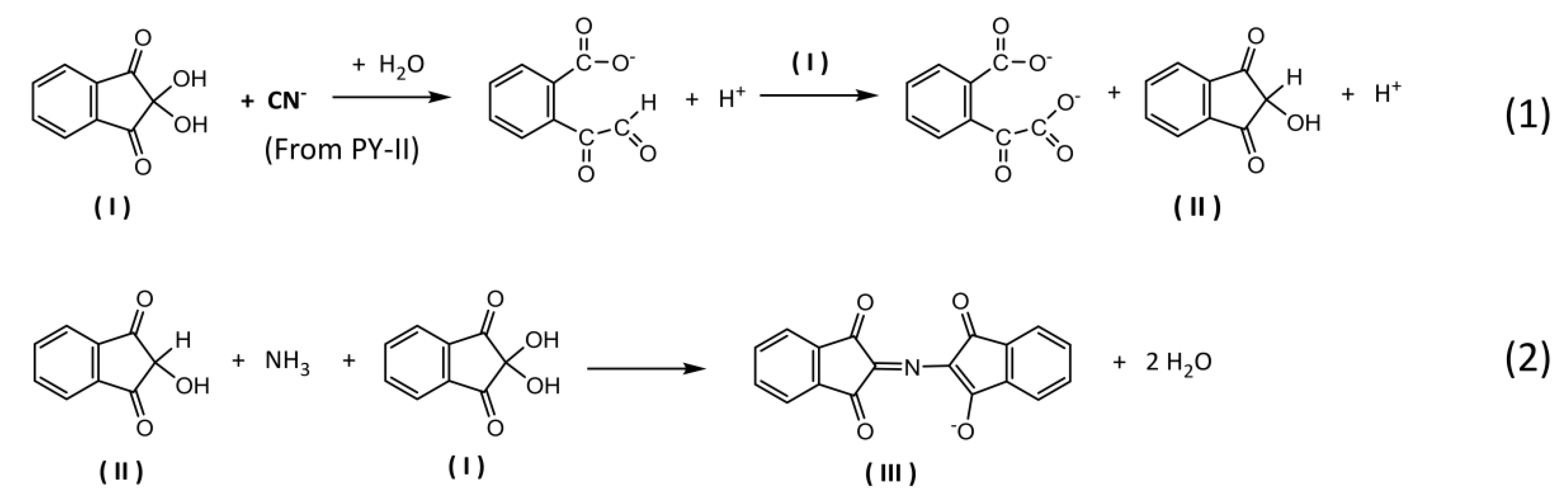
3.2. Detection Mechanism of the Type-II Pyrethroid Targets
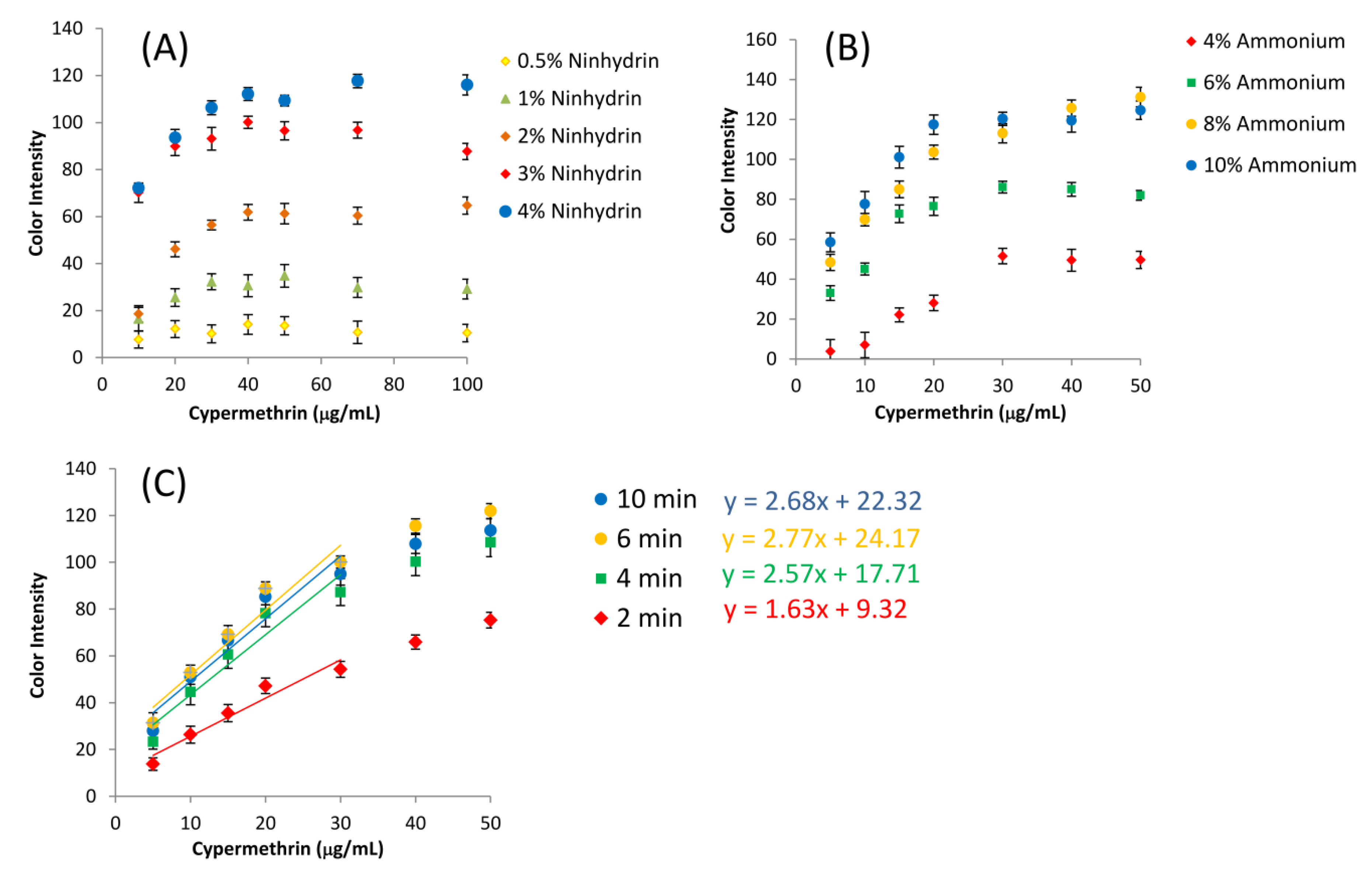
3.3. Assay Optimization and Measurement of Type-II Pyrethroids
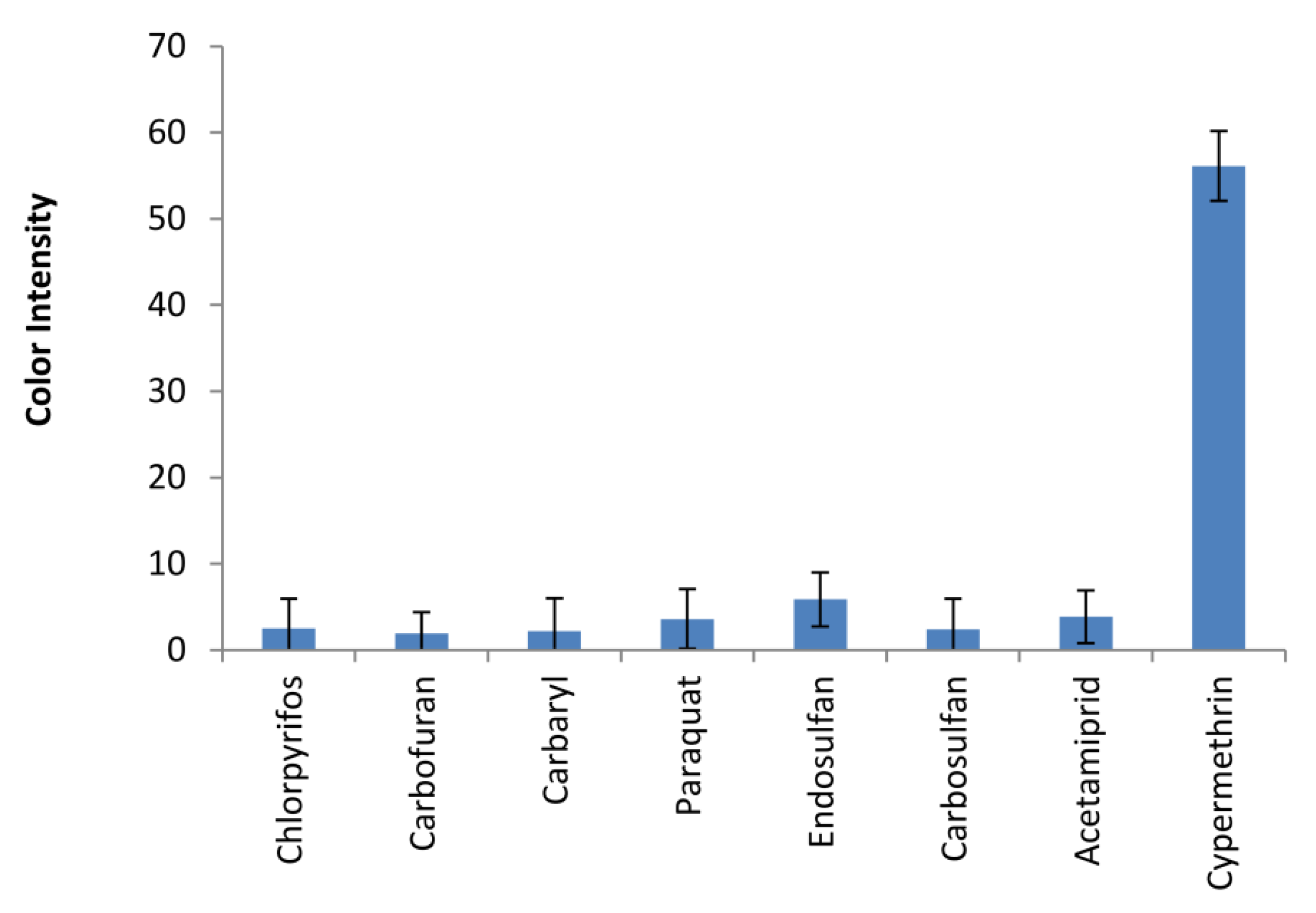
3.4. Assay Selectivity for Type-II Pyrethroids
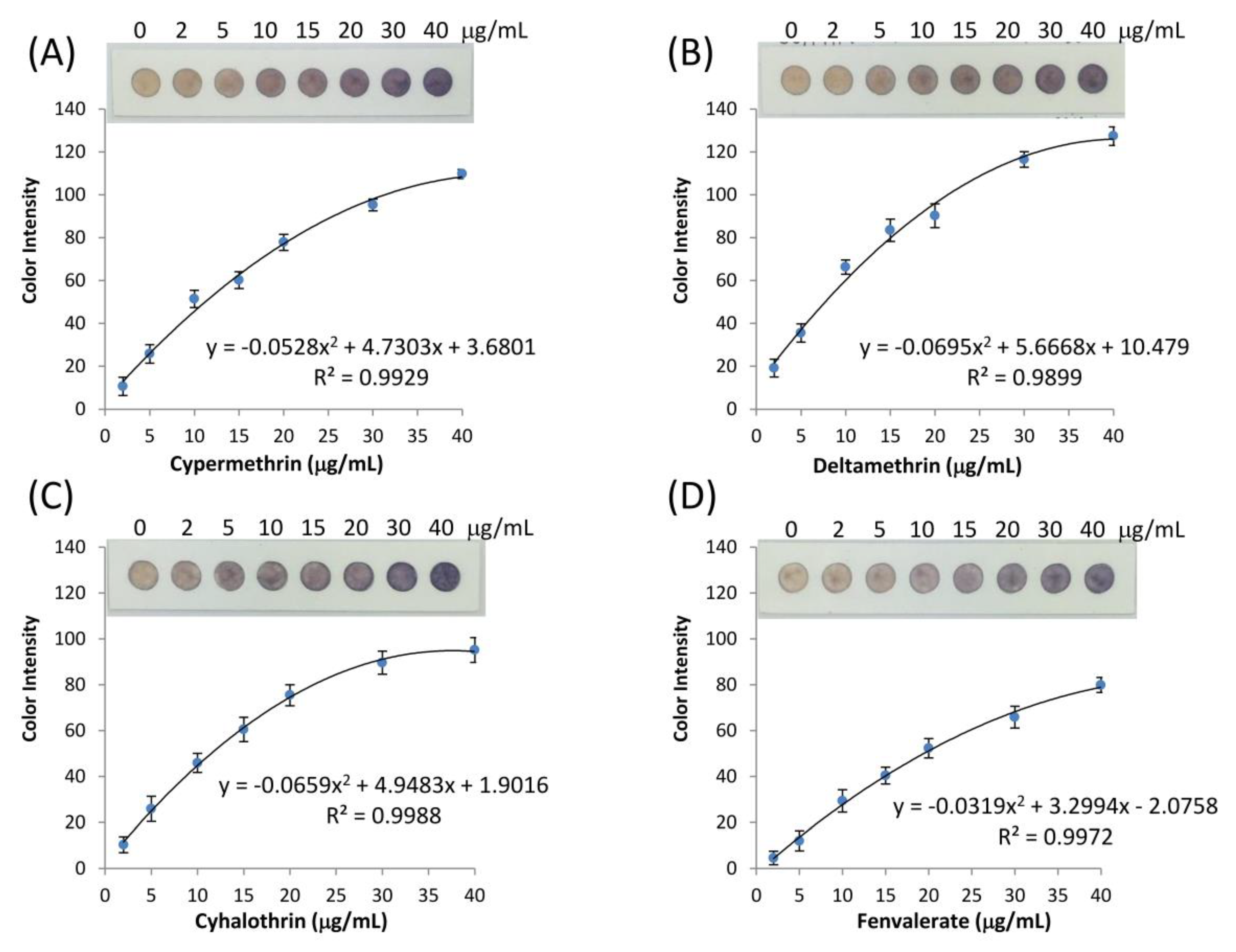
3.5. Calibration Graph of Type-II Pyrethroid Analysis
3.6. Measurement of Type-II pyrethroid Targets in an Environmental Water Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, M.; Sanjay, S.T.; Benhabib, M.; Xu, F.; Li, X. Low-cost bioanalysis on paper-based and its hybrid microfluidic platforms. Talanta 2015, 145, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Capitán, M.; Baldi, A.; Fernández-Sánchez, C. Electrochemical Paper-Based Biosensor Devices for Rapid Detection of Biomarkers. Sensors 2020, 20, 967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busa, L.S.A.; Mohammadi, S.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis. Micromachines 2016, 7, 86. [Google Scholar] [CrossRef]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H.; Volckens, J.; Henry, C.S. Paper-based analytical devices for environmental analysis. Analyst 2016, 141, 1874–1887. [Google Scholar] [CrossRef]
- Yang, Y.; Noviana, E.; Nguyen, M.P.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Paper-Based Microfluidic Devices: Emerging Themes and Applications. Anal. Chem. 2017, 89, 71–91. [Google Scholar] [CrossRef]
- Racicot, J.M.; Mako, T.L.; Olivelli, A.; Levine, M. A Paper-Based Device for Ultrasensitive, Colorimetric Phosphate Detection in Seawater. Sensors 2020, 20, 2766. [Google Scholar] [CrossRef]
- Deng, S.; Yang, T.; Zhang, W.; Ren, C.; Zhang, J.; Zhang, Y.; Cui, T.; Yue, W. Rapid detection of trichlorfon residues by a microfluidic paper-based phosphorus-detection chip (μPPC). New J. Chem. 2019, 43, 7194–7197. [Google Scholar] [CrossRef]
- Wang, Q.; Yin, Q.; Fan, Y.; Zhang, L.; Xu, Y.; Hu, O.; Guo, X.; Shi, Q.; Fu, H.; She, Y. Double quantum dots-nanoporphyrin fluorescence-visualized paper-based sensors for detecting organophosphorus pesticides. Talanta 2019, 199, 46–53. [Google Scholar] [CrossRef]
- Chen, H.; Hu, O.; Fan, Y.; Xu, L.; Zhang, L.; Lan, W.; Hu, Y.; Xie, X.; Ma, L.; She, Y.; et al. Fluorescence paper-based sensor for visual detection of carbamate pesticides in food based on CdTe quantum dot and nano ZnTPyP. Food Chem. 2020, 327, 127075. [Google Scholar] [CrossRef]
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [Google Scholar] [CrossRef]
- Meng, X.; Schultz, C.W.; Cui, C.; Li, X.; Yu, H.-Z. On-site chip-based colorimetric quantitation of organophosphorus pesticides using an office scanner. Sens. Actuators B Chem. 2015, 215, 577–583. [Google Scholar] [CrossRef]
- Nouanthavong, S.; Nacapricha, D.; Henry, C.S.; Sameenoi, Y. Pesticide analysis using nanoceria-coated paper-based devices as a detection platform. Analyst 2016, 141, 1837–1846. [Google Scholar] [CrossRef]
- Apilux, A.; Siangproh, W.; Insin, N.; Chailapakul, O.; Prachayasittikul, V. Paper-based thioglycolic acid (TGA)-capped CdTe QD device for rapid screening of organophosphorus and carbamate insecticides. Anal. Methods 2017, 9, 519–527. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Park, S.J.; Kwon, C.; Noh, H. Development of Colorimetric Paper Sensor for Pesticide Detection Using Competitive-inhibiting Reaction. BioChip J. 2018, 12, 326–331. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, X.; Khan, H.; Tian, M.; Yang, L. Converting solution viscosity to distance-readout on paper substrates based on enzyme-mediated alginate hydrogelation: Quantitative determination of organophosphorus pesticides. Anal. Chim. Acta 2019, 1071, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Shaheen, N.; Xie, L.; Yu, J.; Ahmad, H.; Mao, H. Pesticide Residues Identification by Optical Spectrum in the Time-Sequence of Enzyme Inhibitors Performed on Microfluidic Paper-Based Analytical Devices (µPADs). Molecules 2019, 24, 2428. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Hao, Z.; Zheng, Q.; Chen, H.; Zhu, L.; Wang, C.; Liu, X.; Lu, C. A facile microfluidic paper-based analytical device for acetylcholinesterase inhibition assay utilizing organic solvent extraction in rapid detection of pesticide residues in food. Anal. Chim. Acta 2020, 1100, 215–224. [Google Scholar] [CrossRef]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef]
- Akyazi, T.; Basabe-Desmonts, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialisation. Anal. Chim. Acta 2018, 1001, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pengpumkiat, S.; Wu, Y.; Sumantakul, S.; Remcho, V.T. A Membrane-based Disposable Well-Plate for Cyanide Detection Incorporating a Fluorescent Chitosan-CdTe Quantum Dot. Anal. Sci. 2020, 36, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Top Pesticide Using Countries. Available online: https://www.worldatlas.com/articles/top-pesticide-consuming-countries-of-the-world.html (accessed on 1 June 2020).
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- The Office of Agricultural Regulation, Department of Agriculture. Available online: http://www.doa.go.th/ard/?page_id=386 (accessed on 4 June 2020).
- Iwai, C.B.; Sujira, H.; Somparn, A.; Komarova, T.; Mueller, J.; Noller, B. Monitoring Pesticides in the Paddy Field Ecosystem of North-Eastern Thailand for Environmental and Health Risks. In Rational Environmental Management of Agrochemicals; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2007; Volume 966, pp. 259–273. ISBN 978-0-8412-7420-4. [Google Scholar]
- DeMicco, A.; Cooper, K.R.; Richardson, J.R.; White, L.A. Developmental neurotoxicity of pyrethroid insecticides in zebrafish embryos. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 113, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Gu, A.; Ji, G.; Li, Y.; Di, J.; Jin, J.; Hu, F.; Long, Y.; Xia, Y.; Lu, C.; et al. Developmental toxicity of cypermethrin in embryo-larval stages of zebrafish. Chemosphere 2011, 85, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Prusty, A.K.; Kohli, M.P.S.; Sahu, N.P.; Pal, A.K.; Saharan, N.; Mohapatra, S.; Gupta, S.K. Effect of short term exposure of fenvalerate on biochemical and haematological responses in Labeo rohita (Hamilton) fingerlings. Pestic. Biochem. Physiol. 2011, 100, 124–129. [Google Scholar] [CrossRef]
- Vani, T.; Saharan, N.; Mukherjee, S.C.; Ranjan, R.; Kumar, R.; Brahmchari, R.K. Deltamethrin induced alterations of hematological and biochemical parameters in fingerlings of Catla catla (Ham.) and their amelioration by dietary supplement of vitamin C. Pestic. Biochem. Physiol. 2011, 101, 16–20. [Google Scholar] [CrossRef]
- Ali, M.H.; Sumon, K.A.; Sultana, M.; Rashid, H. Toxicity of cypermethrin on the embryo and larvae of Gangetic mystus, Mystus cavasius. Environ. Sci. Pollut. Res. 2017, 1–7. [Google Scholar] [CrossRef]
- Schettgen, T.; Dewes, P.; Kraus, T. A method for the simultaneous quantification of eight metabolites of synthetic pyrethroids in urine of the general population using gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 5467–5478. [Google Scholar] [CrossRef]
- Nasuti, C.; Cantalamessa, F.; Falcioni, G.; Gabbianelli, R. Different effects of Type I and Type II pyrethroids on erythrocyte plasma membrane properties and enzymatic activity in rats. Toxicology 2003, 191, 233–244. [Google Scholar] [CrossRef]
- The Water Environment Partnership in Asia (WEPA) The Surface Water Quality Standard in Thailand. Available online: http://www.wepa-db.net/policies/law/thailand/std_surface_water.htm (accessed on 2 July 2020).
- The US Environmental Protection Agency. National Recommended Water Quality Criteria—Human Health Criteria. Table. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-human-health-criteria-table (accessed on 2 July 2020).
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, H.; Qiao, J. Miniaturized matrix solid-phase dispersion combined with ultrasound-assisted dispersive liquid–liquid microextraction for the determination of three pyrethroids in soil. J. Sep. Sci. 2012, 35, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Q.; Yang, X.; Wang, W.; Li, Z.; Zhang, L.; Wang, C.; Wang, Z. A zeolitic imidazolate framework based nanoporous carbon as a novel fiber coating for solid-phase microextraction of pyrethroid pesticides. Talanta 2017, 166, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.K.; Patyal, S.K.; Sharma, A. Validation of QuEChERS analytical technique for organochlorines and synthetic pyrethroids in fruits and vegetables using GC-ECD. Environ. Monit. Assess. 2018, 190, 231. [Google Scholar] [CrossRef]
- Nardelli, V.; Casamassima, F.; Gesualdo, G.; Li, D.; Marchesiello, W.M.V.; Nardiello, D.; Quinto, M. Sensitive Screening Method for Determination of Pyrethroids in Chicken Eggs and Various Meat Samples by Gas Chromatography and Electron Capture Detection. J. Agric. Food Chem. 2018, 66, 10267–10273. [Google Scholar] [CrossRef]
- de Oliveira, L.G.; Kurz, M.H.S.; Guimarães, M.C.M.; Martins, M.L.; Prestes, O.D.; Zanella, R.; da Silva Ribeiro, J.N.; Gonçalves, F.F. Development and validation of a method for the analysis of pyrethroid residues in fish using GC–MS. Food Chem. 2019, 297, 124944. [Google Scholar] [CrossRef]
- Mao, X.; Wan, Y.; Li, Z.; Chen, L.; Lew, H.; Yang, H. Analysis of organophosphorus and pyrethroid pesticides in organic and conventional vegetables using QuEChERS combined with dispersive liquid-liquid microextraction based on the solidification of floating organic droplet. Food Chem. 2020, 309, 125755. [Google Scholar] [CrossRef]
- Saito-Shida, S.; Nagata, M.; Nemoto, S.; Akiyama, H. Quantitative analysis of pesticide residues in tea by gas chromatography–tandem mass spectrometry with atmospheric pressure chemical ionization. J. Chromatogr. B 2020, 1143, 122057. [Google Scholar] [CrossRef]
- Tian, F.; Qiao, C.; Luo, J.; Guo, L.; Pang, T.; Pang, R.; Li, J.; Wang, C.; Wang, R.; Xie, H. Method development and validation of ten pyrethroid insecticides in edible mushrooms by Modified QuEChERS and gas chromatography-tandem mass spectrometry. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Yu, X.; Ang, H.C.; Yang, H.; Zheng, C.; Zhang, Y. Low temperature cleanup combined with magnetic nanoparticle extraction to determine pyrethroids residue in vegetables oils. Food Control 2017, 74, 112–120. [Google Scholar] [CrossRef]
- Zhao, P.; Dong, X.; Chen, X.; Guo, X.; Zhao, L. Stereoselective Analysis of Chiral Pyrethroid Insecticides Tetramethrin and α-Cypermethrin in Fruits, Vegetables, and Cereals. J. Agric. Food Chem. 2019, 67, 9362–9370. [Google Scholar] [CrossRef]
- Ccanccapa-Cartagena, A.; Masiá, A.; Picó, Y. Simultaneous determination of pyrethroids and pyrethrins by dispersive liquid-liquid microextraction and liquid chromatography triple quadrupole mass spectrometry in environmental samples. Anal. Bioanal. Chem. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petrarca, M.H.; Ccanccapa-Cartagena, A.; Masiá, A.; Godoy, H.T.; Picó, Y. Comparison of green sample preparation techniques in the analysis of pyrethrins and pyrethroids in baby food by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2017, 1497, 28–37. [Google Scholar] [CrossRef]
- Lehmann, E.; Oltramare, C.; de Alencastro, L.F. Development of a modified QuEChERS method for multi-class pesticide analysis in human hair by GC-MS and UPLC-MS/MS. Anal. Chim. Acta 2018, 999, 87–98. [Google Scholar] [CrossRef] [PubMed]
- De O. Silva, R.; De Menezes, M.G.G.; De Castro, R.C.; De A. Nobre, C.; Milhome, M.A.L.; Do Nascimento, R.F. Efficiency of ESI and APCI ionization sources in LC-MS/MS systems for analysis of 22 pesticide residues in food matrix. Food Chem. 2019, 297, 124934. [Google Scholar] [CrossRef] [PubMed]
- López-García, M.; Romero-González, R.; Garrido Frenich, A. Monitoring of organophosphate and pyrethroid metabolites in human urine samples by an automated method (TurboFlowTM) coupled to ultra-high performance liquid chromatography-Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2019, 173, 31–39. [Google Scholar] [CrossRef]
- Camilleri, P. Alkaline hydrolysis of some pyrethroid insecticides. J. Agric. Food Chem. 1984, 32, 1122–1124. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, J.; Zhang, X.; Huang, L.; Wang, Z.; Li, Y.; Gao, H.; Zhu, S.; Wang, T.; Yang, B. Suppression of the Coffee Ring Effect by Hydrosoluble Polymer Additives. ACS Appl. Mater. Interfaces 2012, 4, 2775–2780. [Google Scholar] [CrossRef] [PubMed]
- Mekebri, A.; Crane, D.B.; Blondina, G.J.; Oros, D.R.; Rocca, J.L. Extraction and Analysis Methods for the Determination of Pyrethroid Insecticides in Surface Water, Sediments and Biological Tissues at Environmentally Relevant Concentrations. Bull. Environ. Contam. Toxicol. 2008, 80, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Albaseer, S.S.; Rao, R.N.; Swamy, Y.V.; Mukkanti, K.; Bandi, B.G. Micro liquid–liquid extraction of synthetic pyrethroids from surface waters for liquid-chromatographic determination. Toxicol. Environ. Chem. 2011, 93, 1309–1318. [Google Scholar] [CrossRef]
- Murdock, R.C.; Shen, L.; Griffin, D.K.; Kelley-Loughnane, N.; Papautsky, I.; Hagen, J.A. Optimization of a Paper-Based ELISA for a Human Performance Biomarker. Anal. Chem. 2013, 85, 11634–11642. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.; Kim, S. Simultaneous quantification of multiple biomarkers on a self-calibrating microfluidic paper-based analytic device. Anal. Chim. Acta 2020, 1097, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nelis, J.L.D.; Bura, L.; Zhao, Y.; Burkin, K.M.; Rafferty, K.; Elliott, C.T.; Campbell, K. The Efficiency of Color Space Channels to Quantify Color and Color Intensity Change in Liquids, pH Strips, and Lateral Flow Assays with Smartphones. Sensors 2019, 19, 5104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottom, C.B.; Hanna, S.S.; Siehr, D.J. Mechanism of the ninhydrin reaction. Biochem. Educ. 1978, 6, 4–5. [Google Scholar] [CrossRef]
- Lamothe, P.J.; McCormick, P.G. Role of hydrindantin in the determination of amino acids using ninhydrin. Anal. Chem. 1973, 45, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Drochioiu, G. Highly selective and sensitive reaction of cyanide with 2,2-dihydroxy-1,3-indanedione. Anal. Bioanal. Chem. 2002, 372, 744–747. [Google Scholar] [CrossRef]
- Drochioiu, G.; Mangalagiu, I.; Avram, E.; Popa, K.; Dirtu, A.C.; Druta, I. Cyanide reaction with ninhydrin: Elucidation of reaction and interference mechanisms. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2004, 20, 1443–1447. [Google Scholar] [CrossRef] [Green Version]
- Mihaescu, I.M.; Drochioiu, G. Cyanide reaction with ninhydrin: The effect of pH changes and UV-Vis radiation upon the analytical results. Revue Roumaine de Chimie 2009, 54, 841–845. [Google Scholar]
- Holstein, C.A.; Griffin, M.; Hong, J.; Sampson, P.D. Statistical Method for Determining and Comparing Limits of Detection of Bioassays. Anal. Chem. 2015, 87, 9795–9801. [Google Scholar] [CrossRef]
- AOAC Appendix F: Guidelines for Standard Method Performance Requirements. Available online: http://www.eoma.aoac.org/app_f.pdf (accessed on 15 May 2020).


| Analyte | Added Concentration | Found Value | Recovery |
|---|---|---|---|
| (µg/mL) | (µg/mL) ± SD | (%) ± SD | |
| Cypermethrin | 0.10 | 0.078 ± 0.013 | 78.31 ± 13.14 |
| 0.15 | 0.141 ± 0.026 | 93.74 ± 17.11 | |
| Deltamethrin | 0.10 | 0.086 ± 0.045 | 86.27 ± 44.66 |
| 0.15 | 0.131 ± 0.037 | 87.14 ± 24.83 | |
| Cyhalothrin | 0.10 | 0.084 ± 0.029 | 83.73 ± 28.93 |
| 0.15 | 0.137 ± 0.032 | 91.07 ± 21.10 | |
| Fenvalerate | 0.10 | 0.085 ± 0.017 | 84.55 ± 17.02 |
| 0.15 | 0.159 ± 0.022 | 106.01 ± 14.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pengpumkiat, S.; Nammoonnoy, J.; Wongsakoonkan, W.; Konthonbut, P.; Kongtip, P. A Microfluidic Paper-Based Analytical Device for Type-II Pyrethroid Targets in an Environmental Water Sample. Sensors 2020, 20, 4107. https://doi.org/10.3390/s20154107
Pengpumkiat S, Nammoonnoy J, Wongsakoonkan W, Konthonbut P, Kongtip P. A Microfluidic Paper-Based Analytical Device for Type-II Pyrethroid Targets in an Environmental Water Sample. Sensors. 2020; 20(15):4107. https://doi.org/10.3390/s20154107
Chicago/Turabian StylePengpumkiat, Sumate, Jintana Nammoonnoy, Watcharaporn Wongsakoonkan, Pajaree Konthonbut, and Pornpimol Kongtip. 2020. "A Microfluidic Paper-Based Analytical Device for Type-II Pyrethroid Targets in an Environmental Water Sample" Sensors 20, no. 15: 4107. https://doi.org/10.3390/s20154107






