Critical Factors for In Vivo Measurements of Human Skin by Terahertz Attenuated Total Reflection Spectroscopy
Abstract
1. Introduction
2. Experimental Methodology, Results and Discussions
2.1. Experimental Setup
2.2. Experimental Processing
2.3. Data Processing
2.4. THz Response with Different Thumb/Prism Relative Positions
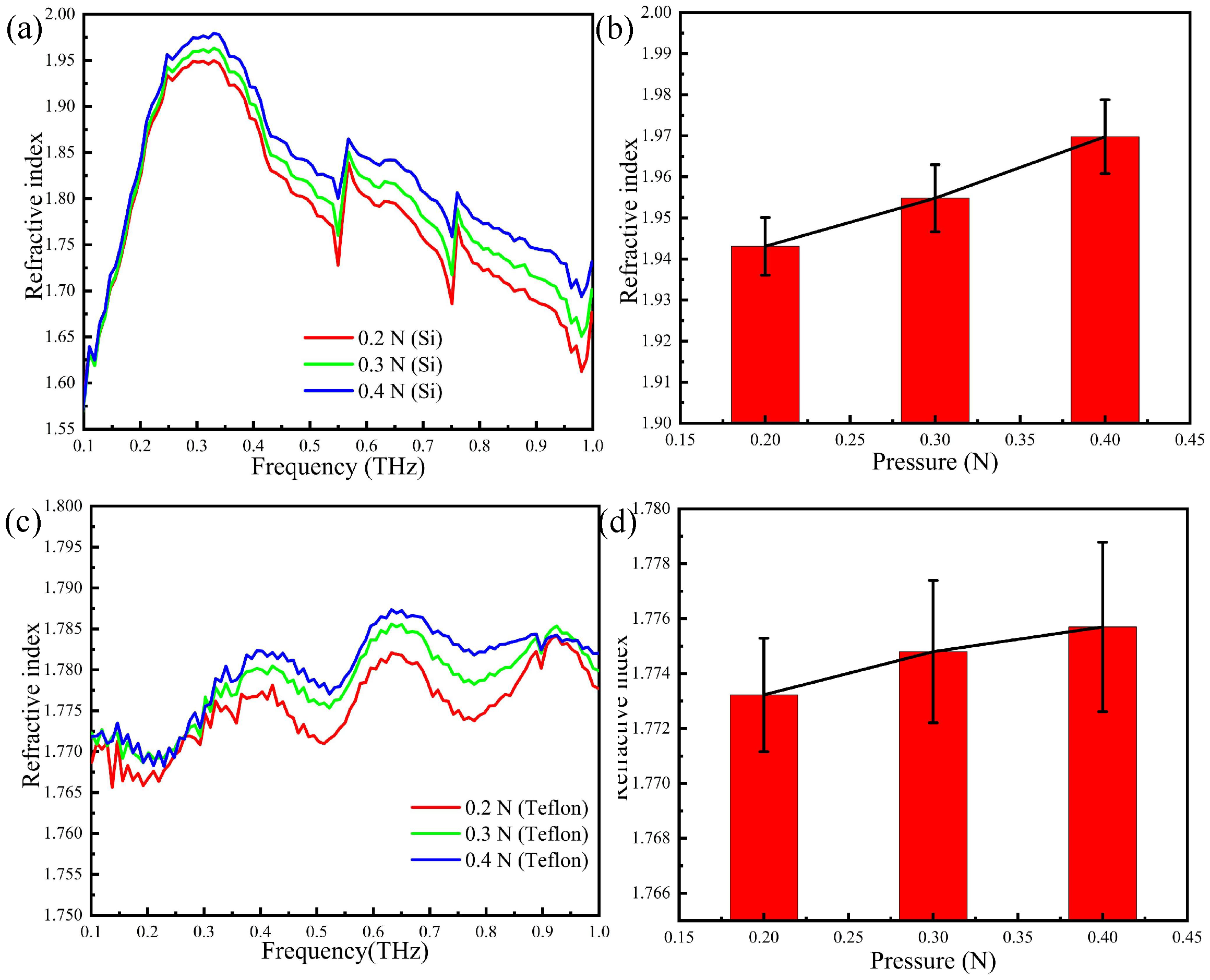
2.5. THz Response with Different Contact Pressure
2.6. THz Response with Different Contact Durations
2.7. THz Response with Prism Made of Different Materials
2.8. Systematic and Random Errors
3. Potential Applications
3.1. THz Response to Different Handcream
3.2. Theory and Simulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koenig, S.; Lopez-Diaz, D.; Antes, J.; Boes, F.; Henneberger, R.; Leuther, A.; Tessmann, A.; Schmogrow, R.; Hillerkuss, D.; Palmer, R.; et al. Wireless sub-THz communication system with high data rate. Nat. Photonics 2013, 7, 977. [Google Scholar] [CrossRef]
- Federici, J.F.; Schulkin, B.; Huang, F.; Gary, D.; Barat, R.; Oliveira, F.; Zimdars, D. THz imaging and sensing for security applications—Explosives, weapons and drugs. Semicond. Sci. Technol. 2005, 20, S266. [Google Scholar] [CrossRef]
- Massaouti, M.; Daskalaki, C.; Gorodetsky, A.; Koulouklidis, A.D.; Tzortzakis, S. Detection of harmful residues in honey using terahertz time-domain spectroscopy. Appl. Spectrosc. 2013, 67, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Chen, Y.; Bastiaans, G.J.; Zhang, X.C. Detection and identification of explosive RDX by THz diffuse reflection spectroscopy. Opt. Express 2006, 14, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Pepper, M.; Taday, P. Terahertz spectroscopy: Signatures and fingerprints. Nat. Photonics 2008, 2, 541. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Z.; Tang, M.; Wang, D.; Wang, H.; Yan, S.; Wei, D.; Cui, H.L. Terahertz Spectroscopic Signatures of Microcystin Aptamer Solution Probed with a Microfluidic Chip. Sensors 2019, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, Y.; Zang, X.; Zhu, Y.; Zhuang, S. Excitation of dark multipolar plasmonic resonances at terahertz frequencies. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Q.; Zhang, Y.; Xu, X.; Zhang, Q.; Zhao, W.; Zhang, B.; Cai, W.; Yao, J.; Xu, J. Enhanced on-chip terahertz sensing with hybrid metasurface/lithium niobate structures. Appl. Phys. Lett. 2019, 114, 121102. [Google Scholar] [CrossRef]
- Hermannsson, P.G.; Sørensen, K.T.; Vannahme, C.; Smith, C.L.; Klein, J.J.; Russew, M.M.; Grützner, G.; Kristensen, A. All-polymer photonic crystal slab sensor. Opt. Express 2015, 23, 16529–16539. [Google Scholar] [CrossRef]
- Sancho-Fornes, G.; Avella-Oliver, M.; Carrascosa, J.; Fernandez, E.; Brun, E.M.; Maquieira, Á. Disk-based one-dimensional photonic crystal slabs for label-free immunosensing. Biosens. Bioelectron. 2019, 126, 315–323. [Google Scholar] [CrossRef]
- Chen, L.; Xu, N.; Singh, L.; Cui, T.; Singh, R.; Zhu, Y.; Zhang, W. Defect-induced Fano resonances in corrugated plasmonic metamaterials. Adv. Opt. Mater. 2017, 5, 1600960. [Google Scholar] [CrossRef]
- Siegel, P.H. Terahertz technology in biology and medicine. IEEE Trans. Microw. Theory Tech. 2004, 52, 2438–2447. [Google Scholar] [CrossRef]
- Ren, A.; Zahid, A.; Fan, D.; Yang, X.; Imran, M.A.; Alomainy, A.; Abbasi, Q.H. State-of-the-art in terahertz sensing for food and water security—A comprehensive review. Trends Food Sci. Technol. 2019, 85, 241–251. [Google Scholar] [CrossRef]
- Mittleman, D.M. Frontiers in terahertz sources and plasmonics. Nat. Photonics 2013, 7, 666–669. [Google Scholar] [CrossRef]
- Son, J.H.; Oh, S.J.; Cheon, H. Potential clinical applications of terahertz radiation. J. Appl. Phys. 2019, 125, 190901. [Google Scholar] [CrossRef]
- Jepsen, P.U.; Cooke, D.G.; Koch, M. Terahertz spectroscopy and imaging–Modern techniques and applications. Laser Photonics Rev. 2011, 5, 124–166. [Google Scholar] [CrossRef]
- Withayachumnankul, W.; Png, G.M.; Yin, X.; Atakaramians, S.; Jones, I.; Lin, H.; Ung, B.S.Y.; Balakrishnan, J.; Ng, B.W.H.; Ferguson, B.; et al. T-ray sensing and imaging. Proc. IEEE 2007, 95, 1528–1558. [Google Scholar] [CrossRef]
- O’Hara, J.F.; Withayachumnankul, W.; Al-Naib, I. A review on thin-film sensing with terahertz waves. J. Infrared Millim. Terahertz Waves 2012, 33, 245–291. [Google Scholar] [CrossRef]
- Soltani, A.; Jahn, D.; Duschek, L.; Castro-Camus, E.; Koch, M.; Withayachumnankul, W. Attenuated total reflection terahertz time-domain spectroscopy: Uncertainty analysis and reduction scheme. IEEE Trans. Terahertz Sci. Technol. 2016, 6, 32–39. [Google Scholar] [CrossRef]
- Mendenhall, M.J.; Nunez, A.S.; Martin, R.K. Human skin detection in the visible and near infrared. Appl. Opt. 2015, 54, 10559–10570. [Google Scholar] [CrossRef]
- Pickwell, E.; Cole, B.E.; Fitzgerald, A.J.; Pepper, M.; Wallace, V.P. In vivo study of human skin using pulsed terahertz radiation. Phys. Med. Biol. 2004, 49, 1595. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.E.; Woodward, R.M.; Crawley, D.A.; Wallace, V.P.; Arnone, D.D.; Pepper, M. Terahertz imaging and spectroscopy of human skin in vivo. In Commercial and Biomedical Applications of Ultrashort Pulse Lasers; Laser Plasma Generation and Diagnostics, Proceedings of the Photonics West 2001-LASE, San Jose, CA, United States, 29 July–3 August 2001; Society of Photo Optical: Bellingham, WA, USA, 2001; Volume 4276, pp. 1–10. [Google Scholar]
- Gusev, S.; Guseva, V.; Simonova, A.; Demchenko, P.; Sedykh, E.; Cherkasova, O.; Khodzitsky, M. Application of terahertz pulsed spectroscopy for the development of non-invasive glucose measuring method. In Proceedings of the 2017 Progress in Electromagnetics Research Symposium-Spring (PIERS), St. Petersburg, Russia, 22–25 May 2017; pp. 3229–3232. [Google Scholar]
- Sun, Q.; Parrott, E.P.; He, Y.; Pickwell-MacPherson, E. In vivo THz imaging of human skin: Accounting for occlusion effects. J. Biophotonics 2018, 11, e201700111. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Stantchev, R.I.; Sun, Q.; Chiu, T.W.; Ahuja, A.T.; MacPherson, E.P. THz in vivo measurements: The effects of pressure on skin reflectivity. Biomed. Opt. Express 2018, 9, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Truong, B.C.; Fitzgerald, A.J.; Fan, S.; Wallace, V.P. Concentration analysis of breast tissue phantoms with terahertz spectroscopy. Biomed. Opt. Express 2018, 9, 1334–1349. [Google Scholar] [CrossRef]
- Fan, S.; Ung, B.S.; Parrott, E.P.; Wallace, V.P.; Pickwell-MacPherson, E. In vivo terahertz reflection imaging of human scars during and after the healing process. J. Biophotonics 2017, 10, 1143–1151. [Google Scholar] [CrossRef]
- Nagai, M.; Yada, H.; Arikawa, T.; Tanaka, K. Terahertz time-domain attenuated total reflection spectroscopy in water and biological solution. Int. J. Infrared Millim. Waves 2006, 27, 505–515. [Google Scholar] [CrossRef]
- Ogawa, Y. A Method for Measuring Water Content in Stratum Corneum with Terahertz Wave. JP. Patent 053,528, 14 April 2016. (in Japanese). [Google Scholar]
- Arikawa, T.; Nagai, M.; Tanaka, K. Characterizing hydration state in solution using terahertz time-domain attenuated total reflection spectroscopy. Chem. Phys. Lett. 2008, 457, 12–17. [Google Scholar] [CrossRef]
- Cherkasova, O.; Nazarov, M.; Shkurinov, A. Noninvasive blood glucose monitoring in the terahertz frequency range. Opt. Quantum Electron. 2016, 48, 217. [Google Scholar] [CrossRef]
- Cherkasova, O.P.; Nazarov, M.M.; Berlovskaya, E.; Angeluts, A.; Makurenkov, A.; Shkurinov, A. Studying human and animal skin optical properties by terahertz time-domain spectroscopy. Bull. Russ. Acad. Sci. Phys. 2016, 80, 479–483. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Xu, D.; Wu, L.; Yan, C.; Yan, D.; Tang, L.; He, Y.; Feng, H.; Yao, J. High-sensitivity attenuated total internal reflection continuous-wave terahertz imaging. J. Phys. Appl. Phys. 2017, 50, 375103. [Google Scholar] [CrossRef]
- Yin, H.; Wang, L.; Chen, L. The study of non-invasive blood glucose measurement based terahertz ATR spectroscopy. Opt. Instrum. 2019, 41, 8–13. (in Chinese). [Google Scholar]
- Crompton, D.; Vickers, A. Assigning the characteristics of an ATR dove prism for use with terahertz frequencies: Supplemented with molecular dynamic simulations. In Proceedings of the 2012 4th Computer Science and Electronic Engineering Conference (CEEC), Colchester, UK, 12–13 September 2012; pp. 47–52. [Google Scholar]
- Cherkasova, O.P.; Nazarov, M.M.; Shkurinov, A.P. The investigation of blood and skin THz response at high glucose concentration. In Proceedings of the 2015 40th International Conference on Infrared, Millimeter, and Terahertz waves (IRMMW-THz), Hong Kong, China, 23–28 August 2015. [Google Scholar]
- Cherkasova, O.; Nazarov, M.; Shkurinov, A. Terahertz spectroscopy for diabetes diagnostics. Epj Web Conf. 2017, 149, 05013. [Google Scholar] [CrossRef]
- Hernandez-Cardoso, G.; Rojas-Landeros, S.; Alfaro-Gomez, M.; Hernandez-Serrano, A.; Salas-Gutierrez, I.; Lemus-Bedolla, E.; Castillo-Guzman, A.; Lopez-Lemus, H.; Castro-Camus, E. Terahertz imaging for early screening of diabetic foot syndrome: A proof of concept. Sci. Rep. 2017, 7, 42124. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, M.M.; Shkurinov, A.P.; Kuleshov, E.; Tuchin, V.V. Terahertz time-domain spectroscopy of biological tissues. Quantum Electron. 2008, 38, 647. [Google Scholar] [CrossRef]
- Chen, L.; Gao, C.; Xu, J.; Zang, X.; Cai, B.; Zhu, Y. Observation of electromagnetically induced transparency-like transmission in terahertz asymmetric waveguide-cavities systems. Opt. Lett. 2013, 38, 1379–1381. [Google Scholar] [CrossRef]
- Chen, L.; Liao, D.; Guo, X.; Zhao, J.; Zhu, Y.; Zhuang, S. Terahertz time-domain spectroscopy and micro-cavity components for probing samples: A review. Front. Inf. Technol. Electron. Eng. 2019, 20, 591–607. [Google Scholar] [CrossRef]
- Chen, L.; Yin, H.; Chen, L.; Zhu, Y. Ultra-sensitive fluid fill height sensing based on spoof surface plasmon polaritons. J. Electromagn. Waves Appl. 2018, 32, 471–482. [Google Scholar] [CrossRef]
- Chen, L.; Ge, Y.; Zang, X.; Xie, J.; Ding, L.; Balakin, A.V.; Shkurinov, A.P.; Zhu, Y. Tunable Phase Transition via Radiative Loss Controlling in a Terahertz Attenuated Total Reflection-Based Metasurface. IEEE Trans. Terahertz Sci. Technol. 2019, 9, 643–650. [Google Scholar] [CrossRef]
- Shiraga, K.; Ogawa, Y.; Suzuki, T.; Kondo, N.; Irisawa, A.; Imamura, M. Determination of the complex dielectric constant of an epithelial cell monolayer in the terahertz region. Appl. Phys. Lett. 2013, 102, 053702. [Google Scholar] [CrossRef]
- Nandi, N.; Bhattacharyya, K.; Bagchi, B. Dielectric relaxation and solvation dynamics of water in complex chemical and biological systems. Chem. Rev. 2000, 100, 2013–2046. [Google Scholar] [CrossRef] [PubMed]
- Coutaz, J.L.; Garet, F.; Wallace, V.P. Principles of Terahertz Time-Domain Spectroscopy; Jenny Stanford Publishing: New York, NY, USA, 2018. [Google Scholar]
- Bennett, D.B.; Li, W.; Taylor, Z.D.; Grundfest, W.S.; Brown, E.R. Stratified media model for terahertz reflectometry of the skin. IEEE Sens. J. 2010, 11, 1253–1262. [Google Scholar] [CrossRef]
- Betzalel, N.; Feldman, Y.; Ishai, P.B. The Modeling of the Absorbance of Sub-THz Radiation by Human Skin. IEEE Trans. Terahertz Sci. Technol. 2017, 7, 521–528. [Google Scholar] [CrossRef]
- Pickwell, E.; Cole, B.; Fitzgerald, A.; Wallace, V.; Pepper, M. Simulation of terahertz pulse propagation in biological systems. Appl. Phys. Lett. 2004, 84, 2190–2192. [Google Scholar] [CrossRef]
- Kindt, J.; Schmuttenmaer, C. Far-infrared dielectric properties of polar liquids probed by femtosecond terahertz pulse spectroscopy. J. Phys. Chem. 1996, 100, 10373–10379. [Google Scholar] [CrossRef]
- Chen, L.; Cao, Z.; Ou, F.; Li, H.; Shen, Q.; Qiao, H. Observation of large positive and negative lateral shifts of a reflected beam from symmetrical metal. Opt. Lett. 2007, 32, 1432–1434. [Google Scholar] [CrossRef] [PubMed]









| Handcream | Handcream 1 | Handcream 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ingredients | Aqua | Glycerol | Caprylic/ Capric Triglyceride | Sorbitan Oleate | Aqua | Isopropyl Myristate | Huile minerale | Glyceryl Stearate |
| Function | Solvent | Moisturize | Parfum | Emulgator | Solvent | Ester | Solvent | Emulgator |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Guilavogui, S.; Yin, H.; Wu, Y.; Zang, X.; Xie, J.; Ding, L.; Chen, L. Critical Factors for In Vivo Measurements of Human Skin by Terahertz Attenuated Total Reflection Spectroscopy. Sensors 2020, 20, 4256. https://doi.org/10.3390/s20154256
Wang L, Guilavogui S, Yin H, Wu Y, Zang X, Xie J, Ding L, Chen L. Critical Factors for In Vivo Measurements of Human Skin by Terahertz Attenuated Total Reflection Spectroscopy. Sensors. 2020; 20(15):4256. https://doi.org/10.3390/s20154256
Chicago/Turabian StyleWang, Lixia, Sayon Guilavogui, Henghui Yin, Yiping Wu, Xiaofei Zang, Jingya Xie, Li Ding, and Lin Chen. 2020. "Critical Factors for In Vivo Measurements of Human Skin by Terahertz Attenuated Total Reflection Spectroscopy" Sensors 20, no. 15: 4256. https://doi.org/10.3390/s20154256
APA StyleWang, L., Guilavogui, S., Yin, H., Wu, Y., Zang, X., Xie, J., Ding, L., & Chen, L. (2020). Critical Factors for In Vivo Measurements of Human Skin by Terahertz Attenuated Total Reflection Spectroscopy. Sensors, 20(15), 4256. https://doi.org/10.3390/s20154256







