Assessment of Human Visual Acuity Using Visual Evoked Potential: A Review
Abstract
:1. Introduction
2. Overview of VEP Visual Acuity Technique
3. Effects of Visual Stimuli
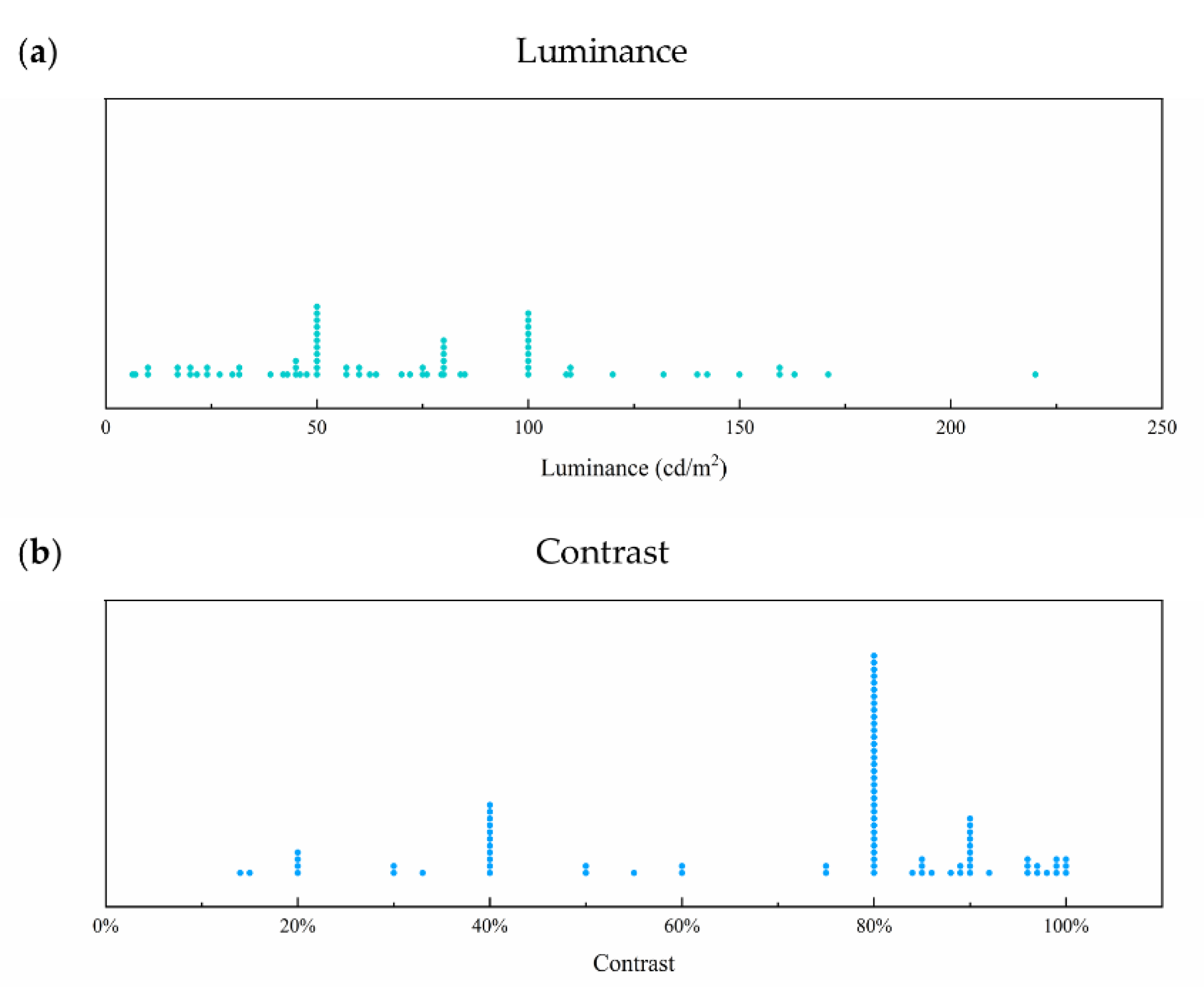
3.1. Luminance
3.2. Contrast
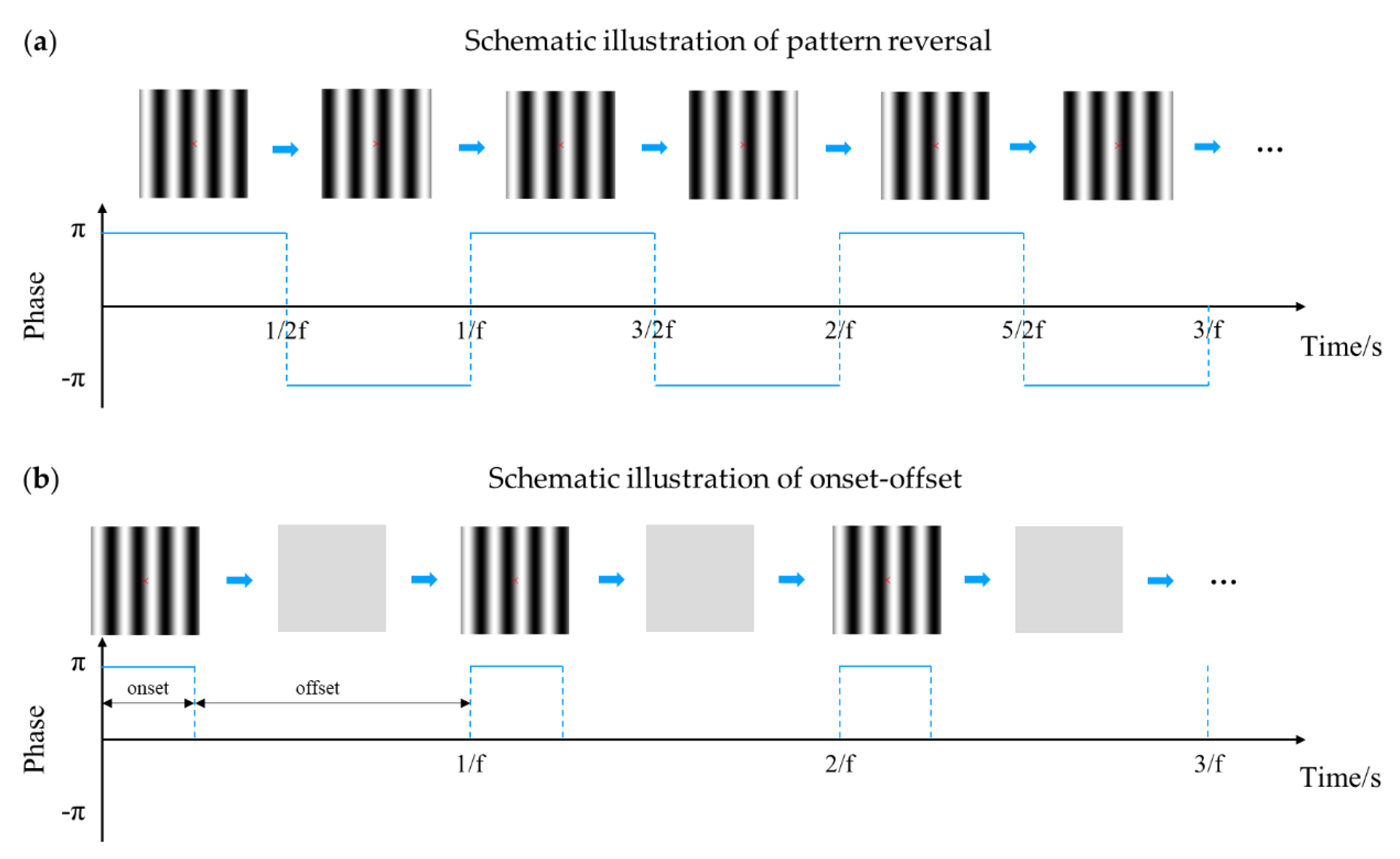
3.3. Stimulus Pattern
3.4. Stimulus Orientation of Grating Pattern
3.5. Stimulus Mode
3.6. Stimulus Field
3.7. Temporal Frequency
3.8. Sweep Parameters
3.8.1. Sweep Mode
3.8.2. Sweep Direction
3.8.3. Sweep Type
3.8.4. Sweep Duration
3.8.5. Sweep Range
4. Effects of Signal Acquisition and Analysis
4.1. VEP Acuity Assessment System
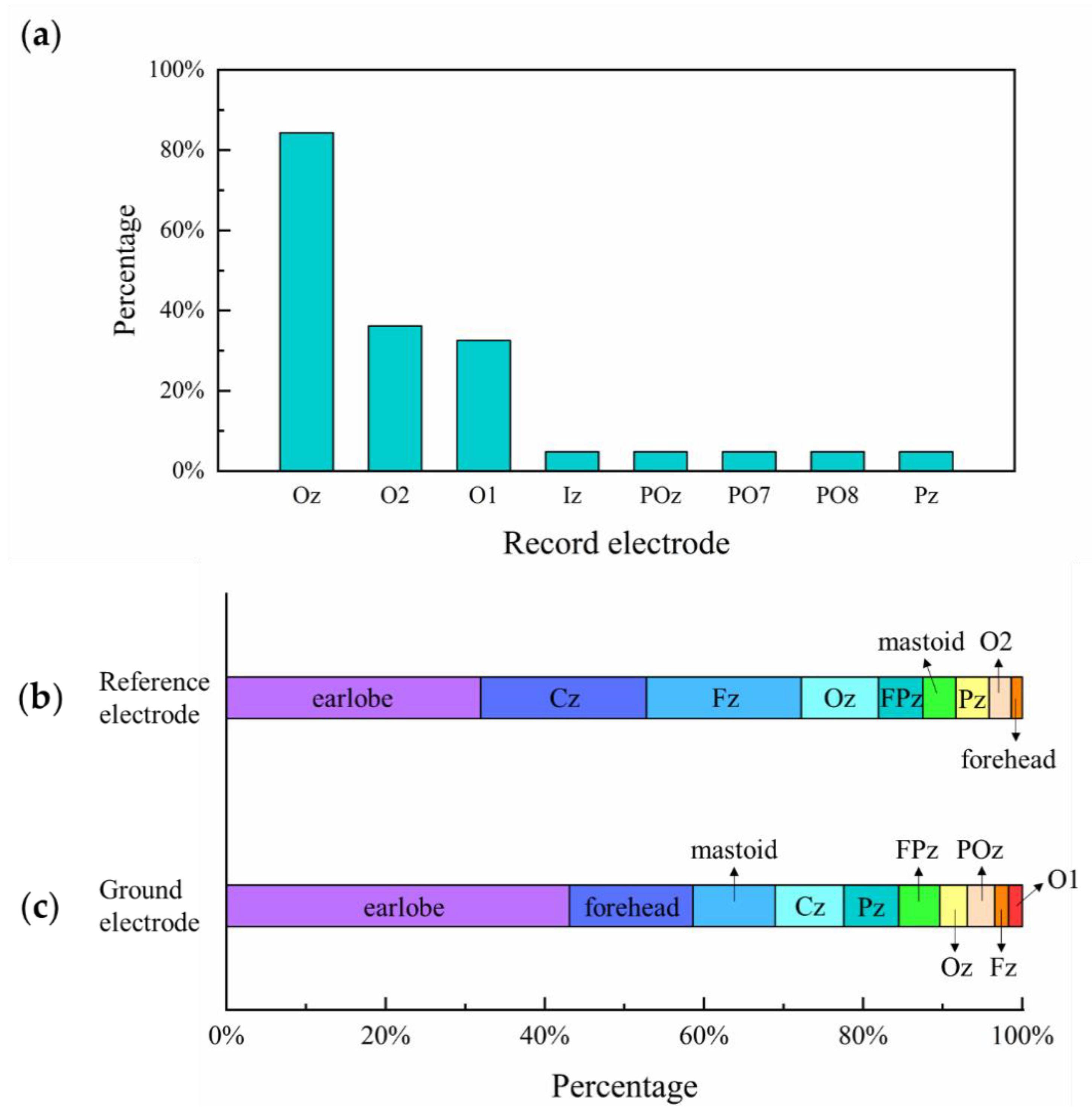
4.2. Electrode Placement
4.3. Threshold Determination Method
| Threshold Determination Method | Description | Studies |
|---|---|---|
| Linear extrapolation | Linear extrapolation from last VEP amplitude peak to 0 µV baseline versus linear spatial frequency | [13,14,17,19,20,36,52,59,62,63,65,66,73,74,75,77,79,80,81,82,85,86,88,90,91,92,93,94,95,96,97,98,99,100,101,102,103,106,108,109,110,117,121,122,123,124,125,126,127,128,129,130] |
| Improved linear extrapolation | Linear extrapolation from last VEP amplitude peak to 0 µV baseline against log visual–angle/log spatial frequency | [18,61] |
| Linear extrapolation from last VEP amplitude peak to noise level baseline against spatial frequency | [46,80,83,121,126,127,129] | |
| Smallest check size technique | Smallest check size that evokes a recognizable and repeatable VEP | [10,53,67,71,72,84,105,111,132] |
| Improved smallest check size technique | Three consecutively increasing spatial frequencies: detection, detection, no detection | [89] |
| Significant response among at least three of the four preceding steps | [87] | |
| Significance of VEP response combined with OR algorithm in Boolean algebra | [7] | |
| Stepwise heuristic algorithm | Optimal range for regression and value for SF0 or failure for all VEP recordings via a set of rules on VEP amplitude and noise estimate | [22,26,28,31,120] |
| Other methods | Extrapolation of curvilinear function of best-fitting quadratic equation to zero amplitude | [55] |
| Second-order polynomial function plotting peak amplitudes against spatial frequency | [18,137] | |
| Nonlinear regression of modified Ricker model fitting sweep VEP peak amplitudes and spatial frequency | [137] | |
| Machine learning approach with small dataset of 108 cases | [27] |
5. Clinical Application
5.1. Studies of the Visual Acuity Development by VEP
5.2. Clinical Studies of VEP Acuity Assessment
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Blindness and Vision Impairment. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 9 May 2020).
- Fricke, T.R.; Tahhan, N.; Resnikoff, S.; Papas, E.; Burnett, A.; Ho, S.M.; Naduvilath, T.; Naidoo, K.S. Global Prevalence of Presbyopia and Vision Impairment from Uncorrected Presbyopia: Systematic Review, Meta-analysis, and Modelling. Ophthalmology 2018, 125, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [Green Version]
- Bailey, I.L.; Lovie, J.E. New Design Principles for Visual-Acuity Letter Charts. Am. J. Optom. Phys. Opt. 1976, 53, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Cedrone, C.; Cerulli, L. Standardized measurement of visual acuity. Ophthalmic Epidemiol. 2009, 5, 41–53. [Google Scholar] [CrossRef]
- Bach, M. The Freiburg Visual Acuity test--automatic measurement of visual acuity. Optom. Vis. Sci. 1996, 73, 49–53. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, G.; Wang, Y.; Han, C.; Du, C.; Yan, W.; Zhang, S.; Liang, R. Objective and quantitative assessment of visual acuity and contrast sensitivity based on steady-state motion visual evoked potentials using concentric-ring paradigm. Doc. Ophthalmol. 2019, 139, 123–136. [Google Scholar] [CrossRef]
- Incesu, A.I.; Sobaci, G. Malingering or simulation in ophthalmology -visual acuity. Int. J. Ophthalmol. 2011, 4, 558–566. [Google Scholar] [CrossRef]
- Regan, D. Rapid Objective Refraction Using Evoked Brain Potentials. Investig. Ophthalmol. 1973, 12, 669–679. [Google Scholar]
- Towle, V.L.; Harter, M.R. Objective Determination of Human Visual-Acuity—Pattern Evoked-Potentials. Investig. Ophthalmol. Vis. Sci. 1977, 16, 1073–1076. [Google Scholar]
- Towle, V.L.; Harter, M.R. Objective determination of human visual acuity from the visual evoked potential. Percept. Psychophys 1979, 25, 497–500. [Google Scholar] [CrossRef]
- Petersen, J. Objective determination of visual acuity by visual evoked potentials. Optimized procedure and clinical value. Dev. Ophthalmol. 1984, 9, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Norcia, A.M.; Tyler, C.W. Infant Vep Acuity Measurements - Analysis of Individual-Differences and Measurement Error. Electroencephalogr. Clin. Neurophysiol. 1985, 61, 359–369. [Google Scholar] [CrossRef]
- Norcia, A.M.; Tyler, C.W. Spatial frequency sweep VEP: Visual acuity during the first year of life. Vis. Res. 1985, 25, 1399–1408. [Google Scholar] [CrossRef]
- Almoqbel, F.; Leat, S.J.; Irving, E. The technique, validity and clinical use of the sweep VEP. Ophthalmic Physiol. Opt. 2008, 28, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Norcia, A.M.; Appelbaum, L.G.; Ales, J.M.; Cottereau, B.R.; Rossion, B. The steady-state visual evoked potential in vision research: A review. J. Vis. 2015, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Almoqbel, F.M.; Yadav, N.K.; Leat, S.J.; Head, L.M.; Irving, E.L. Effects of sweep VEP parameters on visual acuity and contrast thresholds in children and adults. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, A.; Langrova, H.; Messias, A.; Zrenner, E.; Jagle, H. A comparison of the performance of three visual evoked potential-based methods to estimate visual acuity. Doc. Ophthalmol. 2013, 126, 45–56. [Google Scholar] [CrossRef]
- Allen, D.; Bennett, P.J.; Banks, M.S. The Effects of Luminance on Fpl and Vep Acuity in Human Infants. Vis. Res. 1992, 32, 2005–2012. [Google Scholar] [CrossRef]
- Good, W.V.; Hou, C. Sweep visual evoked potential grating acuity thresholds paradoxically improve in low-luminance conditions in children with cortical visual impairment. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3220–3224. [Google Scholar] [CrossRef] [Green Version]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P.; International Society for Clinical Electrophysiology of Vision. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Bach, M.; Maurer, J.P.; Wolf, M.E. Visual evoked potential-based acuity assessment in normal vision, artificially degraded vision, and in patients. Br. J. Ophthalmol. 2008, 92, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, T.S.; Bach, M. Contrast adaptation in human retina and cortex. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2721–2727. [Google Scholar]
- Bach, M.; Meigen, T.; Strasburger, H. Raster-scan cathode-ray tubes for vision research--limits of resolution in space, time and intensity, and some solutions. Spat. Vis. 1997, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Cheng, F.C.; Chiu, Y.S. Efficient Contrast Enhancement Using Adaptive Gamma Correction with Weighting Distribution. IEEE Trans. Image Process. 2013, 22, 1032–1041. [Google Scholar] [CrossRef]
- Bach, M.; Farmer, J.D. Evaluation of the “Freiburg Acuity VEP” on Commercial Equipment. Doc. Ophthalmol. 2020, 140, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Bach, M.; Heinrich, S.P. Acuity VEP: Improved with machine learning. Doc. Ophthalmol. 2019, 139, 113–122. [Google Scholar] [CrossRef]
- Knotzele, J.; Heinrich, S.P. Can VEP-based acuity estimates in one eye be improved by applying knowledge from the other eye? Doc. Ophthalmol. 2019, 139, 161–168. [Google Scholar] [CrossRef]
- Strasburger, H.; Remky, A.; Murray, I.J.; Hadjizenonos, C.; Rentschler, I. Objective measurement of contrast sensitivity and visual acuity with the steady-state visual evoked potential. Ger. J. Ophthalmol. 1996, 5, 42–52. [Google Scholar]
- Hoffmann, M.B.; Brands, J.; Behrens-Baumann, W.; Bach, M. VEP-based acuity assessment in low vision. Doc. Ophthalmol. 2017, 135, 209–218. [Google Scholar] [CrossRef]
- Wenner, Y.; Heinrich, S.P.; Beisse, C.; Fuchs, A.; Bach, M. Visual evoked potential-based acuity assessment: Overestimation in amblyopia. Doc. Ophthalmol. 2014, 128, 191–200. [Google Scholar] [CrossRef]
- Odom, J.V.; Hobson, R.; Coldren, J.T.; Chao, G.M.; Weinstein, G.W. 10-Hz Flash Visual Evoked-Potentials Predict Post-Cataract Extraction Visual-Acuity. Doc. Ophthalmol. 1987, 66, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Vadrevu, V.L.; Cavender, S.; Odom, J.V. Use of 10-Hz Flash Visual Evoked-Potentials in Prediction of Final Visual-Acuity in Diabetic Eyes with Vitreous Hemorrhage. Doc. Ophthalmol. 1992, 79, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Sobaci, G.; Mutlu, F.M.; Soyler, M.; Tatar, T.; Yildirim, E. Using flash visually evoked potential in predicting final visual acuity in severely injured eyes. Ann. Ophthalmol. 2000, 32, 63–65. [Google Scholar] [CrossRef]
- Lenassi, E.; Likar, K.; Stirn-Kranjc, B.; Brecelj, J. VEP maturation and visual acuity in infants and preschool children. Doc. Ophthalmol. 2008, 117, 111–120. [Google Scholar] [CrossRef]
- Simon, F.; Rassow, B. Retinal Visual-Acuity with Pattern Vep Normal Subjects and Reproducibility. Graefes Arch. Clin. Exp. Ophthalmol. 1986, 224, 160–164. [Google Scholar] [CrossRef]
- Han, C.; Xu, G.; Xie, J.; Chen, C.; Zhang, S. Highly Interactive Brain-Computer Interface Based on Flicker-Free Steady-State Motion Visual Evoked Potential. Sci. Rep. 2018, 8, 5835. [Google Scholar] [CrossRef]
- Yan, W.Q.; Xu, G.H.; Xie, J.; Li, M.; Dan, Z.Y. Four Novel Motion Paradigms Based on Steady-State Motion Visual Evoked Potential. IEEE Trans. Biomed. Eng. 2018, 65, 1696–1704. [Google Scholar] [CrossRef]
- Xie, J.; Xu, G.H.; Wang, J.; Li, M.; Han, C.C.; Jia, Y.G. Effects of Mental Load and Fatigue on Steady-State Evoked Potential Based Brain Computer Interface Tasks: A Comparison of Periodic Flickering and Motion-Reversal Based Visual Attention. PLoS ONE 2016, 11, e0163426. [Google Scholar] [CrossRef]
- Tobimatsu, S.; Kurita-Tashima, S.; Nakayama-Hiromatsu, M.; Kato, M. Effect of spatial frequency on transient and steady-state VEPs: Stimulation with checkerboard, square-wave grating and sinusoidal grating patterns. J. Neurol. Sci. 1993, 118, 17–24. [Google Scholar] [CrossRef]
- Jia, F.Q.; Liang, Y.G.; Zhang, X.Y.; Luo, F.L.; Xiong, Y.H.; Cheng, L.L.; Liu, J.H. Characteristics of Pattern Visual Evoked Potential in Two Eyes with Varying Visual Acuity in One Eye and Forensic Application. Curr. Med. Sci. 2018, 38, 342–348. [Google Scholar] [CrossRef]
- Appelle, S. Perception and discrimination as a function of stimulus orientation: The “oblique effect” in man and animals. Psychol. Bull. 1972, 78, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Reisbeck, T.E.; Gegenfurtner, K.R. Effects of contrast and temporal frequency on orientation discrimination for luminance and isoluminant stimuli. Vis. Res. 1998, 38, 1105–1117. [Google Scholar] [CrossRef] [Green Version]
- Camisa, J.M.; Blake, R.; Lema, S. The Effects of Temporal Modulation on the Oblique Effect in Humans. Perception 2016, 6, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Maffei, L.; Campbell, F.W. Neurophysiological localization of the vertical and horizontal visual coordinates in man. Science 1970, 167, 386–387. [Google Scholar] [CrossRef]
- Nelson, J.I.; Kupersmith, M.J.; Seiple, W.H.; Weiss, P.A.; Carr, R.E. Spatiotemporal Conditions Which Elicit or Abolish the Oblique Effect in Man - Direct Measurement with Swept Evoked-Potential. Vis. Res. 1984, 24, 579–586. [Google Scholar] [CrossRef]
- Moskowitz, A.; Sokol, S. Effect of Stimulus Orientation on the Latency and Amplitude of the Vep. Investig. Ophthalmol. Vis. Sci. 1985, 26, 246–248. [Google Scholar]
- Arakawa, K.; Tobimatsu, S.; Kurita-Tashima, S.; Nakayama, M.; Kira, J.I.; Kato, M. Effects of stimulus orientation on spatial frequency function of the visual evoked potential. Exp. Brain Res. 2000, 131, 121–125. [Google Scholar] [CrossRef]
- Cobb, W.A.; Morton, H.B.; Ettlinge, G. Cerebral Potentials Evoked by Pattern Reversal and Their Suppression in Visual Rivalry. Nature 1967, 216, 1123–1125. [Google Scholar] [CrossRef]
- Millodot, M.; Riggs, L.A. Refraction determined electrophysiologically. Responses to alternation of visual contours. Arch. Ophthalmol. 1970, 84, 272–278. [Google Scholar] [CrossRef]
- Spekreijse, H.; Vandertw, L.; Zuidema, T. Contrast Evoked-Responses in Man. Vis. Res. 1973, 13, 1577–1601. [Google Scholar] [CrossRef]
- Cavascan, N.N.; Salomao, S.R.; Sacai, P.Y.; Pereira, J.M.; Rocha, D.M.; Berezovsky, A. Contributing factors to VEP grating acuity deficit and inter-ocular acuity difference in children with cerebral visual impairment. Doc. Ophthalmol. 2014, 128, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Raniel, Y.; Pratt, H.; Neumann, E.; Schacham, S.E. Miniature fiber-optic pattern-reversal stimulator for determination of the visual evoked potential threshold; comparison with Snellen acuity. Graefes Arch. Clin. Exp. Ophthalmol. 1989, 227, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Gottlob, I.; Wizov, S.S.; Odom, J.V.; Reinecke, R.D. Predicting Optotype Visual-Acuity by Swept Spatial Visual-Evoked Potentials. Clin. Vis. Sci. 1993, 8, 417–423. [Google Scholar]
- Jenkins, T.C.A.; Douthwaite, W.A.; Peedle, J.E. The Ver as a Predictor of Normal Visual-Acuity in the Adult Human-Eye. Ophthalmic Physiol. Opt. 1985, 5, 441–449. [Google Scholar] [CrossRef]
- Regan, D. Assessment of visual acuity by evoked potential recording: Ambiguity caused by temporal dependence of spatial frequency selectivity. Vis. Res. 1978, 18, 439–443. [Google Scholar] [CrossRef]
- Spinelli, D.; Pirchio, M.; Sandini, G. Visual-Acuity in the Young Infant Is Highest in a Small Retinal Area. Vis. Res. 1983, 23, 1133–1136. [Google Scholar] [CrossRef]
- Katsumi, O.; Hirose, T.; Tsukada, T. Effect of Number of Elements and Size of Stimulus Field on Recordability of Pattern Reversal Visual Evoked-Response. Investig. Ophthalmol. Vis. Sci. 1988, 29, 922–927. [Google Scholar]
- Tyler, C.W.; Apkarian, P.; Levi, D.M.; Nakayama, K. Rapid assessment of visual function: An electronic sweep technique for the pattern visual evoked potential. Investig. Ophthalmol. Vis. Sci. 1979, 18, 703–713. [Google Scholar]
- Mitsuyu, M.; Zimmer, E.M. Bangerter occlusives versus spherical convex lenses in the evaluation of visial acuity by visually evoked cortical potentials. Dev. Ophthalmol. 1984, 9, 115–122. [Google Scholar]
- Zhou, P.; Zhao, M.W.; Li, X.X.; Hu, X.F.; Wu, X.; Niu, L.J.; Yu, W.Z.; Xu, X.L. A new method of extrapolating the sweep pattern visual evoked potential acuity. Doc. Ophthalmol. 2008, 117, 85–91. [Google Scholar] [CrossRef]
- Katsumi, O.; Denno, S.; Arai, M.; De Lopes Faria, J.; Hirose, T. Comparison of preferential looking acuity and pattern reversal visual evoked response acuity in pediatric patients. Graefes Arch. Clin. Exp. Ophthalmol 1997, 235, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Katsumi, O.; Paranhos, F.R.; Lopes De Faria, J.M.; Hirose, T. Comparison of Snellen acuity and objective assessment using the spatial frequency sweep PVER. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Tobimatsu, S. Transient and steady-state VEPs—reappraisal. Int. Congr. Ser. 2002, 1232, 207–211. [Google Scholar] [CrossRef]
- Norcia, A.M.; Tyler, C.W.; Hamer, R.D. Development of Contrast Sensitivity in the Human Infant. Vis. Res. 1990, 30, 1475–1486. [Google Scholar] [CrossRef]
- Skoczenski, A.M.; Norcia, A.M. Development of VEP Vernier acuity and grating acuity in human infants. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2411–2417. [Google Scholar]
- Westall, C.A.; Ainsworth, J.R.; Buncic, J.R. Which ocular and neurologic conditions cause disparate results in visual acuity scores recorded with visually evoked potential and Teller Acuity Cards? J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2000, 4, 295–301. [Google Scholar] [CrossRef]
- Reed, J.L.; Marx, M.S.; May, J.G. Spatial frequency tuning in the visual evoked potential elicited by sine-wave gratings. Vis. Res. 1984, 24, 1057–1062. [Google Scholar] [CrossRef]
- Cysewska-Sobusiak, A.; Hulewicz, A.; Grzybowski, A. Application of visual evoked potentials to objective visual acuity assessment. In Proceedings of the 4th IET International Conference on Advances in Medical, Signal and Information Processing—MEDSIP 2008, Santa Margherita Ligure, Italy, 14–16 July 2008; pp. 1–4. [Google Scholar]
- Bobak, P.; Khanna, P.; Goodwin, J.; Brigell, M. Pattern visual evoked potentials in cases of ambiguous acuity loss. Doc. Ophthalmol. 1993, 85, 185–192. [Google Scholar] [CrossRef]
- Gundogan, F.C.; Mutlu, F.M.; Altinsoy, H.I.; Tas, A.; Oz, O.; Sobaci, G. Pattern visual evoked potentials in the assessment of objective visual acuity in amblyopic children. Int. Ophthalmol. 2010, 30, 377–383. [Google Scholar] [CrossRef]
- Gundogan, F.C.; Sobaci, G.; Bayer, A. Pattern visual evoked potentials in the assessment of visual acuity in malingering. Ophthalmology 2007, 114, 2332–2337. [Google Scholar] [CrossRef]
- Sokol, S.; Moskowitz, A.; McCormack, G.; Augliere, R. Infant grating acuity is temporally tuned. Vis. Res. 1988, 28, 1357–1366. [Google Scholar] [CrossRef]
- Sokol, S.; Moskowitz, A.; Mccormack, G. Infant Vep and Preferential Looking Acuity Measured with Phase Alternating Gratings. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3156–3161. [Google Scholar]
- Gottlob, I.; Fendick, M.G.; Guo, S.; Zubcov, A.A.; Odom, J.V.; Reinecke, R.D. Visual acuity measurements by swept spatial frequency visual-evoked-cortical potentials (VECPs): Clinical application in children with various visual disorders. J. Pediatr. Ophthalmol. Strabismus 1990, 27, 40–47. [Google Scholar] [PubMed]
- Seiple, W.; Holopigian, K. An Examination of Vep Response Phase. Electroencephalogr. Clin. Neurophysiol. 1989, 73, 520–531. [Google Scholar] [CrossRef]
- Vesely, P. Contribution of sVEP visual acuity testing in comparison with subjective visual acuity. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2015, 159, 616–621. [Google Scholar] [CrossRef]
- Tyler, C.W.; Apkarian, P.; Nakayama, K. Multiple Spatial-Frequency Tuning of Electrical Responses from Human Visual-Cortex. Exp. Brain Res. 1978, 33, 535–550. [Google Scholar] [CrossRef]
- Watson, T.; Orel-Bixler, D.; Haegerstrom-Portnoy, G. VEP vernier, VEP grating, and behavioral grating acuity in patients with cortical visual impairment. Optom. Vis. Sci. 2009, 86, 774–780. [Google Scholar] [CrossRef]
- Ridder, W.H., 3rd. Methods of visual acuity determination with the spatial frequency sweep visual evoked potential. Doc. Ophthalmol. 2004, 109, 239–247. [Google Scholar] [CrossRef]
- Lauritzen, L.; Jorgensen, M.H.; Michaelsen, K.F. Test-retest reliability of swept visual evoked potential measurements of infant visual acuity and contrast sensitivity. Pediatr. Res. 2004, 55, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Lopes de Faria, J.; Katsumi, O.; Cagliero, E.; Nathan, D.; Hirose, T. Neurovisual abnormalities preceding the retinopathy in patients with long-term type 1 diabetes mellitus. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 643–648. [Google Scholar] [CrossRef]
- Ridder, W.H., 3rd. A comparison of contrast sensitivity and sweep visual evoked potential (sVEP) acuity estimates in normal humans. Doc. Ophthalmol. 2019, 139, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Keyser, M.D.; Vissenberg, I.; Neetens, A. Are visually evoked potentials (VEP) useful for determination of visual acuity? A clinical trial. Neuro-Ophthalmology 2009, 10, 153–163. [Google Scholar] [CrossRef]
- Ridder, W.H.; Tong, A.; Floresca, T. Reliability of acuities determined with the sweep visual evoked potential (sVEP). Doc. Ophthalmol. 2012, 124, 99–107. [Google Scholar] [CrossRef]
- da Costa, M.F.; Salomão, S.R.; Berezovsky, A.; de Haro, F.M.; Ventura, D.F. Relationship between vision and motor impairment in children with spastic cerebral palsy: New evidence from electrophysiology. Behav. Brain Res. 2004, 149, 145–150. [Google Scholar] [CrossRef]
- Hemptinne, C.; Liu-Shuang, J.; Yuksel, D.; Rossion, B. Rapid Objective Assessment of Contrast Sensitivity and Visual Acuity With Sweep Visual Evoked Potentials and an Extended Electrode Array. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1144–1157. [Google Scholar] [CrossRef] [Green Version]
- John, F.M.; Bromham, N.R.; Woodhouse, J.M.; Candy, T.R. Spatial vision deficits in infants and children with Down syndrome. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1566–1572. [Google Scholar] [CrossRef]
- Mackay, A.M.; Bradnam, M.S.; Hamilton, R.; Elliot, A.T.; Dutton, G.N. Real-time rapid acuity assessment using VEPs: Development and validation of the step VEP technique. Investig. Ophthalmol. Vis. Sci. 2008, 49, 438–441. [Google Scholar] [CrossRef]
- Good, W.V. Development of a quantitative method to measure vision in children with chronic cortical visual impairment. Trans. Am. Ophthalmol. Soc. 2001, 99, 253–269. [Google Scholar]
- Suttle, C.M.; Banks, M.S.; Candy, T.R. Does a front-end nonlinearity confound VEP acuity measures in human infants? Vis. Res. 2000, 40, 3665–3675. [Google Scholar] [CrossRef] [Green Version]
- Watson, T.; Orel-Bixler, D.; Haegerstrom-Portnoy, G. Early Visual-Evoked Potential Acuity and Future Behavioral Acuity in Cortical Visual Impairment. Optom. Vis. Sci. 2010, 87, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Prager, T.C.; Zou, Y.L.; Jensen, C.L.; Fraley, J.K.; Anderson, R.E.; Heird, W.C. Evaluation of methods for assessing visual function of infants. J. Aapos. 1999, 3, 275–282. [Google Scholar] [CrossRef]
- Ridder, W.H.; Rouse, M.W. Predicting potential acuities in amblyopes - Predicting post-therapy acuity in amblyopes. Doc. Ophthalmol. 2007, 114, 135–145. [Google Scholar] [CrossRef]
- Ridder, W.H.; McCulloch, D.; Herbert, A.M. Stimulus duration, neural adaptation, and sweep visual evoked potential acuity estimates. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2759–2768. [Google Scholar]
- Iyer, K.K.; Bradley, A.P.; Wilson, S.J. Conducting shorter VEP tests to estimate visual acuity via assessment of SNR. Doc. Ophthalmol. 2013, 126, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ridder, W.H., 3rd; Waite, B.S.; Melton, T.F. Comparing enfant and PowerDiva sweep visual evoked potential (sVEP) acuity estimates. Doc. Ophthalmol. 2014, 129, 105–114. [Google Scholar] [CrossRef]
- Kharauzov, A.K.; Pronin, S.V.; Sobolev, A.F.; Koskin, S.A.; Boiko, É.V.; Shelepin, Y.E. Objective measurement of human visual acuity by visual evoked potentials. Neurosci. Behav. Phys. 2006, 36, 1021–1030. [Google Scholar] [CrossRef]
- Salomao, S.R.; Ejzenbaum, F.; Berezovsky, A.; Sacai, P.Y.; Pereira, J.M. Age norms for monocular grating acuity measured by sweep-VEP in the first three years of age. Arq. Bras. Oftalmol. 2008, 71, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Sokol, S. Measurement of Infant Visual-Acuity from Pattern Reversal Evoked-Potentials. Vis. Res. 1978, 18, 33–39. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Gibson, R.A. Perinatal characteristics may influence the outcome of visual acuity. Lipids 2001, 36, 897–900. [Google Scholar] [CrossRef]
- Steele, M.; Seiple, W.H.; Carr, R.E.; Klug, R. The clinical utility of visual-evoked potential acuity testing. Am. J. Ophthalmol. 1989, 108, 572–577. [Google Scholar] [CrossRef]
- Thompson, D.A.; Moller, H.; Russell-Eggitt, I.; Kriss, A. Visual acuity in unilateral cataract. Br. J. Ophthalmol. 1996, 80, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Li, Q.Q.; Liu, X.Q.; Yang, L.; Xia, W.T.; Tao, L.Y. Visual acuity evaluated by pattern-reversal visual-evoked potential is affected by check size/visual angle. Neurosci. Bull. 2012, 28, 737–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, R.; Bradnam, M.S.; Dutton, G.N.; Yan, A.L.L.C.; Lavy, T.E.; Livingstone, I.; Mackay, A.M.; Mackinnon, J.R. Sensitivity and specificity of the step VEP in suspected functional visual acuity loss. Doc. Ophthalmol. 2013, 126, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sokol, S.; Hansen, V.C.; Moskowitz, A.; Greenfield, P.; Towle, V.L. Evoked potential and preferential looking estimates of visual acuity in pediatric patients. Ophthalmology 1983, 90, 552–562. [Google Scholar] [CrossRef]
- McBain, V.A.; Robson, A.G.; Hogg, C.R.; Holder, G.E. Assessment of patients with suspected non-organic visual loss using pattern appearance visual evoked potentials. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 502–510. [Google Scholar] [CrossRef]
- Katsumi, O.; Arai, M.; Wajima, R.; Denno, S.; Hirose, T. Spatial frequency sweep pattern reversal VER acuity vs. Snellen visual acuity: Effect of optical defocus. Vis. Res. 1996, 36, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Almoqbel, F.M.; Irving, E.L.; Leat, S.J. Visual Acuity and Contrast Sensitivity Development in Children: Sweep Visually Evoked Potential and Psychophysics. Optom. Vis. Sci. 2017, 94, 830–837. [Google Scholar] [CrossRef]
- Ghasia, F.; Brunstom, J.; Tychsen, L. Visual acuity and visually evoked responses in children with cerebral palsy: Gross Motor Function Classification Scale. Br. J. Ophthalmol. 2009, 93, 1068–1072. [Google Scholar] [CrossRef]
- Alves Pereira, S.; Costa, M.F. Visual acuity evaluation in children with hydrocephalus: An electrophysiological study with sweep visual evoked potential. World J. Neurosci. 2012, 2, 36–43. [Google Scholar] [CrossRef]
- Jeon, J.; Oh, S.; Kyung, S. Assessment of visual disability using visual evoked potentials. BMC Ophthalmol. 2012, 12, 36. [Google Scholar] [CrossRef] [Green Version]
- Bach, M. Freiburg Evoked Potentials (EP2000). Available online: https://michaelbach.de/sci/stim/ep2000/ (accessed on 8 May 2020).
- American Clinical Neurophysiology Society. Guideline 5: Guidelines for standard electrode position nomenclature. Am. J. Electroneurodiagnostic. Technol. 2006, 46, 222–225. [Google Scholar] [CrossRef]
- Wegener, V.; Jorysz, G.; Arnoldi, A.; Utzschneider, S.; Wegener, B.; Jansson, V.; Heimkes, B. Normal radiological unossified hip joint space and femoral head size development during growth in 675 children and adolescents. Clin. Anat. 2017, 30, 267–275. [Google Scholar] [CrossRef]
- Wright, C.M.; Inskip, H.M.; Godfrey, K.; Williams, A.F.; Ong, K.K. Monitoring head size and growth using the new UK-WHO growth standard. Arch. Dis. Child 2011, 96, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Good, W.V.; Norcia, A.M. Detection of Amblyopia Using Sweep VEP Vernier and Grating Acuity. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1435–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Xu, G.; Chen, L.; Zheng, X. Steady-State Motion Visual Evoked Potential (SSMVEP) Enhancement Method Based on Time-Frequency Image Fusion. Comput. Intell. Neurosci. 2019, 2019, 9439407. [Google Scholar] [CrossRef]
- McFarland, D.J.; McCane, L.M.; David, S.V.; Wolpaw, J.R. Spatial filter selection for EEG-based communication. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 386–394. [Google Scholar] [CrossRef]
- Heinrich, S.P.; Bock, C.M.; Bach, M. Imitating the effect of amblyopia on VEP-based acuity estimates. Doc. Ophthalmol. 2016, 133, 183–187. [Google Scholar] [CrossRef]
- Riddell, P.M.; Ladenheim, B.; Mast, J.; Catalano, T.; Nobile, R.; Hainline, L. Comparison of measures of visual acuity in infants: Teller acuity cards and sweep visual evoked potentials. Optom. Vis. Sci. 1997, 74, 702–707. [Google Scholar] [CrossRef]
- Wiener, D.E.; Wellish, K.; Nelson, J.I.; Kupersmith, M.J. Comparisons among Snellen, Psychophysical, and Evoked-Potential Visual-Acuity Determinations. Am. J. Optom. Phys. Opt. 1985, 62, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, Y.S.; France, T.D.; Verhoeve, J.; Gangnon, R.E. Sweep visual evoked potential testing as a predictor of recognition acuity in albinism. Arch. Ophthalmol. 2007, 125, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Hamer, R.D.; Norcia, A.M.; Tyler, C.W.; Hsu-Winges, C. The development of monocular and binocular VEP acuity. Vis. Res. 1989, 29, 397–408. [Google Scholar] [CrossRef]
- Chan, H.; Odom, J.V.; Coldren, J.; Dove, C.; Chao, G.M. Acuity Estimated by Visually Evoked-Potentials Is Affected by Scaling. Doc. Ophthalmol. 1986, 62, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Odom, J.V.; Hoyt, C.S.; Marg, E. Effect of Natural Deprivation and Unilateral Eye Patching on Visual-Acuity of Infants and Children—Evoked-Potential Measurements. Arch. Ophthalmol. 1981, 99, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Odom, J.V.; Hoyt, C.S.; Marg, E. Eye patching and visual evoked potential acuity in children four months to eight years old. Am. J. Optom. Physiol. Opt. 1982, 59, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Furuskog, P.; Wanger, P. Visual-Acuity Measurement Using Evoked-Potentials and Fast Fourier-Transform. Acta. Ophthalmol. 1986, 64, 352–355. [Google Scholar] [CrossRef]
- Odom, J.V.; Green, M. Visually evoked potential (VEP) acuity: Testability in a clinical pediatric population. Acta. Ophthalmol. 1984, 62, 993–998. [Google Scholar] [CrossRef]
- Tinelli, F.; Pei, F.; Guzzetta, A.; Bancale, A.; Mazzoth, S.; Baldassi, S.; Cioni, G. The assessment of visual acuity in children with periventricular damage: A comparison of behavioural and electrophysiological techniques. Vis. Res. 2008, 48, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Meigen, T.; Bach, M. On the statistical significance of electrophysiological steady-state responses. Doc. Ophthalmol. 1999, 98, 207–232. [Google Scholar] [CrossRef]
- Rao, G.X.; Wu, B.W.; Zhang, L.L. The exact estimation of visual acuity by VEP technology: A report of 726 cases of eye injury. J. Huazhong Univ. Sci. Technol. Med Sci. 2010, 30, 138–140. [Google Scholar] [CrossRef]
- Joost, W.; Bach, M. Variability of the steady-state visually evoked potential: Interindividual variance and intraindividual reproducibility of spatial frequency tuning. Doc. Ophthalmol. 1990, 75, 59–66. [Google Scholar] [CrossRef]
- Tyler, C.W.; Apkarian, P.A. Properties of localized pattern evoked potentials. Ann. N. Y. Acad. Sci. 1982, 388, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Strasburger, H. The Analysis of Steady-State Evoked-Potentials Revisited. Clin. Vision Sci. 1987, 1, 245–256. [Google Scholar]
- Parry, N.R.A.; Murray, I.J.; Hadjizenonos, C. Spatio-temporal tuning of VEPs: Effect of mode of stimulation. Vis. Res. 1999, 39, 3491–3497. [Google Scholar] [CrossRef]
- Strasser, T.; Nasser, F.; Langrova, H.; Zobor, D.; Lisowski, L.; Hillerkuss, D.; Sailer, C.; Kurtenbach, A.; Zrenner, E. Objective assessment of visual acuity: A refined model for analyzing the sweep VEP. Doc. Ophthalmol. 2019, 138, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Teller, D.Y.; Mcdonald, M.A.; Preston, K.; Sebris, S.L.; Dobson, V. Assessment of Visual-Acuity in Infants and Children - the Acuity Card Procedure. Dev. Med. Child Neurol. 1986, 28, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Sturm, V.; Cassel, D.; Eizenman, M. Objective estimation of visual acuity with preferential looking. Investig. Ophthalmol. Vis. Sci. 2011, 52, 708–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, M.C. Terminology and Classification of Cerebral Palsy. Dev. Med. Child Neurol. 1964, 6, 295–297. [Google Scholar] [CrossRef]
- Bax, M.; Goldstein, M.; Rosenbaum, P.; Leviton, A.; Paneth, N. Proposed definition and classification of cerebral palsy, April 2005—Introduction. Dev. Med. Child Neurol. 2005, 47, 571–576. [Google Scholar] [CrossRef]
- Regan, D. Speedy assessment of visual acuity in amblyopia by the evoked potential method. Ophthalmologica 1977, 175, 159–164. [Google Scholar] [CrossRef]
- Hanawa, T.; Fujimoto, N.; Miyauchi, O.; Adachi-Usami, E. Pattern visual evoked cortical potentials predict postoperative visual acuity after cataract surgery in patients with glaucoma. Ophthalmologica 2002, 216, 164–167. [Google Scholar] [CrossRef]
- Heinrich, S.P.; Kruger, K.; Bach, M. The effect of optotype presentation duration on acuity estimates revisited. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Bokariya, P.; Singh, S.; Singh, R. A Comprehensive Review on Methodologies Employed for Visual Evoked Potentials. Scientifica (Cairo) 2016, 2016, 9852194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, P.; Cheng, K.; Yao, D. Multivariate synchronization index for frequency recognition of SSVEP-based brain-computer interface. J. Neurosci. Methods 2014, 221, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Stawicki, P.; Gembler, F.; Rezeika, A.; Volosyak, I. A Novel Hybrid Mental Spelling Application Based on Eye Tracking and SSVEP-Based BCI. Brain. Sci. 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed]







| First Author | Year | Subjects’ Age Range | Results |
|---|---|---|---|
| Sokol [100] | 1978 | Infants: 2–6 months Adults | VEP acuity improved from 20/150 at 2 months to 20/20 by 6 months. |
| Norcia [13] | 1985 | Infants: 17–25 weeks | Temporal frequency of 6 or 10 Hz did not affect estimation of sVEP acuity. Sweep technique was also a robust method for measuring visual acuity. |
| Norcia [14] | 1985 | Infants: 1–53 weeks Adults | VEP acuity increased from 4.5 cpd in the first month to about 20 cpd at 8–13 months. By 8 months, VEP acuity reached adult level. |
| Sokol [73] | 1988 | Infants: 2–10 months Adults | Grating acuity was temporally tuned at 7.5 or 14 rps for infants at 3 months and older. Difference between VEP and PL acuity decreased from 2.0 octaves at 2 months to 0.5 octaves at 12 months. |
| Hamer [124] | 1989 | Infants: 2–52 weeks | Monocular and binocular acuity growth functions were nearly identical; monocular and binocular VEP acuity increased from 6 cpd at 2–10 weeks to 14 cpd by 20–30 weeks. |
| Norcia [65] | 1990 | Infants: 2–40 weeks Adults | SVEP estimated grating acuity showed gradual increase with age, ranging from 2.5–9 cpd in the first 2 months to about 10–20 cpd after 30 weeks. |
| Sokol [74] | 1992 | Infants: 2–13 months Children: 1–5 years Adults: 22–48 years | VEP and PL acuity developed at different rates, reaching a nearly equivalent level by 12 months. PL acuity in infants older than 2 years was found to be not temporally tuned. |
| Allen [19] | 1992 | Infants: 15–20 weeks Adults | FPL acuity improved slightly more with luminance than did VEP acuity. Acuity levels of VEP and FPL were comparable, with VEP slightly higher. |
| Riddell [121] | 1997 | Pre-term infants: 2–8 months Full-term infants: 3 weeks to 1 year | VEP acuity was generally higher than TAC acuity, but the rate of development was higher for TAC than VEP. TAC acuity reached VEP acuity at about 14 months. There was no difference between pre-term and full-term infants in VEP and TAC acuity. |
| Skoczenski [66] | 1999 | Infants: 8–80 weeks Adults | VEP Vernier and grating acuity developed at different rates, the former approaching adult levels earlier than the latter. Vernier acuity increased by a factor of 4.5 between 10 and 100 weeks; grating acuity improved by a factor of 2.3. |
| Prager [93] | 1999 | Infants: 4–8 months | Correlations among transient VEP, sVEP, and TAC acuity were poor, but expected changes in visual maturation from 4 to 8 months were detected with all methods. SVEP acuity increased from 9.61 cpd at 4 months to 10.39 cpd at 8 months. |
| Suttle [91] | 2000 | Infants: 6–17 weeks | Most infants did not exhibit clear VEP to whole-field flicker alone. Estimated VEP acuity was generally not confounded by front-end nonlinear distortion products. |
| Maria [101] | 2001 | Infants: 15.2–17.7 weeks | Perinatal characteristics including birth weight, gender, and number of smokers in the household needed to be considered for VEP acuity. |
| Lauritzen [81] | 2004 | Infants: 6–40 weeks | Mean rather than maximum threshold best estimated visual acuity. VEP method was well suited to describe visual development in infants, which increased by 0.64 octaves per doubling in age for acuity. |
| Salomão [99] | 2008 | Infants/children: 1–36 months | Age norms for grating acuity were determined using sweep VEP technique. Sweep VEP grating acuity ranged from 0.80 logMAR in the first month to 0.06 logMAR at 36 months. |
| Lenassi [35] | 2008 | Infants/children: 1.5 months to 7.5 years | VEP latency was strongly associated with visual acuity, recommending VEP latency as a reliable parameter for evaluating the integrity of the afferent visual pathway. |
| Almoqbel [109] | 2017 | Children: 6–7, 8–9, 10–12 years Adults | Results of various procedures (sweep VEP, psychophysical logMAR letter, and grating visual acuity) were in agreement. There were age-related changes in the visual acuity threshold after 6 years of age and visual acuity did not become adult-like until 8 to 9 years at the earliest. |
| Categorization | Detailed Disorder Types | Studies |
|---|---|---|
| Cortical visual impairment | Hypoxic injury, infection, hydrocephalus | [20,52,67,75,79,90,92,111] |
| Cerebral palsy | Tetraplegic, diplegic, hemiplegic, periventricular leukomalacia | [67,86,110,129,130] |
| Amblyopia | Refractive amblyopia, strabismic amblyopia, deprivation | [31,54,62,63,70,71,75,94,106,112,117,123,126,127,142] |
| Cataract | [22,32,53,62,75,102,103,106,126,137,143] | |
| Glaucoma | [53,62,63,70,75,102,143] | |
| Albinism | [54,75,80,123] | |
| Diabetes | Type 1 diabetes mellitus, background diabetic retinopathy, diabetes with vitreous hemorrhage | [33,80,82,102] |
| Down syndrome | [88] | |
| Functional visual loss | [102,105,107,112] | |
| Nystagmus | Congenital nystagmus, infantile nystagmus, spasmus nutans | [53,54,67,75,106,123] |
| Macular diseases | Macular gliosis, macular holes, macular degeneration, age-related macular degeneration, cystoid macular edema, maculopathy, neurosensory macular detachment, macular abnormality, foveal hypoplasia, retinal pigment epithelium macular detachment, Stargardt’s disease, central serous retinopathy | [22,53,63,67,80,102,123,137] |
| Retinal diseases | Retinitis pigmentosa, retinal reattachment, congenital retinoschisis, cone dysfunction syndrome, congenital retinoschisis, retina coloboma, retinopathy of prematurity, diabetic retinopathy, retinal myelin, rod/cone dystrophy, lattice degeneration, peripheral retinal holes, juvenile X-linked retinoschisis, retinal detachment, retinal perforation, epiretinal gliosis, chorioretinitis | [12,22,54,62,63,67,80,102,106,132,137] |
| Optic nerve disorders | Optic neuritis, optic atrophy, optic nerve hypoplasia, optic glioma, Leber’s atrophy, toxic optic neuropathy, cortical blindness, 3rd nerve palsies | [54,63,70,75,84,102,106,112,129,137] |
| Structural anomalies | High myopia, persistent hyperplastic primary vitreous, vitreous hemorrhage, refractive error, ptosis, iris and choroid coloboma, persistent hyperplastic primary vitreous, retrolental fibroplasia, vitreous hemorrhage, sub-hyaloid hemorrhage, vitreous opacity, aphakia, microphthalmia, corneal clouding | [22,53,54,62,63,75,102,106,123,126,137] |
| Eye trauma | Severe eye trauma with opaque media, eyelid contusion, hyphema, traumatic lens lesion, vitreous hematocele, retinal lesion, optic nerve contusion | [34,70,79,80,132] |
| Delayed visual maturation | [35,75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Xu, G.; Zhang, K.; Liang, R.; Yan, W.; Tian, P.; Jia, Y.; Zhang, S.; Du, C. Assessment of Human Visual Acuity Using Visual Evoked Potential: A Review. Sensors 2020, 20, 5542. https://doi.org/10.3390/s20195542
Zheng X, Xu G, Zhang K, Liang R, Yan W, Tian P, Jia Y, Zhang S, Du C. Assessment of Human Visual Acuity Using Visual Evoked Potential: A Review. Sensors. 2020; 20(19):5542. https://doi.org/10.3390/s20195542
Chicago/Turabian StyleZheng, Xiaowei, Guanghua Xu, Kai Zhang, Renghao Liang, Wenqiang Yan, Peiyuan Tian, Yaguang Jia, Sicong Zhang, and Chenghang Du. 2020. "Assessment of Human Visual Acuity Using Visual Evoked Potential: A Review" Sensors 20, no. 19: 5542. https://doi.org/10.3390/s20195542
APA StyleZheng, X., Xu, G., Zhang, K., Liang, R., Yan, W., Tian, P., Jia, Y., Zhang, S., & Du, C. (2020). Assessment of Human Visual Acuity Using Visual Evoked Potential: A Review. Sensors, 20(19), 5542. https://doi.org/10.3390/s20195542





