Microsensor Electrodes for 3D Inline Process Monitoring in Multiphase Microreactors
Abstract
:1. Introduction
2. Methods and Experimental
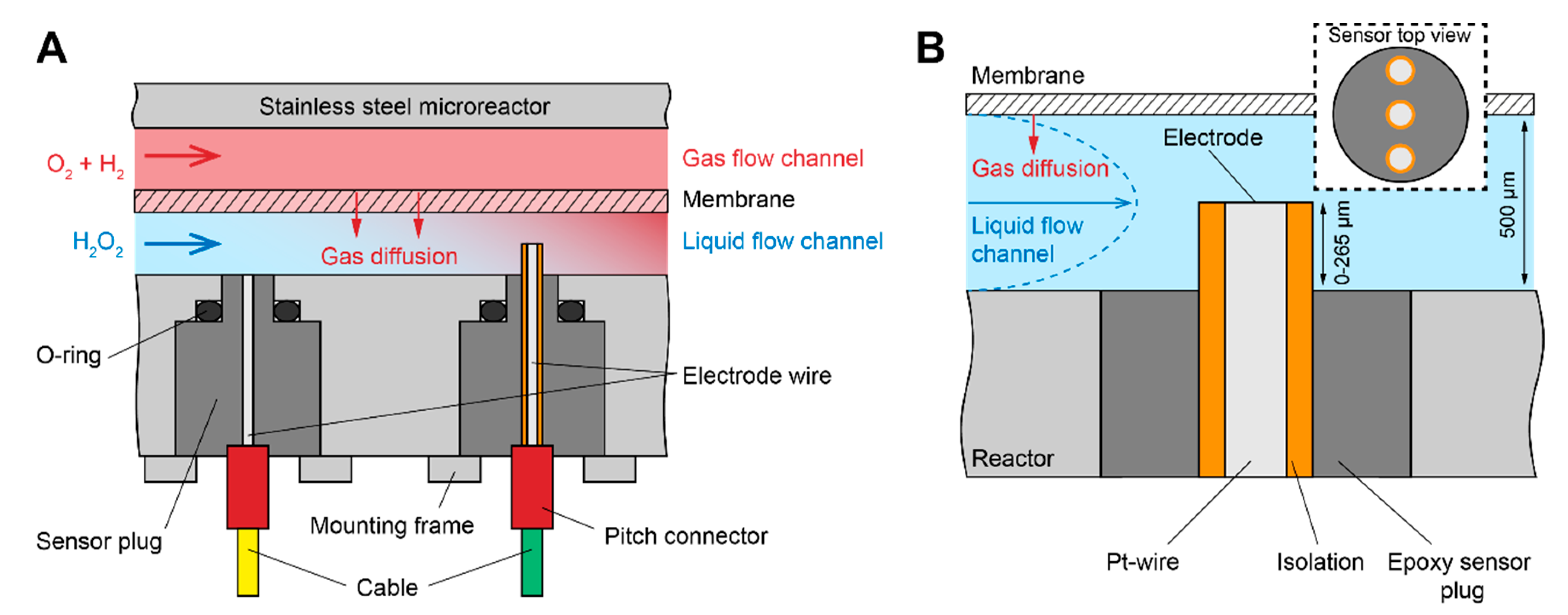
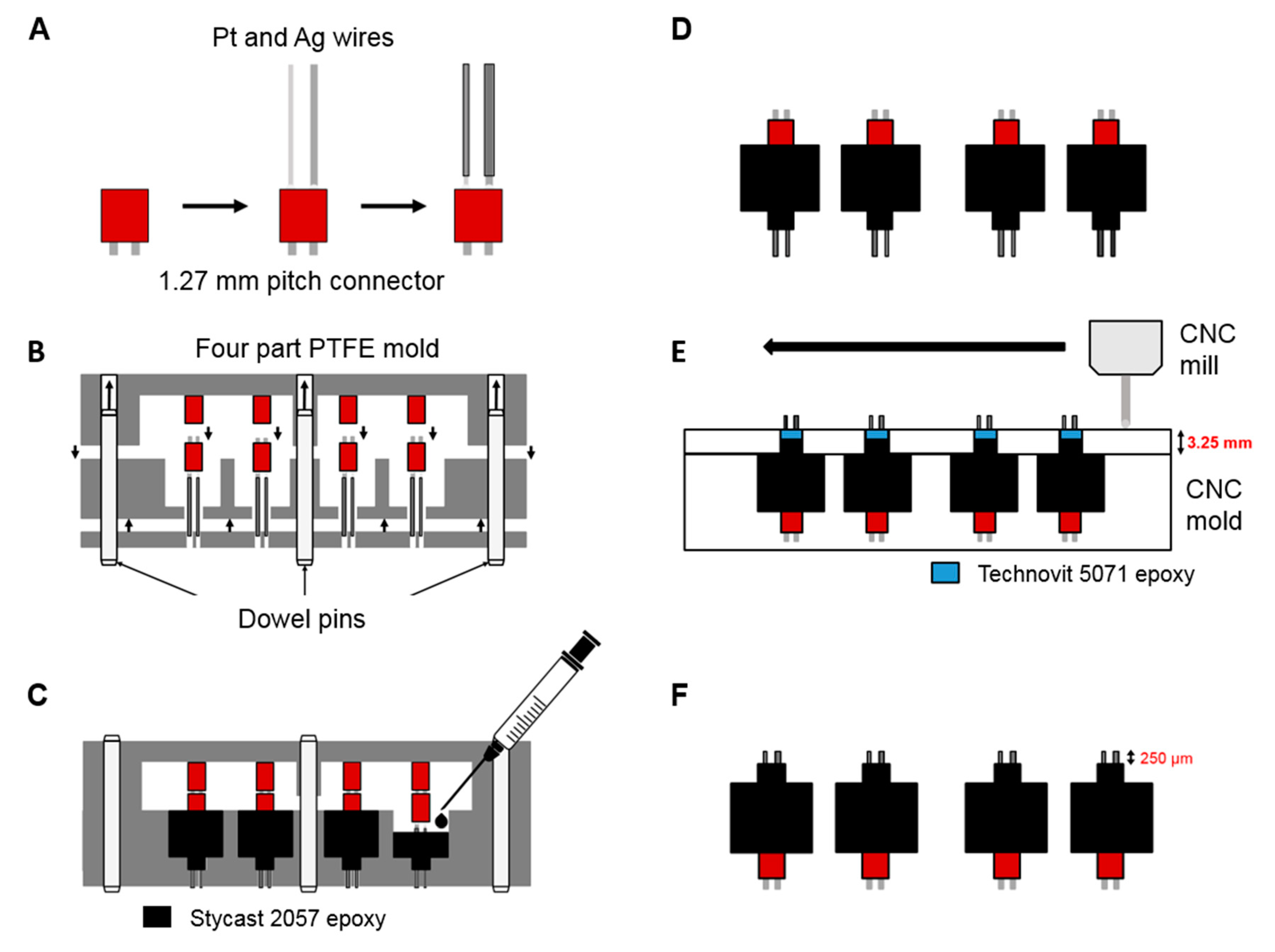
2.1. Sensor Device Fabrication
2.2. Electrochemical Measurements
3. Results and Discussion
3.1. Sensor Calibration
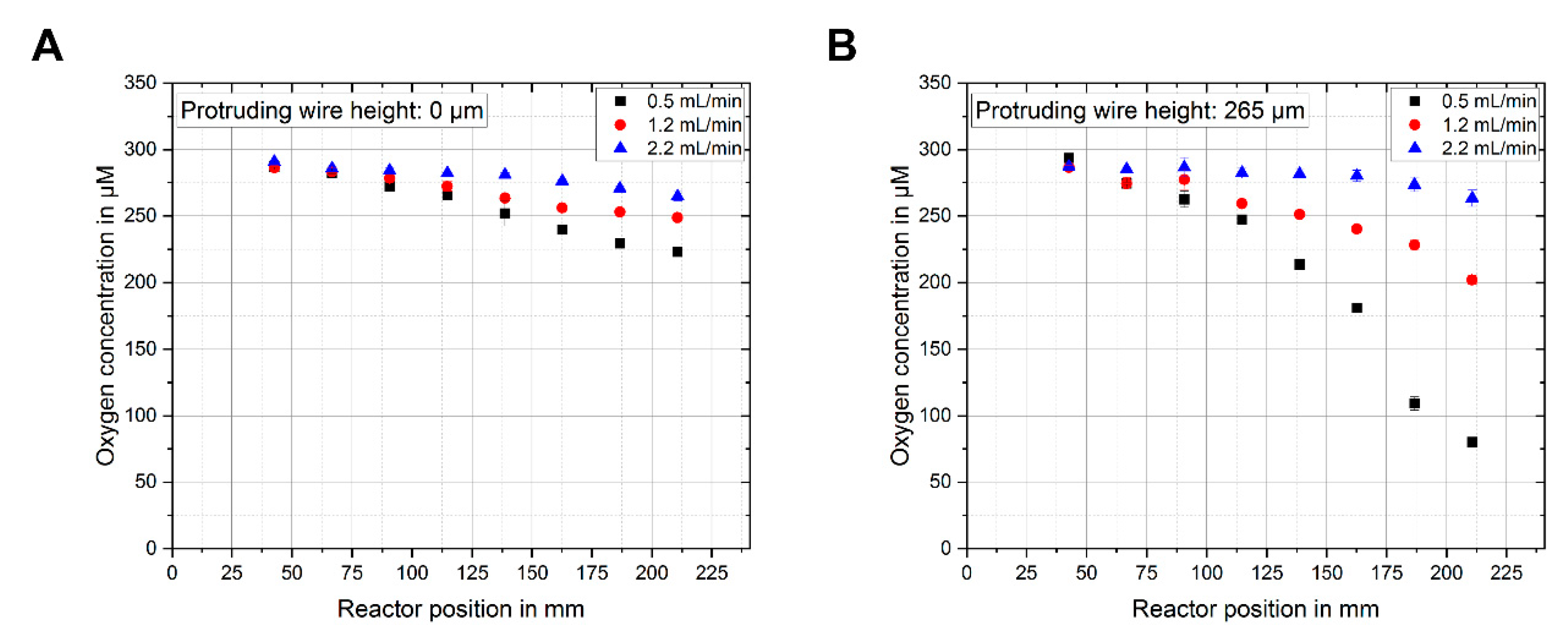
3.2. Oxygen Measurement at Different Reactor Positions and Sensor Heights
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen Peroxide: A Key Chemical for Today’s Sustainable Development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- García-Serna, J.; Moreno, T.; Biasi, P.; Cocero, M.J.; Mikkola, J.-P.; Salmi, T.O. Engineering in direct synthesis of hydrogen peroxide: Targets, reactors and guidelines for operational conditions. Green Chem. 2014, 16, 2320. [Google Scholar] [CrossRef] [Green Version]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chemie Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Pennemann, H.; Kolb, G. Review: Microstructured reactors as efficient tool for the operation of selective oxidation reactions. Catal. Today 2016, 278, 3–21. [Google Scholar] [CrossRef]
- Goor, G.; Glenneberg, J.; Jacobi, S. Hydrogen Peroxide. In Ullmann’s Encyclopedia of Industrial Chemistry; Ley, C., Elvers, B., Bellussi, G., Bus, J., Drauz, K., Greim, H., Hessel, V., Kleemann, A., Kutscher, B., Meijer, G., et al., Eds.; Wiley-VCH: Weinheim, Germany, 2000; ISBN 9783527306732. [Google Scholar]
- Riedl, H.J.; Pfleiderer, G. Production of Hydrogen Peroxide. U.S. Patent 2158525A, 16 May 1939. [Google Scholar]
- Huerta, I.; Biasi, P.; García-Serna, J.; Cocero, M.J.; Mikkola, J.P.; Salmi, T. Effect of low hydrogen to palladium molar ratios in the direct synthesis of H2O2 in water in a trickle bed reactor. Catal. Today 2015, 248, 91–100. [Google Scholar] [CrossRef]
- Samanta, C.; Choudhary, V.R. Direct formation of H2O2 from H2 and O2 and decomposition/hydrogenation of H2O2 in aqueous acidic reaction medium over halide-containing Pd/SiO2 catalytic system. Catal. Commun. 2007, 8, 2222–2228. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, L.; Li, G.; Guo, H. A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: Noble-metal catalytic method fuel-cell method and plasma method. Catal. Sci. Technol. 2016, 6, 1593–1610. [Google Scholar] [CrossRef]
- Samanta, C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: An overview of recent developments in the process. Appl. Catal. A Gen. 2008, 350, 133–149. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Abate, S. Direct Synthesis of Hydrogen Peroxide: Recent Advances. In Modern Heterogeneous Oxidation Catalysis; Mizuno, N., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 253–287. ISBN 9783527627547. [Google Scholar]
- Crole, D.A.; Freakley, S.J.; Edwards, J.K.; Hutchings, G.J. Direct synthesis of hydrogen peroxide in water at ambient temperature. Proc. R. Soc. A Math. Phys. Eng. Sci. 2016, 472, 20160156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallina, G.; García-Serna, J.; Salmi, T.O.; Canu, P.; Biasi, P. Bromide and Acids: A Comprehensive Study on Their Role on the Hydrogen Peroxide Direct Synthesis. Ind. Eng. Chem. Res. 2017, 56, 13367–13378. [Google Scholar] [CrossRef]
- Biasi, P.; Mikkola, J.P.; Sterchele, S.; Salmi, T.; Gemo, N.; Shchukarev, A.; Centomo, P.; Zecca, M.; Canu, P.; Rautio, A.R.; et al. Revealing the role of bromide in the H2O2 direct synthesis with the catalyst wet pretreatment method (CWPM). AIChE J. 2017, 63, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Hessel, V.; Hardt, S.; Löwe, H. Chemical Micro Process Engineering: Fundamentals, Modelling and Reactions; Wiley-VCH: Weinheim, Germany, 2004; ISBN 3527307419. [Google Scholar]
- Selinsek, M.; Bohrer, M.; Vankayala, B.K.; Haas-Santo, K.; Kraut, M.; Dittmeyer, R. Towards a new membrane micro reactor system for direct synthesis of hydrogen peroxide. Catal. Today 2016, 268, 85–94. [Google Scholar] [CrossRef]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Privett, B.J.; Jae, H.S.; Schoenfisch, M.H. Electrochemical sensors. Anal. Chem. 2008, 80, 4499–4517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism from 2D culture to organ-on-chip. Lab Chip. 2018, 18, 1274–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, S.; Weltin, A.; Flamm, H.; Kieninger, J.; Deschner, B.J.; Kraut, M.; Dittmeyer, R.; Urban, G.A. Electrochemical multisensor system for monitoring hydrogen peroxide, hydrogen and oxygen in direct synthesis microreactors. Sens. Actuators B Chem. 2018, 273, 973–982. [Google Scholar] [CrossRef]
- Weltin, A.; Enderle, B.; Kieninger, J.; Urban, G.A. Multiparametric, flexible microsensor platform for metabolic monitoring in vivo. IEEE Sens. J. 2014, 14, 3345–3351. [Google Scholar] [CrossRef]
- Urban, S.; Kieninger, J.; Deschner, B.J.; Kraut, M.; Dittmeyer, R.; Urban, G.A.; Weltin, A. Multiparametric, Spatially Resolved Detection of H2O2 and O2 with Electrochemical Microsensor Array in Synthesis Membrane Microreactors. In Proceedings of the 2019 20th Int. Conf. Solid-State Sensors, Actuators Microsystems Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019. [Google Scholar]
- Hall, S.B.; Khudaish, E.A.; Hart, A.L. Electrochemical oxidation of hydrogen peroxide at platinum electrodes. Part 1. An adsorption-controlled mechanism. Electrochim. Acta 1998, 43, 579–588. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urban, S.; Tamilselvi Sundaram, V.; Kieninger, J.; Urban, G.A.; Weltin, A. Microsensor Electrodes for 3D Inline Process Monitoring in Multiphase Microreactors. Sensors 2020, 20, 4876. https://doi.org/10.3390/s20174876
Urban S, Tamilselvi Sundaram V, Kieninger J, Urban GA, Weltin A. Microsensor Electrodes for 3D Inline Process Monitoring in Multiphase Microreactors. Sensors. 2020; 20(17):4876. https://doi.org/10.3390/s20174876
Chicago/Turabian StyleUrban, Sebastian, Vinayaganataraj Tamilselvi Sundaram, Jochen Kieninger, Gerald A. Urban, and Andreas Weltin. 2020. "Microsensor Electrodes for 3D Inline Process Monitoring in Multiphase Microreactors" Sensors 20, no. 17: 4876. https://doi.org/10.3390/s20174876





