Development of Soft sEMG Sensing Structures Using 3D-Printing Technologies †
Abstract
:1. Introduction
1.1. EMG
1.2. Conductive 3D Printing
2. Methods
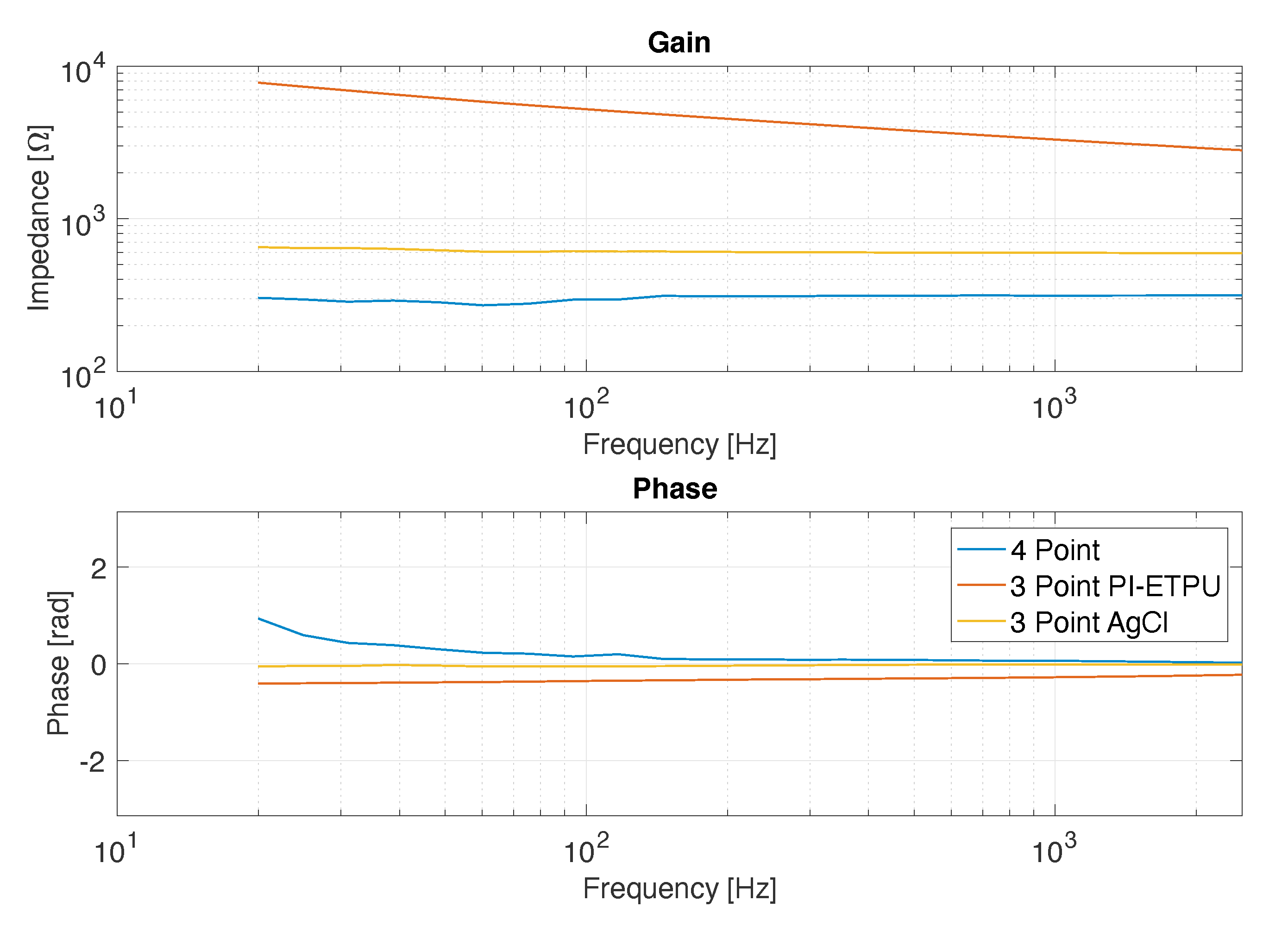
2.1. Electrode Impedance
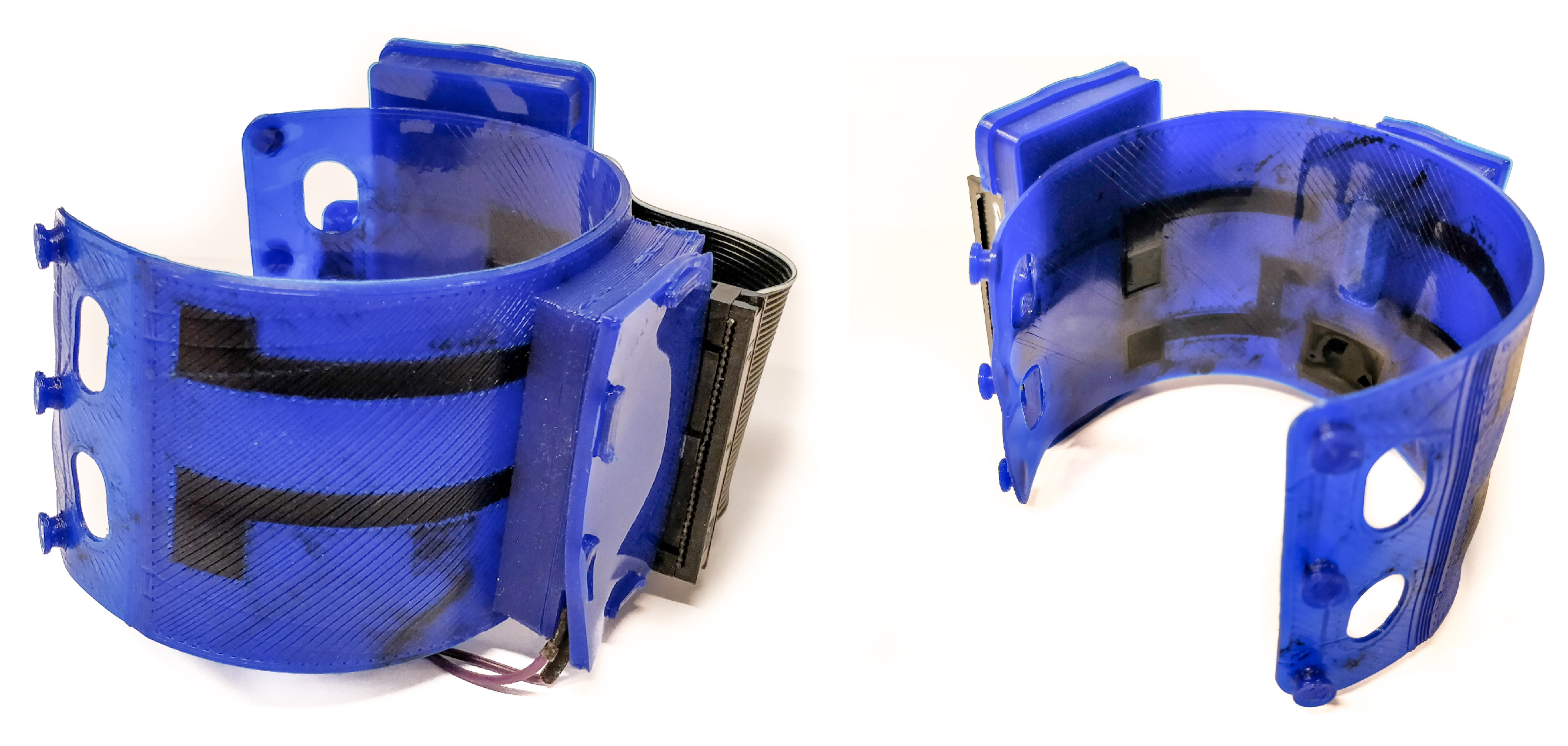
2.2. Side-by-Side sEMG Electrode Characterization
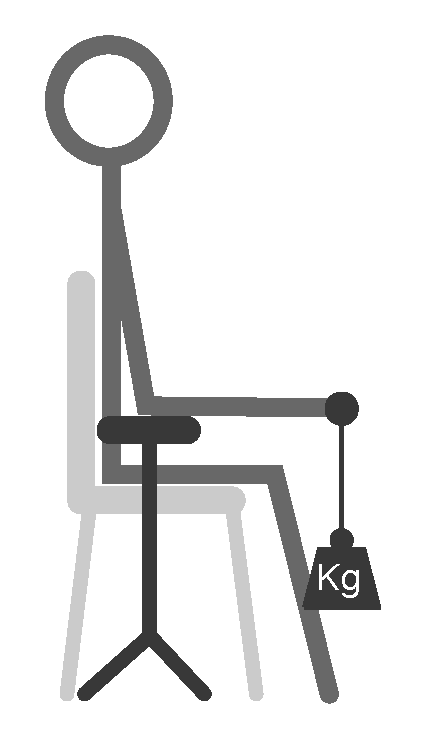
2.2.1. Study Design
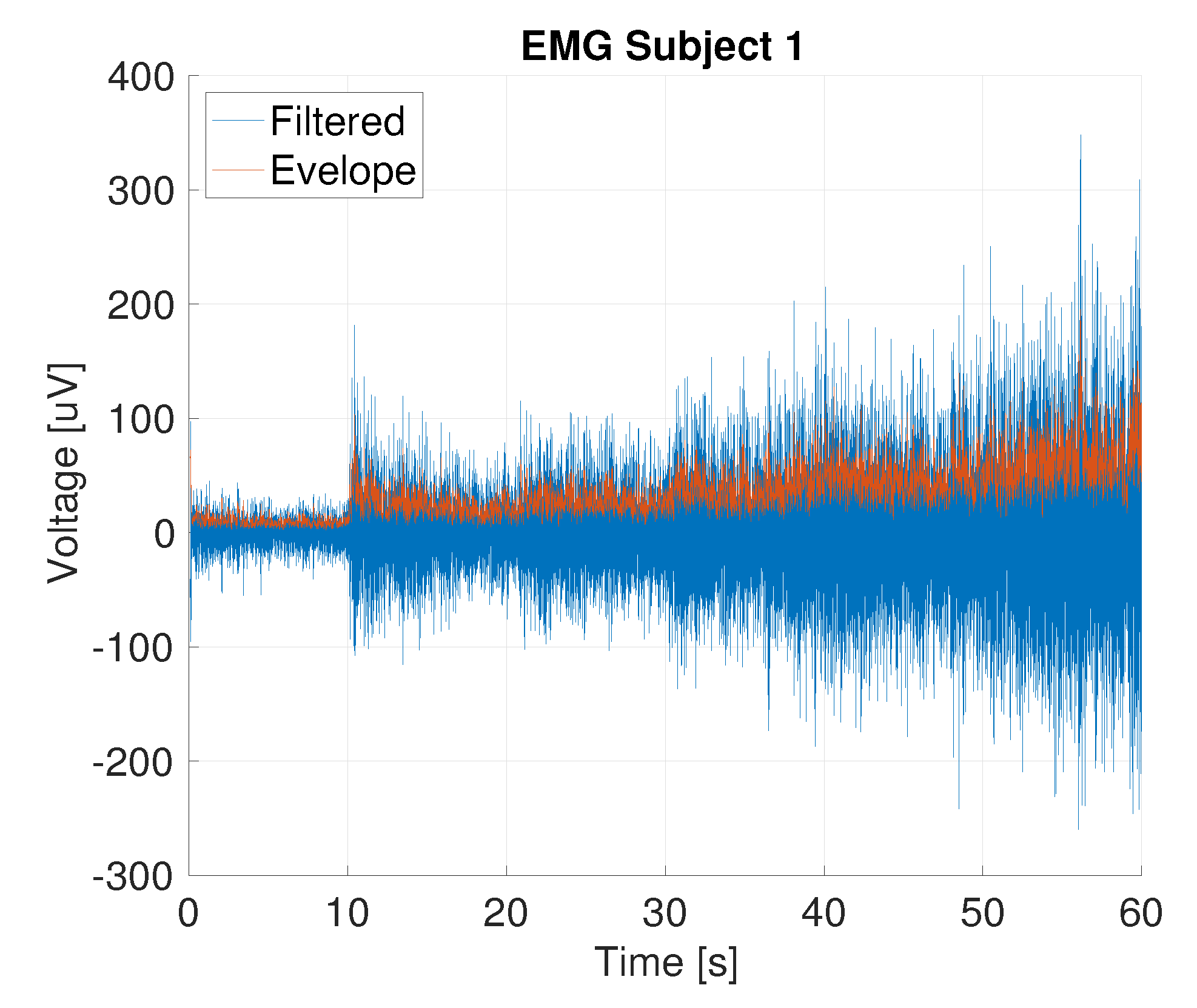
2.2.2. Data Processing
2.2.3. Statistical Analysis
2.3. Functional Analysis: Classifier Approach
- Inactivity: for 9 s, the subject stands still to obtain the EMG in the neutral position.
- The following process is repeated for each class:
- -
- Determining maximum voluntary contraction (MVC): 9 s recording with 3 s of inactivity and 6 s of full contraction.
- -
- 3 s of inactivity followed by 6 s of contraction at 30%, 50% and 70% of the previously determined MVC. The subject receives real-time feedback about the target EMG and MVC.
- Offline generation of the classifier: building the LDA and SVM classifiers. Performing a 10-fold cross validation using the training data to obtain the confusion matrix.
- Online testing of the classifier: real-time EMG data are analyzed by the classifier to get real-time feedback of the user’s gesture.
3. Results
3.1. Impedance Measurements
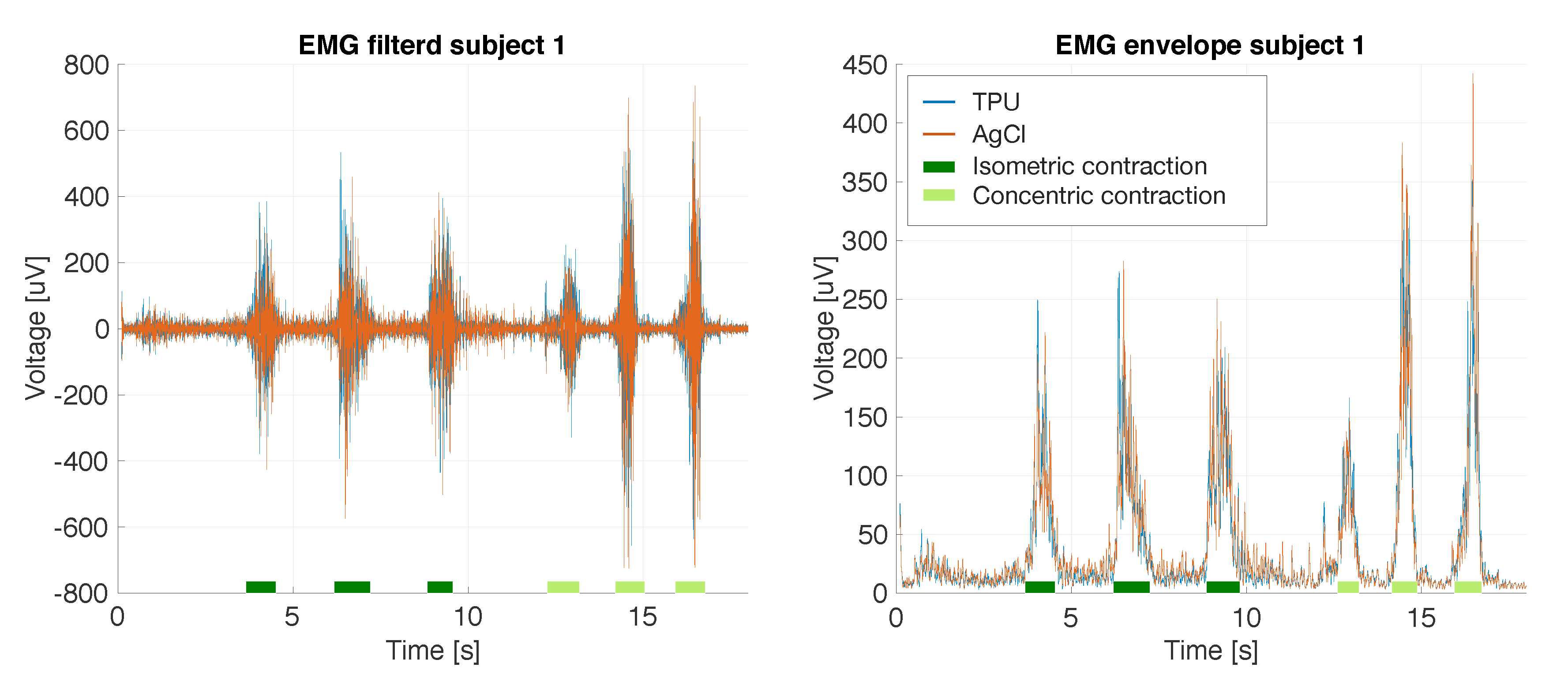
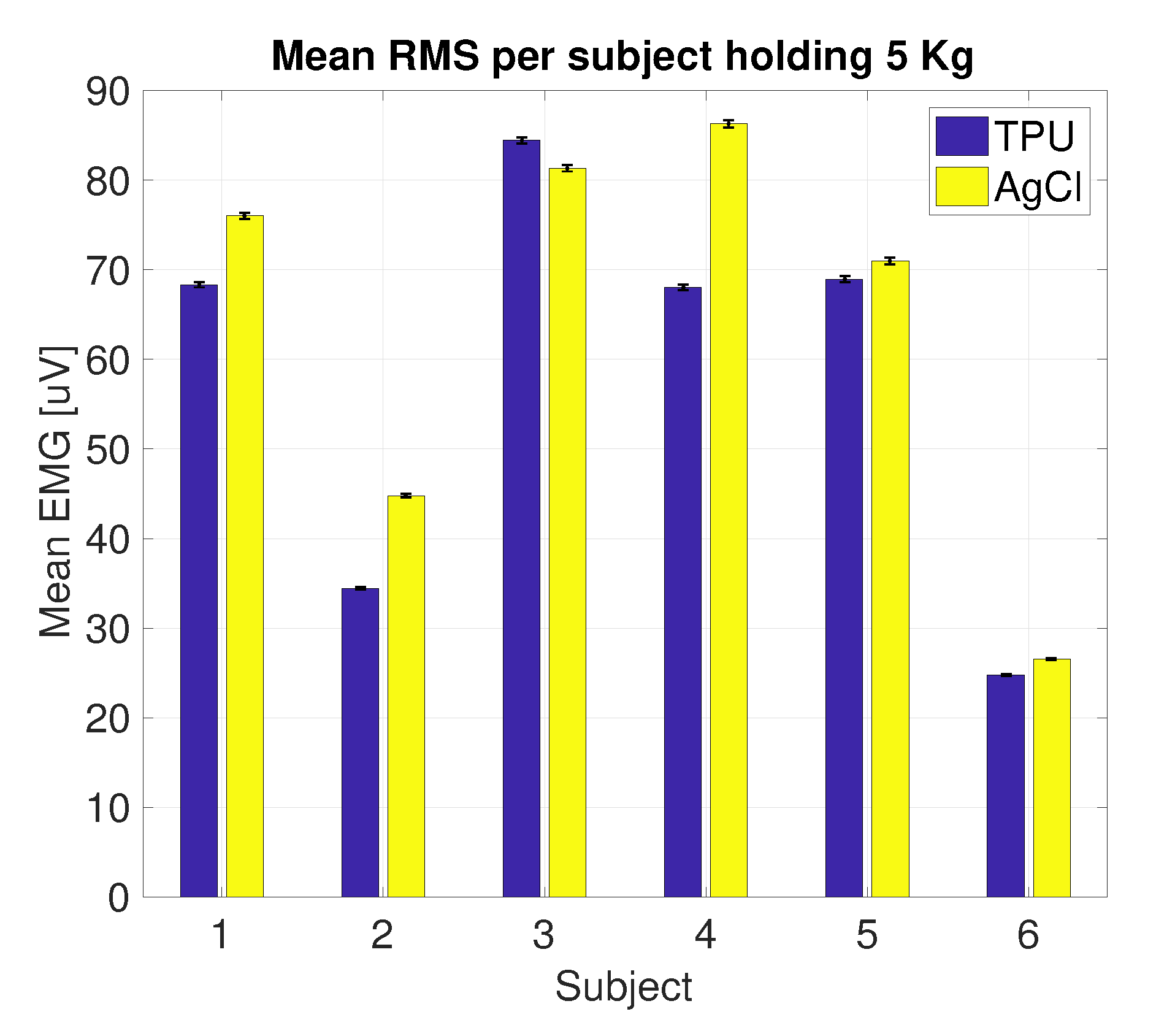
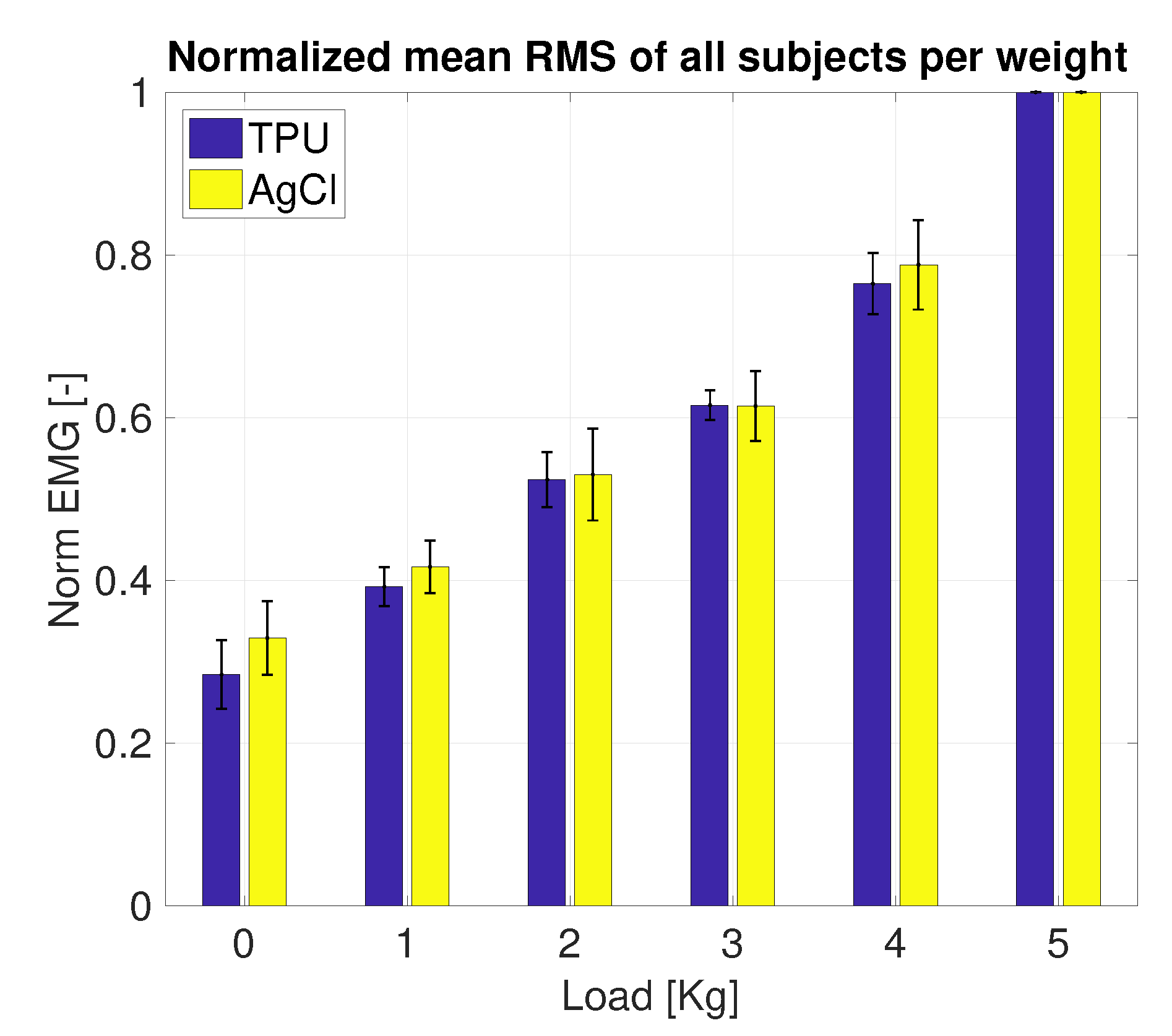
3.2. Side-by-Side sEMG Electrode Characterization
3.2.1. Printed vs. Conventional AgCl Electrodes
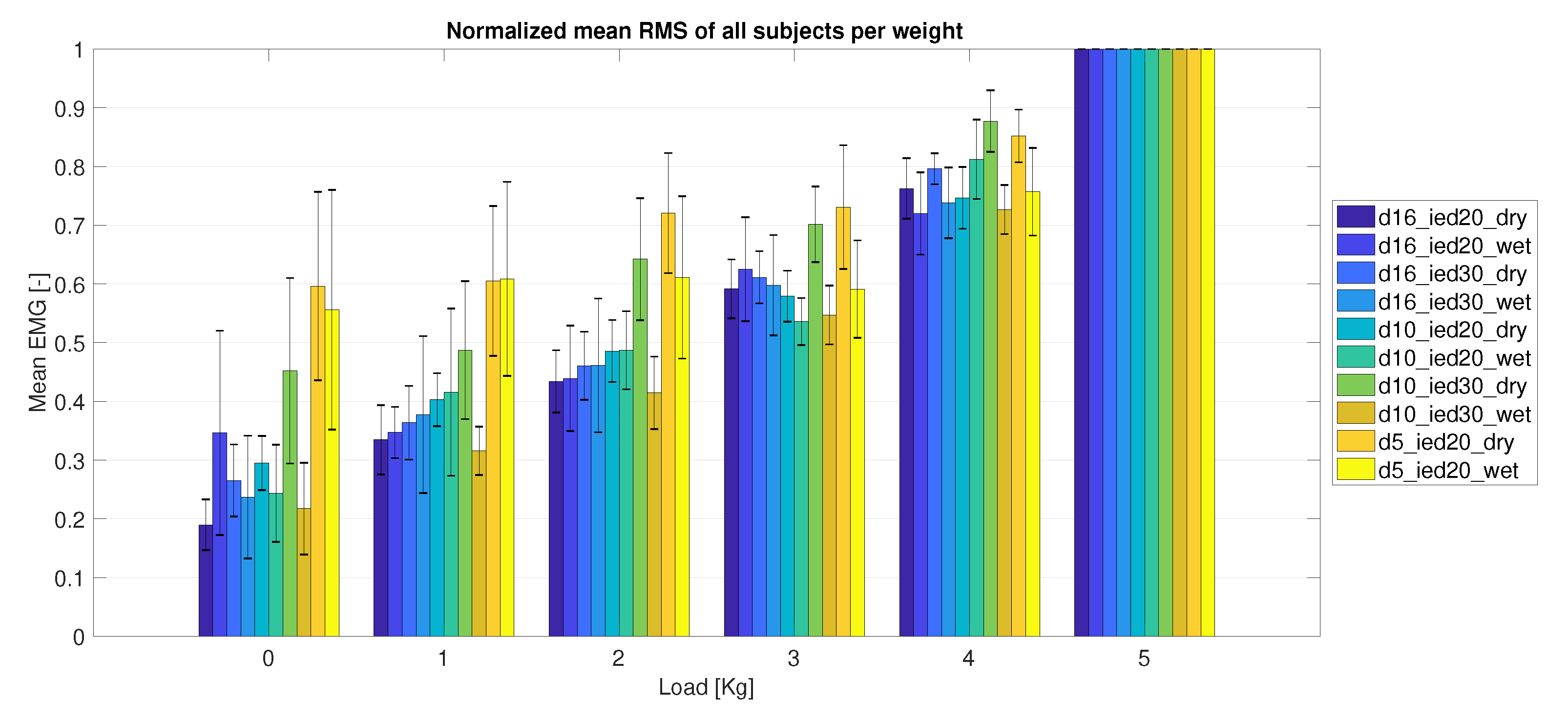
3.2.2. Electrode Size
3.3. Functional Analysis
4. Discussion
4.1. FDM Printing Process
4.2. Contact Interface
4.3. Electrode Evaluation
4.4. Hand Posture Classification
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Staudenmann, D.; Roeleveld, K.; Stegeman, D.F.; van Dieen, J.H. Methodological aspects of SEMG recordings for force estimation—A tutorial and review. J. Electromyogr. Kinesiol. 2010, 20, 375–387. [Google Scholar] [CrossRef]
- Merletti, R.; Botter, A.; Troiano, A.; Merlo, E.; Minetto, M.A. Technology and instrumentation for detection and conditioning of the surface electromyographic signal: State of the art. Clin. Biomech. 2009, 24, 122–134. [Google Scholar] [CrossRef]
- Reaz, M.B.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications (Correction). Biol. Proc. Online 2006, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Piotrkiewicz, M.; Kuraszkiewicz, B. Afterhyperpolarization of human motoneurons firing double and triple discharges. Front. Hum. Neurosci. 2014, 8, 373. [Google Scholar] [CrossRef] [Green Version]
- Scalisi, R.G.; Paleari, M.; Favetto, A.; Stoppa, M.; Ariano, P.; Pandolfi, P.; Chiolerio, A. Inkjet printed flexible electrodes for surface electromyography. Org. Electron. 2015, 18, 89–94. [Google Scholar] [CrossRef]
- Schultz, A.E.; Kuiken, T.A. Neural interfaces for control of upper limb prostheses: The state of the art and future possibilities. PM&R 2011, 3, 55–67. [Google Scholar]
- Godfrey, S.; Ajoudani, A.; Catalano, M.; Grioli, G.; Bicchi, A. A synergy-driven approach to a myoelectric hand. In Proceedings of the 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR), Seattle, WA, USA, 24–26 June 2013; pp. 1–6. [Google Scholar]
- TMS-International B.V. Available online: https://www.tmsi.com/ (accessed on 24 May 2017).
- Manero, A.; Smith, P.; Sparkman, J.; Dombrowski, M.; Courbin, D.; Kester, A.; Womack, I.; Chi, A. Implementation of 3D printing technology in the field of prosthetics: Past, present, and future. Int. J. Environ. Res. Public Health 2019, 16, 1641. [Google Scholar] [CrossRef] [Green Version]
- Wolterink, G.; Sanders, R.; Krijnen, G. A flexible, three material, 3D-printed, shear force sensor for use on finger tips. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 2–5. [Google Scholar] [CrossRef] [Green Version]
- Dijkshoorn, A.; Werkman, P.; Welleweerd, M.; Wolterink, G.; Eijking, B.; Delamare, J.; Sanders, R.; Krijnen, G.J. Embedded sensing: Integrating sensors in 3-D printed structures. J. Sens. Sens. Syst. 2018, 7, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Postolache, G.; Paiva, A.; Carvalho, H.; Catarino, A.; Postolache, O.; Postolache, G. Development of Dry Textile Electrodes for Electromiography. In Proceedings of the 2015 9th International Conference on Sensing Technology (ICST), Auckland, New Zealand, 8–10 December 2015; pp. 447–451. [Google Scholar]
- Bareket, L.; Inzelberg, L.; Rand, D.; David-Pur, M.; Rabinovich, D.; Brandes, B.; Hanein, Y. Temporary-tattoo for long-term high fidelity biopotential recordings. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Guo, X.; Kong, B.; Zhang, M.; Qian, X.; Mi, S.; Sun, W. The Boom in 3D-Printed Sensor Technology. Sensors 2017, 17, 1166. [Google Scholar] [CrossRef] [PubMed]
- Wolterink, G.; Sanders, R.; Krijnen, G. Thin, flexible, capacitive force sensors based on anisotropy in 3D-printed structures. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar]
- Webster, J.G.; Clark, J.W. Medical Instrumentation: Application And Design, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; 713p. [Google Scholar]
- Luca, C.J.D. Surface Electromyography: Detection and Recording. Available online: https://www.delsys.com/downloads/TUTORIAL/semg-detection-and-recording.pdf (accessed on 29 July 2020).
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Xie, L.; Yang, G.; Xu, L.; Seoane, F.; Chen, Q.; Zheng, L. Characterization of dry biopotential electrodes. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 1478–1481. [Google Scholar] [CrossRef]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D-printed dry electrode for ECG/EEG recording. Sens. Actuators Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, R47–R69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Op de Beeck, M.; Vanderheyden, L.; Carrette, E.; Mihajlovic, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; Van Hoof, C. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Chang, C.L.; Chang, C.W.; Lai, S.C.; Chien, T.F.; Huang, H.Y.; Chiou, J.C.; Luo, C.H. A low-power bio-potential acquisition system with flexible PDMS dry electrodes for portable ubiquitous healthcare applications. Sensors 2013, 13, 3077–3091. [Google Scholar] [CrossRef]
- Manabe, H.; Fukumoto, M.; Yagi, T. Conductive rubber electrodes for earphone-based eye gesture input interface. Pers. Ubiquitous Comput. 2015, 19, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Pylatiuk, C.; Muller-Riederer, M.; Kargov, A.; Schulz, S.; Schill, O.; Reischl, M.; Bretthauer, G. Comparison of surface EMG monitoring electrodes for long-term use in rehabilitation device control. In Proceedings of the 2009 IEEE International Conference on Rehabilitation Robotics, Kyoto, Japan, 23–26 June 2009; pp. 300–304. [Google Scholar]
- Chi, Y.M.; Jung, T.P.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, K.P.; Ruff, R.; Poppendieck, W. Long-term characterization of electrode materials for surface electrodes in biopotential recording. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; Volume 1, pp. 2239–2242. [Google Scholar] [CrossRef]
- TMS-International B.V. Technical Specifications Refa Revision 4. Available online: https://info.tmsi.com/hubfs/Technical%20Specifications_Refa-Rev4.pdf (accessed on 29 July 2020).
- Swensen, J.P.; Odhner, L.U.; Araki, B.; Dollar, A.M. Printing three-dimensional electrical traces in additive manufactured parts for injection of low melting temperature metals. J. Mech. Robot. 2015, 7, 021004. [Google Scholar] [CrossRef]
- Vatani, M.; Lu, Y.; Engeberg, E.D.; Choi, J.W. Combined 3D printing technologies and material for fabrication of tactile sensors. Int. J. Precis. Eng. Manuf. 2015, 16, 1375–1383. [Google Scholar] [CrossRef]
- Wu, S.Y.; Yang, C.; Hsu, W.; Lin, L. 3D-printed microelectronics for integrated circuitry and passive wireless sensors. Microsyst. Nanoeng. 2015, 1, 1–9. [Google Scholar]
- Welleweerd, M. 3D Printing a Three Degree of Freedom Force Sensor. Master’s Thesis, University of Twente, Enschede, The Nederlands, 2017. [Google Scholar]
- Valentine, A.D.; Busbee, T.A.; Boley, J.W.; Raney, J.R.; Chortos, A.; Kotikian, A.; Berrigan, J.D.; Durstock, M.F.; Lewis, J.A. Hybrid 3D Printing of Soft Electronics. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H.; Ntagios, M.; Navaraj, W.T.; Dahiva, R. Multi-Material 3D-printed Bendable Smart Sensing Structures. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018; pp. 3–6. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.Z.; Qiu, K.; Meng, F.; Park, S.H.; McAlpine, M.C. 3D-printed Stretchable Tactile Sensors. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.J.; Duoss, E.B.; Malkowski, T.F.; Motala, M.J.; Ahn, B.Y.; Nuzzo, R.G.; Bernhard, J.T.; Lewis, J.A. Conformal printing of electrically small antennas on three-dimensional surfaces. Adv. Mater. 2011, 23, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Odent, J.; Wallin, T.J.; Pan, W.; Kruemplestaedter, K.; Shepherd, R.F.; Giannelis, E.P. Highly Elastic, Transparent, and Conductive 3D-Printed Ionic Composite Hydrogels. Adv. Funct. Mater. 2017, 27, 1–10. [Google Scholar] [CrossRef]
- Fenner Drives. Ninjatek.com. Available online: https://ninjatek.com (accessed on 31 January 2017).
- Polymaker. PolyFlex. Available online: http://www.polymaker.com/shop/polyflex/ (accessed on 1 May 2016).
- Diabase Engineering. X60 Ultra-Flexible Filament-White. Available online: https://flexionextruder.com/shop/x60-ultra-flexible-filament-white/ (accessed on 28 April 2020).
- Lay-Filaments. 4D Meta Material: The PoroLAY Series. Available online: http://lay-filaments.com (accessed on 28 April 2017).
- Palmiga Innovation. Material Info for PI-ETPU 95-250 Carbon Black the Conductive and Flexible 3D Printing Filament. Available online: http://rubber3Dprinting.com/pi-etpu-95-250-carbon-black/ (accessed on 26 May 2017).
- ProtoPlant, Makers of Proto-Pasta. Composite PLA-Electrically Conductive Graphite. Available online: https://www.proto-pasta.com/ (accessed on 31 January 2017).
- Graphene 3D Lab. Conductive Graphene PLA Filament. Available online: http://www.blackmagic3D.com/Conductive-p/grphn-pla.htm (accessed on 31 January 2017).
- 3D-Prima. 3D-Prima Conductive ABS. Available online: https://www.3Dprima.com/en/filaments-for-3D-printers/abs-175mm/3D-prima-conductive-abs-175mm-1-kg-spool-black.html (accessed on 13 October 2016).
- Multi3D. Electrifi Conductive Filament. Available online: https://www.multi3Dllc.com/product/electrifi/ (accessed on 28 April 2020).
- EEL 3D Filament. Safety Data Sheet. Available online: http://ninjatek.com/wp-content/uploads/2018/12/SDS_EEL.pdf (accessed on 29 July 2020).
- EEL 3D Filament. Full Conductive, Flexible Filament for 3D Printers. Available online: http://ninjatek.com/wp-content/uploads/2018/12/Eel-TDS.pdf (accessed on 29 July 2020).
- Gubbels, F.; Blacher, S.; Vanlathem, E.; Jérôme, R.; Deltour, R.; Brouers, F.; Teyssie, P. Design of electrical composites: Determining the role of the morphology on the electrical properties of carbon black filled polymer blends. Macromolecules 1995, 28, 1559–1566. [Google Scholar] [CrossRef]
- Munson-McGee, S.H. Estimation of the critical concentration in an anisotropic percolation network. Phys. Rev. B 1991, 43, 3331. [Google Scholar] [CrossRef]
- Flandin, L.; Hiltner, A.; Baer, E. Interrelationships between electrical and mechanical properties of a carbon black-filled ethylene–octene elastomer. Polymer 2001, 42, 827–838. [Google Scholar] [CrossRef]
- SENIAM. Available online: www.seniam.org (accessed on 23 March 2017).
- The MathWorks, Inc. Discrete-Time, Direct-Form II Filter. Available online: https://mathworks.com/help/signal/ref/dfilt.df2.html (accessed on 25 March 2020).
- Dijkshoorn, A.; Schouten, M.; Wolterink, G.; Sanders, R.; Krijnen, G. Characterizing the Electrical Properties of Anisotropic, 3D-Printed Conductive Sheets. In Proceedings of the 2019 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Glasgow, UK, 8–10 July 2019; pp. 1–3. [Google Scholar]
- Daley, H.; Englehart, K.; Hargrove, L.; Kuruganti, U. High density electromyography data of normally limbed and transradial amputee subjects for multifunction prosthetic control. J. Electromyogr. Kinesiol. 2012, 22, 478–484. [Google Scholar] [CrossRef]
- Li, G.; Schultz, A.E.; Kuiken, T.A. Quantifying pattern recognition- based myoelectric control of multifunctional transradial prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 185–192. [Google Scholar] [CrossRef] [Green Version]







| Filament/Material | Host | Doping | Volume Resistivity (cm) |
|---|---|---|---|
| Proto-Pasta [43] | PLA | Graphite | |
| BlackMagic3D Conductive Graphene [44] | PLA | Graphene | 0.6 |
| 3D Prima Conductive ABS * [45] | ABS | Carbon fibres | – |
| Multi3D, Electrify [46] | Biodegradable polyester | Copper | 0.006 |
| PI-ETPU [42] | TPU | Carbon black | <300 |
| EEL [47,48] | TPU | Carbon black |
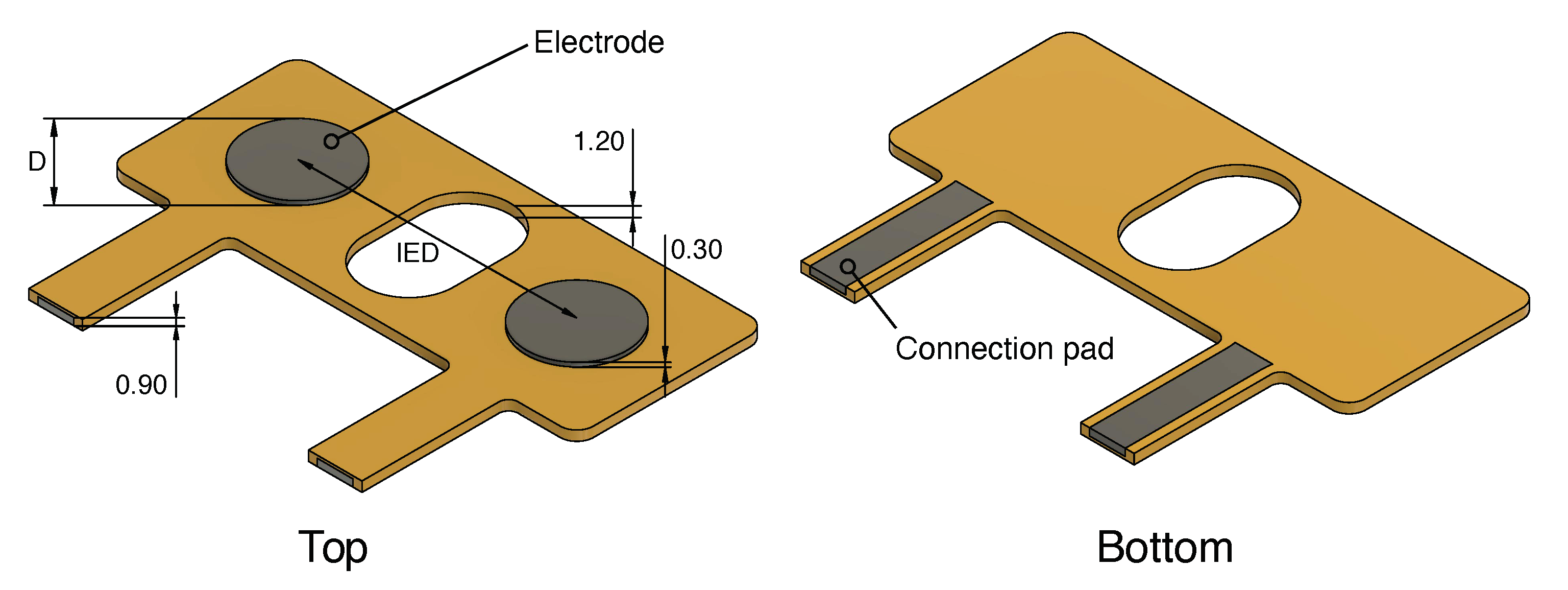
| Name | Diameter (D) (mm) | Inter-Electrode Distance (IED) (mm) |
|---|---|---|
| AgCl | 16 | 20 |
| D16_IED20 | 16 | 20 |
| D16_IED30 | 16 | 30 |
| D10_IED20 | 10 | 20 |
| D10_IED30 | 10 | 30 |
| D05_IED20 | 5 | 20 |
| D05_IED10 | 5 | 10 |
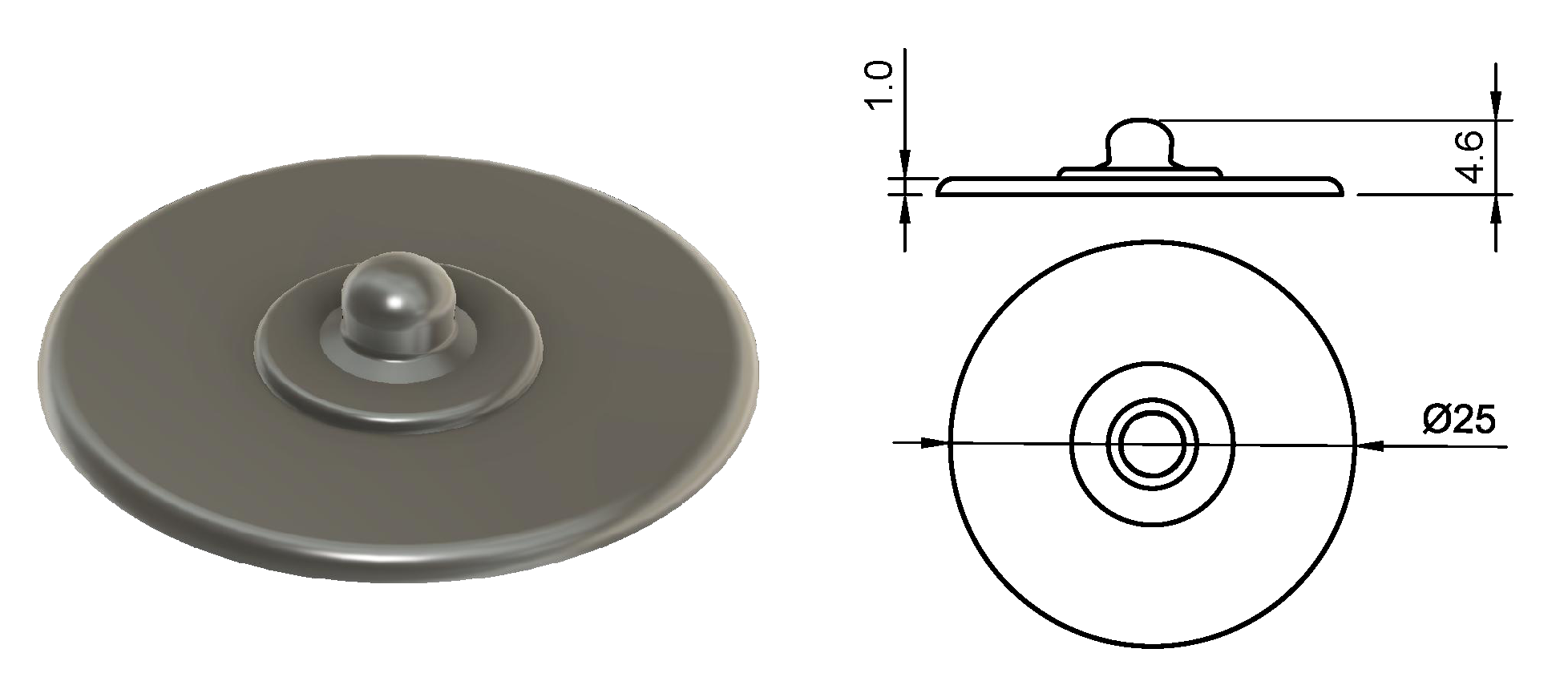
| Snap | 25 | - |
| Subject: | 1 | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|---|
| Classifier | AgCl | ETPU | ETPU | ETPU | Avg ETPU | Std ETPU | |
| LDA | 3 Class | 85.5% | 88.6% | 78.1% | 82.5% | 83.1% | 5.3% |
| 5 Class | 81.6% | 86.5% | 77.5% | 80.2% | 81.4% | 4.6% | |
| SVM | 3 Class | 90.4% | 90.9% | 81.1% | 85.9% | 86.0% | 4.9% |
| 5 Class | 86.8% | 90.6% | 83.1% | 87.1% | 86.9% | 3.8% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolterink, G.; Dias, P.; Sanders, R.G.P.; Muijzer, F.; Beijnum, B.-J.v.; Veltink, P.; Krijnen, G. Development of Soft sEMG Sensing Structures Using 3D-Printing Technologies. Sensors 2020, 20, 4292. https://doi.org/10.3390/s20154292
Wolterink G, Dias P, Sanders RGP, Muijzer F, Beijnum B-Jv, Veltink P, Krijnen G. Development of Soft sEMG Sensing Structures Using 3D-Printing Technologies. Sensors. 2020; 20(15):4292. https://doi.org/10.3390/s20154292
Chicago/Turabian StyleWolterink, Gerjan, Pedro Dias, Remco G. P. Sanders, Frodo Muijzer, Bert-Jan van Beijnum, Peter Veltink, and Gijs Krijnen. 2020. "Development of Soft sEMG Sensing Structures Using 3D-Printing Technologies" Sensors 20, no. 15: 4292. https://doi.org/10.3390/s20154292





