Turning Detection During Gait: Algorithm Validation and Influence of Sensor Location and Turning Characteristics in the Classification of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Demographics and Clinical Measures
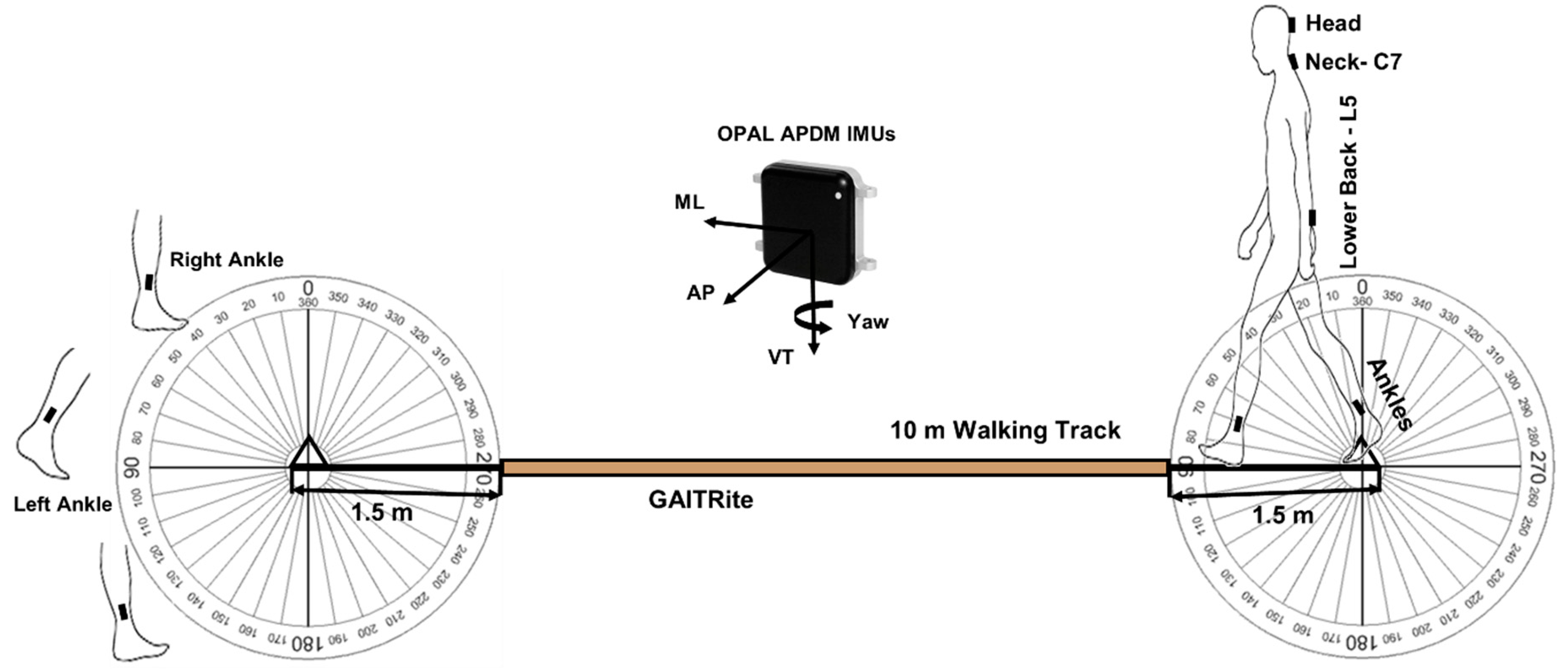
2.3. Testing Protocol and Equipment
2.4. Turn Detection and Algorithm Development
| Algorithm 1. Pseudo code for turning start and end detection. |
| 01: #Data Access 02: for each IMU attachment #head(HD), Neck(C7), L5, Ankles(LA,RA) 03: store calibrated ω, a, m 04: end for 05: #Data Filtering: Noise & Drift Removal 06: Access ω 07: Compensation Algorithm: for HD, C7, L5 08: Access ω, a, m 09: Kalman Filtering (KF) Algorithm: for Ankles (LA,RA) 10: #Every possible body segment rotation around VT 11: Access VT of ω 12: Zero-Crossing: for HD, C7, L5, Ankles 13: #Orientation Estimation 14: Integration ω VT to get Yaw: for HD, C7, L5 15: Sensor fusion with KF to Euler to Yaw: for Ankles 16: #Final Turn Detection and Estimation for HD, C7, L5 17: Yaw angle (ᴪ) extraction based on zero-crossing 18: for every ᴪ 19: #Gradual Turns Combination 20: if ᴪ > 10° and for next consecutive turns if intra turn 21: duration < 0.5 s and ᴪ > 10° and same Direction 22: Combine these gradual turns 23: end 24: #Turns from HD & C7 25: if turn angle (θ) ≥ 30° and 0.5 s ≤ turn duration < 10 s 26: Save turn start and end 27: Save final gradual turn magnitude vector 28: end 29: #Turns from L5 30: if turn angle (θ) ≥ 90° and 0.5 s ≤ turn duration < 10 s 31: Save turn start and end 32: Save final gradual turn magnitude vector 33: end 34: end for 35: #Turn Transition and Estimation from Ankles (LA, RA) 36: for every turn from L5 37: Access the turn start and turn end from L5 38: Access the turn direction from L5 39: #Inner and outer turn detection to overcome direction biases 40: if L5 direction is right 41: Inner turn = RA 42: Outer turn = LA 43: else (L5 direction is left) 44: Outer turn = RA 45: Inner turn = LA 46: end 47: end for 48: #Final turn transition from Ankles 49: for angles from Inner and Outer turn 50: if inner turn angle ≥ 30° 51: save the turn start and turn end 52: save the turn magnitude vector 53: end 54: if outer turn angle ≥ 30° 55: save the turn start and turn end 56: save the turn magnitude vector 57: end 58: end for #Turning features extraction based on turn start and end time |
2.5. Algorithm Validation (Aim 1)
2.6. Feature Extraction (Aim 2)
2.7. Classification Modeling and Importance of Turning Characteristics (Aim 3)
| Algorithm 2. Pseudo code for turning features extraction. |
| 01: #Detected Turns 02: Access turn start and end timings with turning angle magnitude 03: #Spatiotemporal turning characteristics 04: for total turns detected 05: Turn time = mean of (turn end time − turn start time) 06: Turn angle = mean of turning angle magnitude vector 07: minimum, maximum, and variability in turn time and angle vector 08: #Full turn angular velocity 09: Angular velocity = Turn angle/Turn time 10: #Angular frequency (angular velocity) in start, mid & end of turn 11: selection of 0.1 s in the start, mid, end of within turn 12: mean & variability in (max of angular frequency in overall turn) 13: mean & variability in (mean of 0.1 s window in the start, mid, end) 14: #Direction of turn 15: left turn if angle magnitude is negative or right otherwise 16: #Number of turn/transitions 17: length of start and end time vector 18: end for 19: #Signal based turning characteristics 20: Accessing the ω, a in VT, AP & ML directions 21: Detrend the ω, a 22: Data filtering: 4th order low-pass Butterworth filter at 20 Hz cut-off 23: Getting resultant magnitude of VT,AP & ML for ω, a 24: for each start, mid, and end of turn 25: RMS for ω, a in VT, AP, ML & R #Turn overall, start, mid & end 26: #Jerk: rate of change of a 27: RMS, max, min, range of each turn jerk in VT, AP, ML & R #Turn 28: overall, start, mid & End 29: #Angular acceleration: rate of change of ω 30: RMS, max, min, range of each turn angular acceleration in VT, AP, 31: ML & R #Turn overall, start, mid & end 32: end for #classification modeling and statistical analysis |
2.8. Statistical Analysis
3. Results
3.1. Turning Algorithm Validation (Aim 1)
3.1.1. Agreement between Raters
3.1.2. Agreement between the Raters and Algorithm
3.2. Extraction of Turning Characteristics (Aim 2)
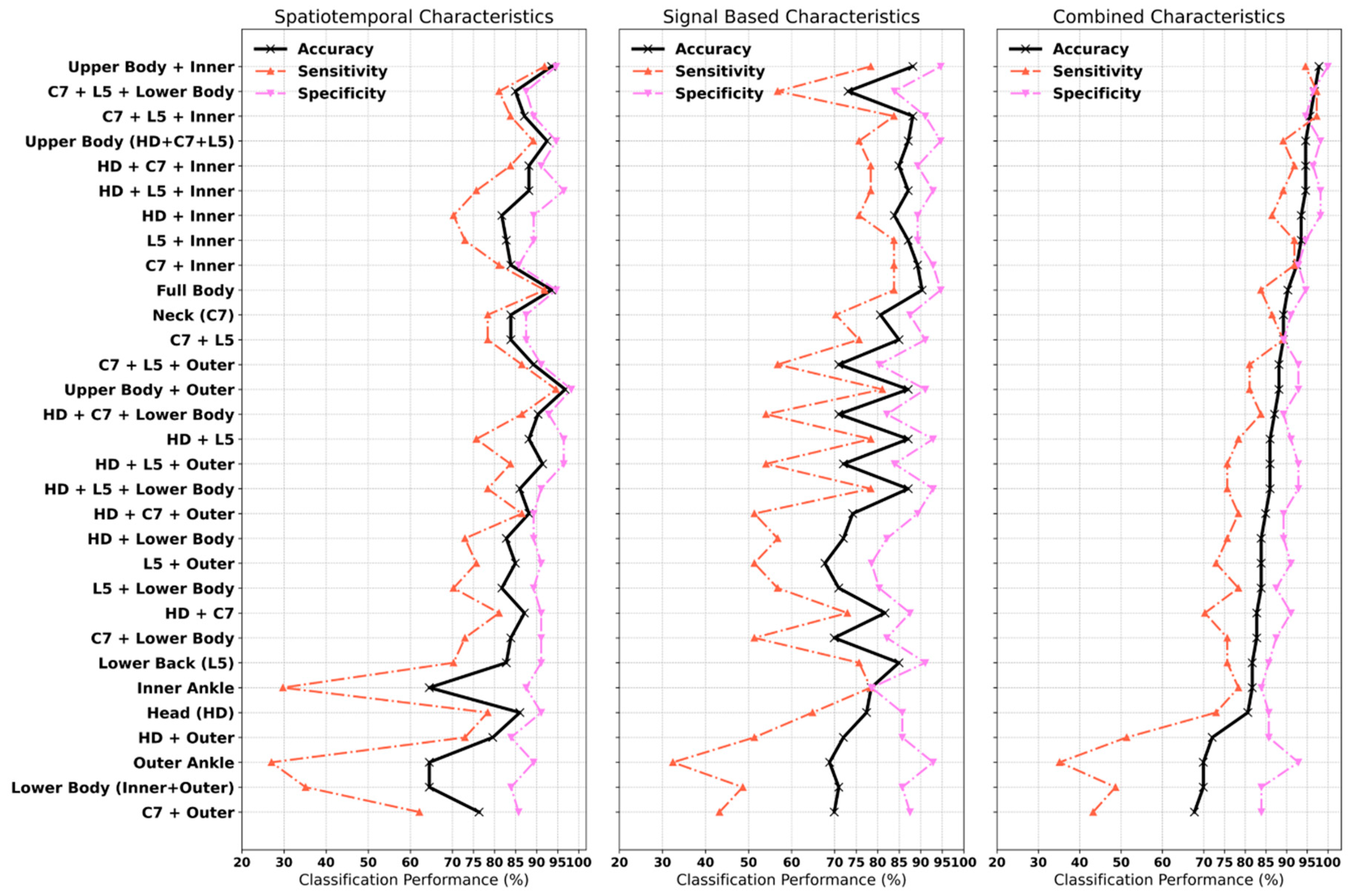
3.3. Classification of PD (Aim 3)
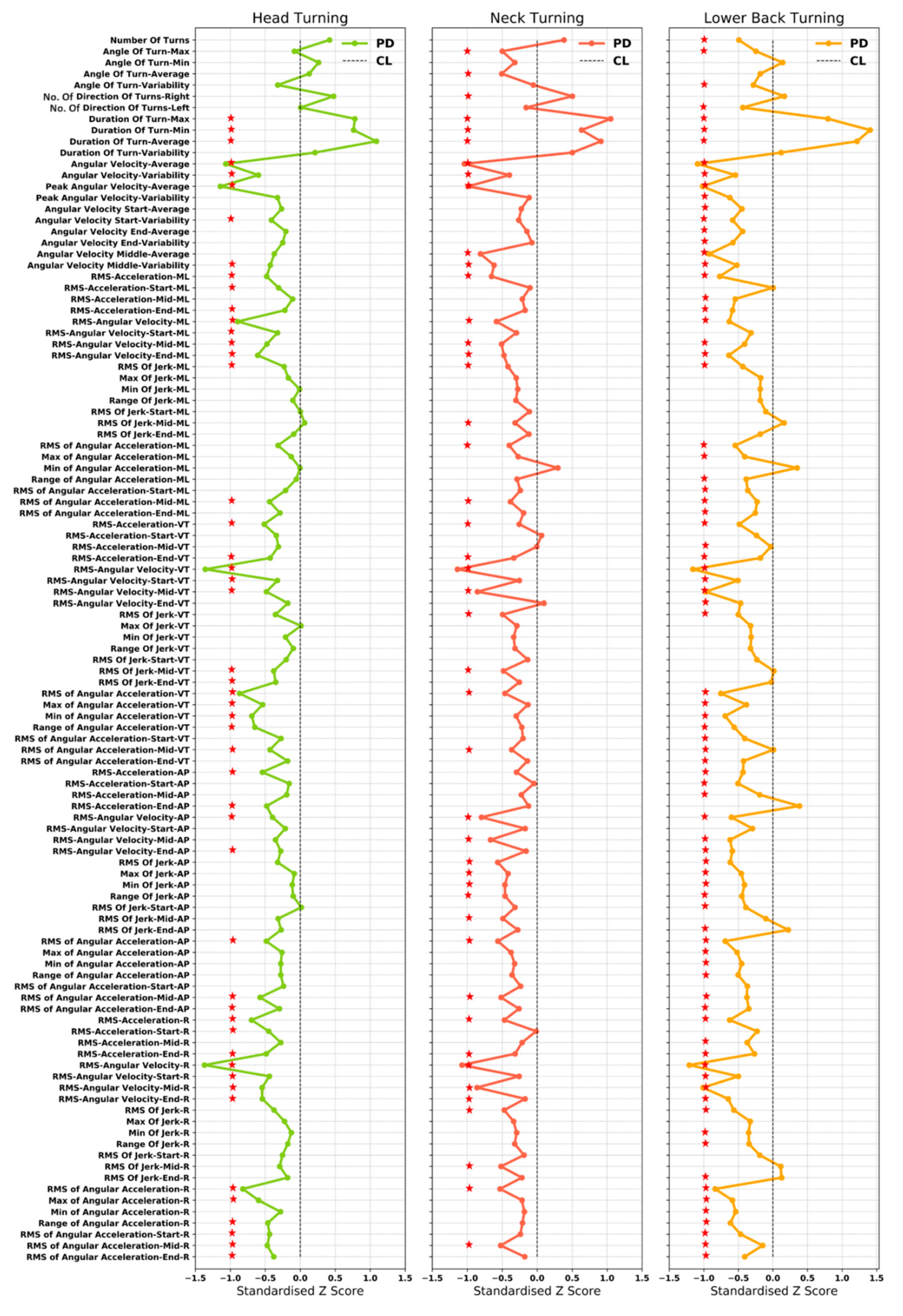
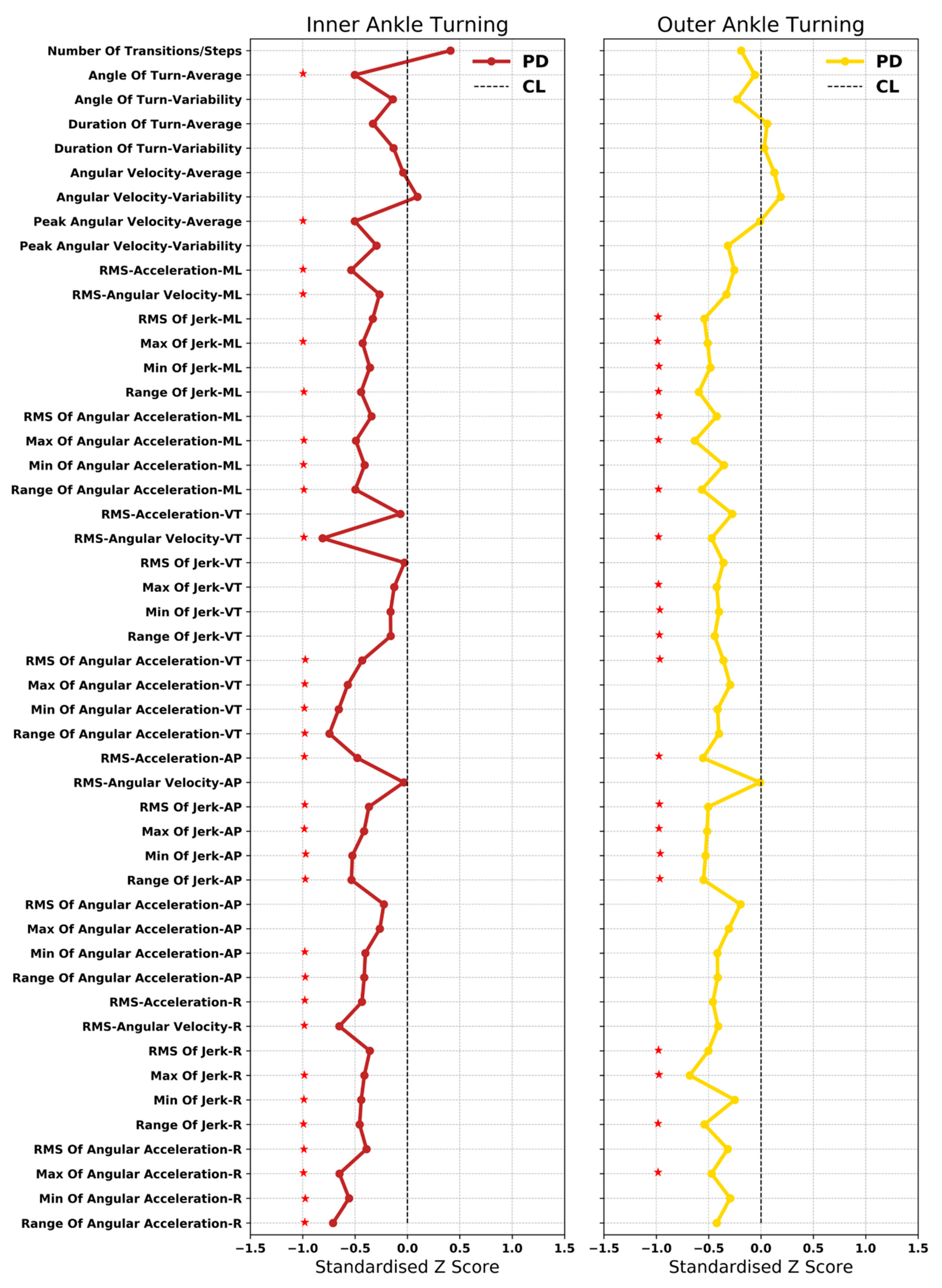
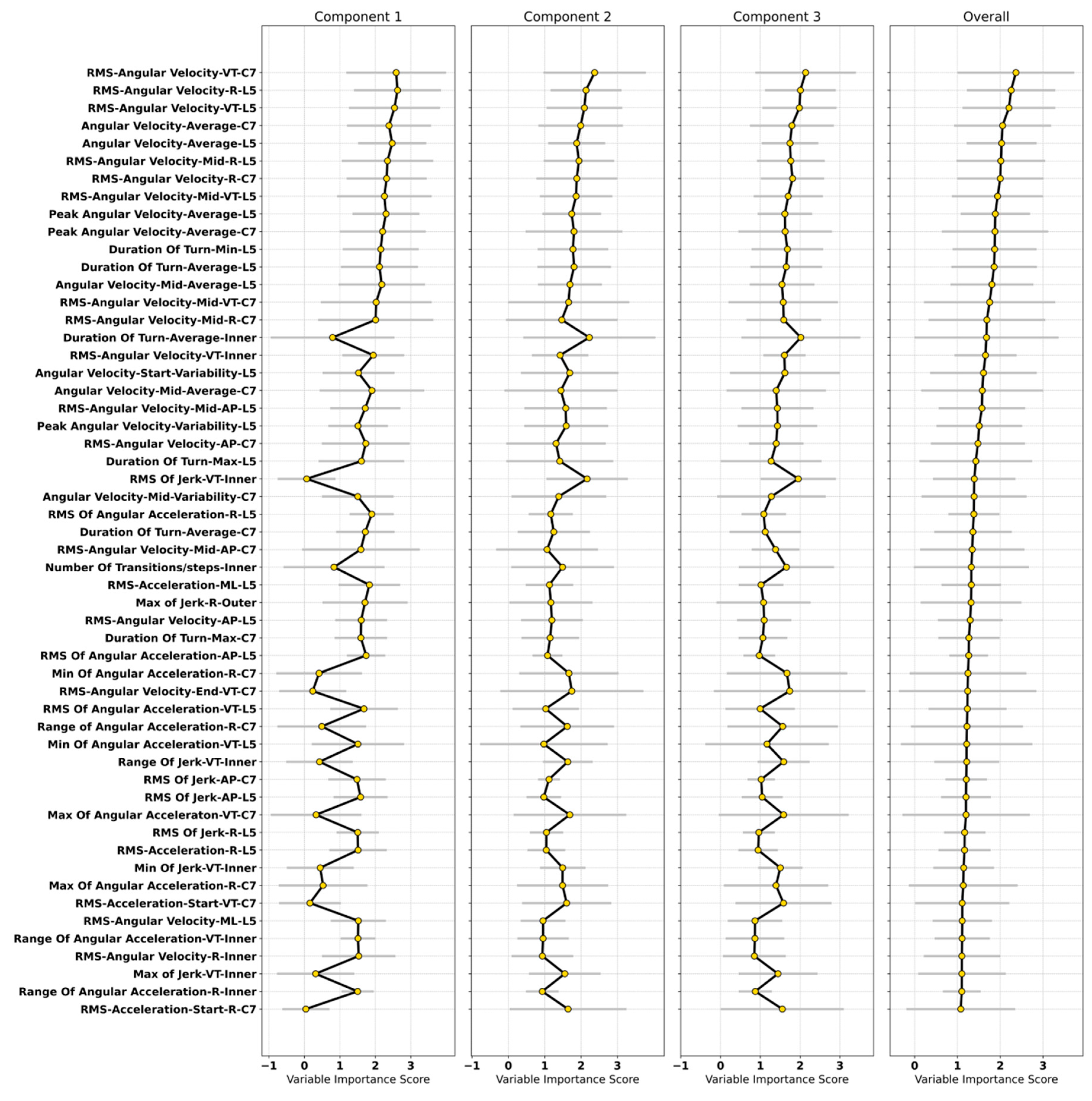
3.4. Important Characteristics in the Classification Model
4. Discussion
4.1. Turning Algorithm Validation
4.2. Contribution of Turning in Classification of PD
4.2.1. Classification of PD
4.2.2. Important Turning Characteristics
4.3. Study Strengths, Limitations, and Directions for Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sensor Attachment | Spatiotemporal | Signal-Based | Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Specificity | Sensitivity | Accuracy | Specificity | Sensitivity | Accuracy | Specificity | Sensitivity | Accuracy | |
| Head (HD) | 91.07 | 78.38 | 86.02 | 85.71 | 64.86 | 77.42 | 85.71 | 72.97 | 80.65 |
| Neck (C7) | 87.50 | 78.38 | 83.87 | 87.50 | 70.27 | 80.65 | 91.07 | 86.49 | 89.25 |
| Lower Back (L5) | 91.07 | 70.27 | 82.80 | 91.07 | 75.68 | 84.95 | 85.71 | 75.68 | 81.72 |
| HD + C7 | 91.07 | 81.08 | 87.10 | 87.50 | 72.97 | 81.72 | 91.07 | 70.27 | 82.80 |
| HD + L5 | 96.43 | 75.68 | 88.17 | 92.86 | 78.38 | 87.10 | 91.07 | 78.38 | 86.02 |
| C7 + L5 | 87.50 | 78.38 | 83.87 | 91.07 | 75.68 | 84.95 | 89.29 | 89.19 | 89.25 |
| Upper Body | 94.64 | 89.19 | 92.47 | 94.64 | 75.68 | 87.10 | 98.21 | 89.19 | 94.62 |
| Inner Ankle | 87.50 | 29.73 | 64.52 | 78.57 | 78.38 | 78.49 | 83.93 | 78.38 | 81.72 |
| Outer Ankle | 89.29 | 27.03 | 64.52 | 92.86 | 32.43 | 68.82 | 92.86 | 35.14 | 69.89 |
| Lower Body | 83.93 | 35.14 | 64.52 | 85.71 | 48.65 | 70.97 | 83.93 | 48.65 | 69.89 |
| HD + Inner | 89.29 | 70.27 | 81.72 | 89.29 | 75.68 | 83.87 | 98.21 | 86.49 | 93.55 |
| HD + Outer | 83.93 | 72.97 | 79.57 | 85.71 | 51.35 | 72.04 | 85.71 | 51.35 | 72.04 |
| HD + Lower Body | 89.29 | 72.97 | 82.80 | 82.14 | 56.76 | 72.04 | 89.29 | 75.68 | 83.87 |
| C7 + Inner Ankle | 85.71 | 81.08 | 83.87 | 92.86 | 83.78 | 89.25 | 92.86 | 91.89 | 92.47 |
| C7 + Outer Ankle | 85.71 | 62.16 | 76.34 | 87.50 | 43.24 | 69.89 | 83.93 | 43.24 | 67.74 |
| C7 + Lower Body | 91.07 | 72.97 | 83.87 | 82.14 | 51.35 | 69.89 | 87.50 | 75.68 | 82.80 |
| L5 + Inner Ankle | 89.29 | 72.97 | 82.80 | 89.29 | 83.78 | 87.10 | 94.64 | 91.89 | 93.55 |
| L5 + Outer Ankle | 91.07 | 75.68 | 84.95 | 78.57 | 51.35 | 67.74 | 91.07 | 72.97 | 83.87 |
| L5 + Lower Body | 89.29 | 70.27 | 81.72 | 80.36 | 56.76 | 70.97 | 87.50 | 78.38 | 83.87 |
| HD + C7 + Inner Ankle | 91.07 | 83.78 | 88.17 | 89.29 | 78.38 | 84.95 | 96.43 | 91.89 | 94.62 |
| HD + C7 + Outer Ankle | 89.29 | 86.49 | 88.17 | 89.29 | 51.35 | 74.19 | 89.29 | 78.38 | 84.95 |
| HD + C7 + Lower Body | 92.86 | 86.49 | 90.32 | 82.14 | 54.05 | 70.97 | 89.29 | 83.78 | 87.10 |
| HD + L5 + Inner Ankle | 96.43 | 75.68 | 88.17 | 92.86 | 78.38 | 87.10 | 98.21 | 89.19 | 94.62 |
| HD + L5 + Outer Ankle | 96.43 | 83.78 | 91.40 | 83.93 | 54.05 | 72.04 | 92.86 | 75.68 | 86.02 |
| HD + L5 + Lower Body | 91.07 | 78.38 | 86.02 | 92.86 | 78.38 | 87.10 | 92.86 | 75.68 | 86.02 |
| C7 + L5 + Inner Ankle | 89.29 | 83.78 | 87.10 | 91.07 | 83.78 | 88.17 | 94.64 | 97.30 | 95.70 |
| C7 + L5 + Outer Ankle | 91.07 | 86.49 | 89.25 | 80.36 | 56.76 | 70.97 | 92.86 | 81.08 | 88.17 |
| C7 + L5 + Lower Body | 87.50 | 81.08 | 84.95 | 83.93 | 56.76 | 73.12 | 96.43 | 97.30 | 96.77 |
| Upper Body + Inner Ankle | 94.64 | 91.89 | 93.55 | 94.64 | 78.38 | 88.17 | 100.00 | 94.59 | 97.85 |
| Upper Body + Outer Ankle | 98.21 | 94.59 | 96.77 | 91.07 | 81.08 | 87.10 | 92.86 | 81.08 | 88.17 |
| Full Body | 94.64 | 91.89 | 93.55 | 94.64 | 83.78 | 90.32 | 94.64 | 83.78 | 90.32 |
| Raters | Accuracy | Sensitivity | Specificity |
|---|---|---|---|
| Rater 1 | 77.38 | 71.43 | 81.63 |
| Rater 2 | 75.00 | 80.00 | 71.43 |

References
- Parkinson’s UK. The Incidence and Prevalence of Parkinson’s in the UK; Parkinson’s UK: London, UK, 2018; Available online: https://www.parkinsons.org.uk/professionals/resources/incidence-and-prevalence-parkinsons-uk-report (accessed on 18 September 2020).
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; Collado-Mateo, D. Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Poewe, W.; Mahlknecht, P. The Clinical Progression of Parkinson’s Disease. Parkinsonism Relat. Disord. 2009, 15, S28–S32. [Google Scholar] [CrossRef]
- El-Gohary, M.; Pearson, S.; McNames, J.; Mancini, M.; Horak, F.; Mellone, S.; Chiari, L. Continuous Monitoring of Turning in Patients with Movement Disability. Sensors 2014, 14, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.; Ashburn, A.; Robert, L.; Verheyden, G. A Narrative Review of Turning deficits in people with parkinson’s disease. Disabil. Rehabil. 2015, 37, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Spildooren, J.; Vercruysse, S.; Heremans, E.; Galna, B.; Vandenbossche, J.; Desloovere, K.; Vandenberghe, W.; Nieuwboer, A. Head-pelvis coupling is increased during turning in patients with parkinson’s disease and freezing of gait. Mov. Disord. 2013, 28, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Mellone, S.; Mancini, M.; King, L.A.; Horak, F.B.; Chiari, L. The quality of turning in parkinson’s disease: A compensatory strategy to prevent postural instability? J. Neuroeng. Rehabil. 2016, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Amar, K.; Stack, E.; Fitton, C.; Ashburn, A.; Roberts, H. Fall frequency, predicting falls and participating in falls research: Similarities among people with parkinson’s disease with and without cognitive impairment. Parkinsonism Relat. Disord. 2015, 21, 55–60. [Google Scholar] [CrossRef]
- Bertoli, M.; Della Croce, U.; Cereatti, A.; Mancini, M. Objective measures to investigate turning impairments and freezing of gait in people with parkinson’s disease. Gait Posture 2019, 74, 187–193. [Google Scholar] [CrossRef]
- Bloem, B.R.; Grimbergen, Y.A.; Cramer, M.; Willemsen, M.; Zwinderman, A.H. Prospective assessment of falls in parkinson’s disease. J. Neurol. 2001, 248, 950–958. [Google Scholar] [CrossRef]
- Haertner, L.; Elshehabi, M.; Zaunbrecher, L.; Pham, M.H.; Maetzler, C.; van Uem, J.M.; Hobert, M.A.; Hucker, S.; Nussbaum, S.; Berg, D. Effect of fear of falling on turning performance in parkinson’s disease in the lab and at home. Front. Aging Neurosci. 2018, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Schlueter, H.; El-Gohary, M.; Mattek, N.; Duncan, C.; Kaye, J.; Horak, F.B. Continuous monitoring of turning mobility and its association to falls and cognitive function: A pilot study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Mancini, M.; Priest, K.; Salarian, A.; Rodrigues-de-Paula, F.; Horak, F. Do clinical scales of balance reflect turning abnormalities in people with parkinson’s disease? J. Neurol. Phys. Ther. 2012, 36, 25. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Alcock, L.; McArdle, R.; Rehman, R.Z.U.; Del Din, S.; Mazzà, C.; Yarnall, A.J.; Rochester, L. The role of movement analysis in diagnosing and monitoring neurodegenerative conditions: Insights from gait and postural control. Brain Sci. 2019, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Ornelas-Vences, C.; Sanchez-Fernandez, L.P.; Sanchez-Perez, L.A.; Garza-Rodriguez, A.; Villegas-Bastida, A. Fuzzy inference model evaluating turn for parkinson’s disease patients. Comput. Biol. Med. 2017, 89, 379–388. [Google Scholar] [CrossRef]
- Ashburn, A.; Stack, E.; Ballinger, C.; Fazakarley, L.; Fitton, C. The circumstances of falls among people with parkinson’s disease and the use of falls diaries to facilitate reporting. Disabil. Rehabil. 2008, 30, 1205–1212. [Google Scholar] [CrossRef]
- Cheng, F.-Y.; Yang, Y.-R.; Wang, C.-J.; Wu, Y.-R.; Cheng, S.-J.; Wang, H.-C.; Wang, R.-Y. Factors influencing turning and its relationship with falls in individuals with parkinson’s disease. PLoS ONE 2014, 9, e93572. [Google Scholar] [CrossRef]
- Mancini, M.; El-Gohary, M.; Pearson, S.; McNames, J.; Schlueter, H.; Nutt, J.G.; King, L.A.; Horak, F.B. Continuous monitoring of turning in parkinson’s disease: Rehabilitation potential. NeuroRehabilitation 2015, 37, 3–10. [Google Scholar] [CrossRef]
- Haji Ghassemi, N.; Hannink, J.; Roth, N.; Gaßner, H.; Marxreiter, F.; Klucken, J.; Eskofier, B.M. Turning analysis during standardized test using on-shoe wearable sensors in parkinson’s disease. Sensors 2019, 19, 3103. [Google Scholar] [CrossRef]
- Mariani, B.; Jiménez, M.C.; Vingerhoets, F.J.; Aminian, K. On-shoe wearable sensors for gait and turning assessment of patients with parkinson’s disease. IEEE Trans. Biomed. Eng. 2012, 60, 155–158. [Google Scholar] [CrossRef]
- Mariani, B.; Hoskovec, C.; Rochat, S.; Büla, C.; Penders, J.; Aminian, K. 3d gait assessment in young and elderly subjects using foot-worn inertial sensors. J. Biomech. 2010, 43, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.H.; Elshehabi, M.; Haertner, L.; Heger, T.; Hobert, M.A.; Faber, G.S.; Salkovic, D.; Ferreira, J.J.; Berg, D.; Sanchez-Ferro, Á. Algorithm for turning detection and analysis validated under home-like conditions in patients with parkinson’s disease and older adults using a 6 degree-of-freedom inertial measurement unit at the lower back. Front. Neurol. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Ayachi, F.; Lavigne–Pelletier, C.; Blamoutier, M.; Rahimi, F.; Boissy, P.; Jog, M.; Duval, C. Auto detection and segmentation of physical activities during a timed-up-and-go (tug) task in healthy older adults using multiple inertial sensors. J. Neuroeng. Rehabil. 2015, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.; Goršič, M.; Podobnik, J.; Munih, M. Toward real-time automated detection of turns during gait using wearable inertial measurement units. Sensors 2014, 14, 18800–18822. [Google Scholar] [CrossRef] [PubMed]
- Fleury, A.; Noury, N.; Vuillerme, N. A fast algorithm to track changes of direction of a person using magnetometers. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007. [Google Scholar]
- Shah, V.V.; McNames, J.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Lapidus, J.A.; Horak, F.B.; Curtze, C. Digital biomarkers of mobility in parkinson’s disease during daily living. J. Parkinson’s Dis. 2020, 10, 1099–1111. [Google Scholar]
- Hasegawa, N.; Shah, V.V.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B.; Mancini, M. How to select balance measures sensitive to parkinson’s disease from body-worn inertial sensors—Separating the trees from the forest. Sensors 2019, 19, 3320. [Google Scholar] [CrossRef]
- Hulbert, S.; Ashburn, A.; Roberts, L.; Verheyden, G. Dance for parkinson’s—The effects on whole body co-ordination during turning around. Complement. Ther. Med. 2017, 32, 91–97. [Google Scholar] [CrossRef]
- Rehman, R.Z.U.; Buckley, C.; Micó-Amigo, M.E.; Kirk, C.; Dunne-Willows, M.; Mazzà, C.; Shi, J.Q.; Alcock, L.; Rochester, L.; Del Din, S. Accelerometry-based digital gait characteristics for classification of parkinson’s disease: What counts? IEEE Open J. Eng. Med. Biol. 2020, 1, 65–73. [Google Scholar] [CrossRef]
- Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Coleman, S.; O’Brien, J.T.; Brooks, D.J.; Barker, R.A.; Burn, D.J. The spectrum of nonmotor symptoms in early parkinson disease. Neurology 2013, 80, 276–281. [Google Scholar] [CrossRef]
- Hughes, A.J.; Ben-Shlomo, Y.; Daniel, S.E.; Lees, A.J. What features improve the accuracy of clinical diagnosis in parkinson’s disease: A clinicopathologic study. Neurology 1992, 42, 1142. [Google Scholar] [CrossRef]
- Association, W.M. World medical association declaration of helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.E.; Myers, A.M. The activities-specific balance confidence (abc) scale. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, M28–M34. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1998, 50, 318. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (mds-updrs): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Collerton, D.; Taylor, J.-P.; Burn, D.J. Cognitive decline and quality of life in incident parkinson’s disease: The role of attention. Parkinsonism Relat. Disord. 2016, 27, 47–53. [Google Scholar] [CrossRef]
- Nieuwboer, A.; Rochester, L.; Herman, T.; Vandenberghe, W.; Emil, G.E.; Thomaes, T.; Giladi, N. Reliability of the new freezing of gait questionnaire: Agreement between patients with parkinson’s disease and their carers. Gait Posture 2009, 30, 459–463. [Google Scholar] [CrossRef]
- Galna, B.; Lord, S.; Rochester, L. Is gait variability reliable in older adults and parkinson’s disease? Towards an optimal testing protocol. Gait Posture 2013, 37, 580–585. [Google Scholar] [CrossRef]
- Jaiswal, R.; Nair, R.C.; Yarlagadda, N.K.; Senapati, A.A.K.; Mulage, P. Adaptive gyroscope drift compensation based on temporal noise modelling. In Proceedings of the 2016 International Conference on Microelectronics, Computing and Communications (MicroCom), Durgapur, India, 23–25 January 2016. [Google Scholar]
- Fordellone, M.; Bellincontro, A.; Mencarelli, F. Partial least squares discriminant analysis: A dimensionality reduction method to classify hyperspectral data. arXiv 2018, arXiv:1806.09347. [Google Scholar]
- Maitra, S.; Yan, J. Principle component analysis and partial least squares: Two dimension reduction techniques for regression. Appl. Multivar. Stat. Models 2008, 79, 79–90. [Google Scholar]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikström, C.; Wold, S. Multi-and Megavariate Data Analysis Part 1: Basic Principles and Applications; Umetrics: Umeå, Sweden, 2006; pp. 1–103. [Google Scholar]
- Pérez-Enciso, M.; Tenenhaus, M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (pls-da) approach. Hum. Genet. 2003, 112, 581–592. [Google Scholar]
- Hartmann, A.; Luzi, S.; Murer, K.; de Bie, R.A.; de Bruin, E.D. Concurrent validity of a trunk tri-axial accelerometer system for gait analysis in older adults. Gait Posture 2009, 29, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2009; Volume 892. [Google Scholar]
- Rehman, R.Z.U.; Del Din, S.; Shi, J.Q.; Galna, B.; Lord, S.; Yarnall, A.J.; Guan, Y.; Rochester, L. Comparison of walking protocols and gait assessment systems for machine learning-based classification of parkinson’s disease. Sensors 2019, 19, 5363. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.Z.U.; Del Din, S.; Guan, Y.; Yarnall, A.J.; Shi, J.Q.; Rochester, L. Selecting clinically relevant gait characteristics for classification of early parkinson’s disease: A comprehensive machine learning approach. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Akram, S.; Frank, J.S.; Jog, M. Parkinson’s disease and segmental coordination during turning: I. Standing turns. Can. J. Neurol. Sci. 2013, 40, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Patla, A.E.; Adkin, A.; Ballard, T. Online steering: Coordination and control of body center of mass, head and body reorientation. Exp. Brain Res. 1999, 129, 629–634. [Google Scholar] [CrossRef]
- Huxham, F.; Baker, R.; Morris, M.E.; Iansek, R. Footstep adjustments used to turn during walking in parkinson’s disease. Mov. Disord. 2008, 23, 817–823. [Google Scholar] [CrossRef]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Calabrese, E.; Mazzoleni, P.; Nemni, R.; Ferrarin, M. The association between impaired turning and normal straight walking in parkinson’s disease. Gait Posture 2007, 26, 172–178. [Google Scholar] [CrossRef]
- Bhatt, H.; Pieruccini-Faria, F.; Almeida, Q.J. Dynamics of turning sharpness influences freezing of gait in parkinson’s disease. Parkinsonism Relat. Disord. 2013, 19, 181–185. [Google Scholar] [CrossRef]
- Mak, M.K.; Patla, A.; Hui-Chan, C. Sudden turn during walking is impaired in people with parkinson’s disease. Exp. Brain Res. 2008, 190, 43–51. [Google Scholar] [CrossRef]
- Stack, E.; Ashburn, A. Dysfunctional turning in parkinson’s disease. Disabil. Rehabil. 2008, 30, 1222–1229. [Google Scholar] [CrossRef]
- Stack, E.; Ashburn, A.; Rassoulian, H. Turning difficulties associated with parkinson’s disease (pd). Mov. Disord. 2002, 17, S115. [Google Scholar]

| Demographics | CL (n = 56) | PD (n = 37) | p |
|---|---|---|---|
| Sex (Male/Female) | 32/24 | 26/11 | 0.338 |
| Age (years) | 71.0 ± 7.1 | 70.1 ± 9.3 | 0.610 |
| Height (m) | 1.71 ± 0.08 | 1.68 ± 0.08 | 0.130 |
| Mass (kg) | 80.0 ± 12.9 | 77.2 ± 17.3 | 0.388 |
| BMI (kg/m2) | 27 ± 5 | 27 ± 6 | 0.799 |
| MMSE (0–30) | 29 ± 2 | 28 ± 2 | 0.137 |
| ABCs (0–100)% | 92 ± 11 | 77 ± 20 | <0.001 |
| Gait Speed (m/s) | 1.12 ± 0.49 | 1.09 ± 0.36 | 0.724 |
| LEDD, mg/day | 534 ± 278 | ||
| Number of Freezers | 7 | ||
| Disease Duration (years) | 3.1 ± 0.2 | ||
| Hoehn and Yahr Stage (n) | HY II: 31 | ||
| HY III: 6 | |||
| MDS-UPDRS III | 41.1 ± 12.0 | ||
| HY II: (39.6 ± 12.1) | |||
| HY III: (48.7 ± 8.5) |
| Rater 1 vs. Rater 2 | ||||||||
| Turn | Control | Parkinson’s Disease | ||||||
| RMSE | rho | ICC(2,1) | LOA | RMSE | rho | ICC(2,1) | LOA | |
| Start (s) | 0.33 | 0.99 | 0.99 | 0.66 (4.6%) | 0.42 | 0.98 | 0.99 | 0.83 (4.8%) |
| End (s) | 0.44 | 0.99 | 0.99 | 0.87 (3.8%) | 0.35 | 0.99 | 0.99 | 0.70 (3%) |
| Rater 1 vs. Algorithm | ||||||||
| Turn | Control | Parkinson’s Disease | ||||||
| RMSE | rho | ICC(2,1) | LOA | RMSE | rho | ICC(2,1) | LOA | |
| Start (s) | 0.50 | 0.99 | 0.99 | 0.97 (8.7%) | 0.48 | 0.99 | 0.99 | 0.93 (5%) |
| End (s) | 0.60 | 0.99 | 0.99 | 1.2 (6.7%) | 0.59 | 0.99 | 0.99 | 1.2 (5.7%) |
| Rater 2 vs. Algorithm | ||||||||
| Turn | Control | Parkinson’s Disease | ||||||
| RMSE | rho | ICC(2,1) | LOA | RMSE | rho | ICC(2,1) | LOA | |
| Start (s) | 0.44 | 0.99 | 0.99 | 0.86 (8.8%) | 0.39 | 0.99 | 0.99 | 0.77 (5.4%) |
| End (s) | 0.60 | 0.99 | 0.99 | 1.2 (6.3%) | 0.54 | 0.99 | 0.99 | 1.1 (6%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, R.Z.U.; Klocke, P.; Hryniv, S.; Galna, B.; Rochester, L.; Del Din, S.; Alcock, L. Turning Detection During Gait: Algorithm Validation and Influence of Sensor Location and Turning Characteristics in the Classification of Parkinson’s Disease. Sensors 2020, 20, 5377. https://doi.org/10.3390/s20185377
Rehman RZU, Klocke P, Hryniv S, Galna B, Rochester L, Del Din S, Alcock L. Turning Detection During Gait: Algorithm Validation and Influence of Sensor Location and Turning Characteristics in the Classification of Parkinson’s Disease. Sensors. 2020; 20(18):5377. https://doi.org/10.3390/s20185377
Chicago/Turabian StyleRehman, Rana Zia Ur, Philipp Klocke, Sofia Hryniv, Brook Galna, Lynn Rochester, Silvia Del Din, and Lisa Alcock. 2020. "Turning Detection During Gait: Algorithm Validation and Influence of Sensor Location and Turning Characteristics in the Classification of Parkinson’s Disease" Sensors 20, no. 18: 5377. https://doi.org/10.3390/s20185377
APA StyleRehman, R. Z. U., Klocke, P., Hryniv, S., Galna, B., Rochester, L., Del Din, S., & Alcock, L. (2020). Turning Detection During Gait: Algorithm Validation and Influence of Sensor Location and Turning Characteristics in the Classification of Parkinson’s Disease. Sensors, 20(18), 5377. https://doi.org/10.3390/s20185377









