Performance Validation of a Planar Hall Resistance Biosensor through Beta-Amyloid Biomarker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PHR Sensors
2.3. Characterization of Dynamic Range and Sensitivity of PHR Sensors
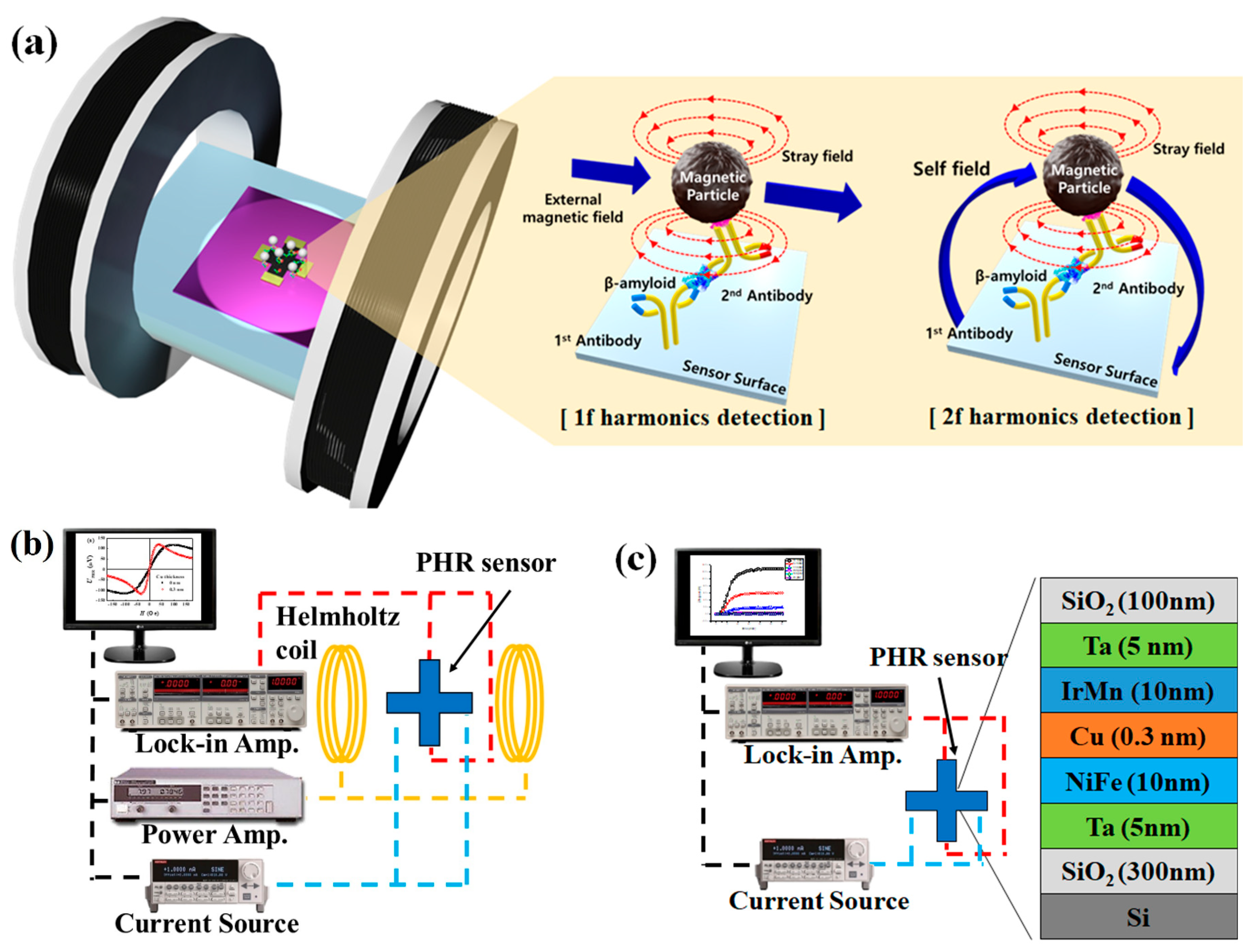
2.4. Detection of Magnetic Particles Using PHE Sensors in the 1f and 2f Modes
2.5. Immobilization of Biomolecules and Magnetic Particles onto the Surface of the Sensor
3. Results
3.1. Optimization of the Layer Composition of the PHR Sensor
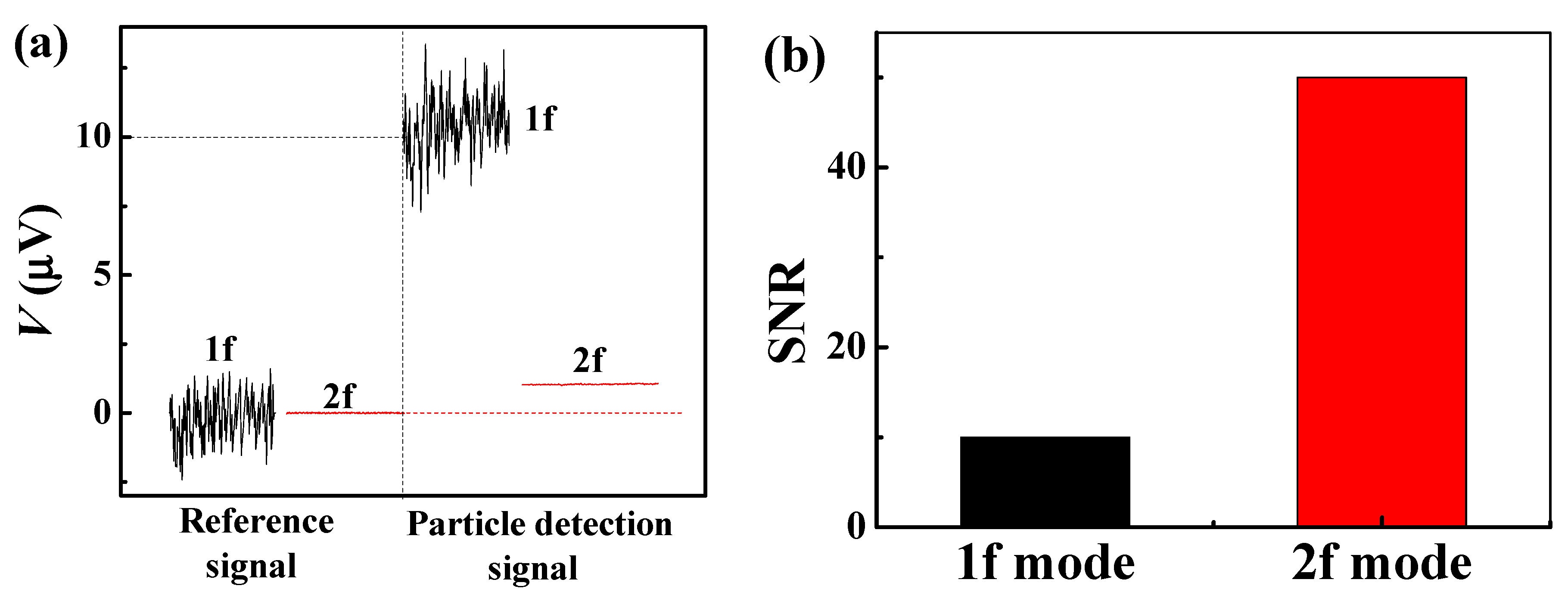
3.2. Operation of the Optimized PHR Sensor in 1f and 2f Detection Modes
3.3. Real-Time Detection of β-Amyloid
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gani, A.W.; Wei, W.; Shi, R.-Z.; Ng, E.; Nguyen, M.; Chua, M.-S.; So, S.; Wang, S.X. An Automated, Quantitative, and Multiplexed Assay Suitable for Point-of-Care Hepatitis B Virus Diagnostics. Sci. Rep. 2019, 9, 15615. [Google Scholar] [CrossRef] [PubMed]
- Barroso, T.G.; Martins, R.C.; Fernandes, E.; Cardoso, S.; Rivas, J.; Freitas, P.P. Detection of BCG bacteria using a magnetoresistive biosensor: A step towards a fully electronic platform for tuberculosis point-of-care detection. Biosens. Bioelectron. 2018, 100, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Klein, T.; Krishna, V.D.; Su, D.; Perez, A.M.; Wang, J.P. Portable GMR Handheld Platform for the Detection of Influenza A Virus. ACS Sens. 2017, 2, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.L.; Ferreira, H.A.; Freitas, P.P. Magnetoresistive-based biosensors and biochips. Trends Biotechnol. 2004, 22, 455–462. [Google Scholar] [CrossRef]
- Gaster, R.S.; Hall, D.A.; Nielsen, C.H.; Osterfeld, S.J.; Yu, H.; Mach, K.E.; Wilson, R.J.; Murmann, B.; Liao, J.C.; Gambhir, S.S.; et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat. Med. 2009, 15, 1327–1332. [Google Scholar] [CrossRef]
- Denmark, D.J.; Bustos-Perez, X.; Swain, A.; Phan, M.-H.; Mohapatra, S.; Mohapatra, S.S. Readiness of Magnetic Nanobiosensors for Point-of-Care Commercialization. J. Electron. Mater. 2019, 48, 4749–4761. [Google Scholar] [CrossRef]
- Van Reenen, A.; de Jong, A.M.; den Toonder, J.M.J.; Prins, M.W.J. Integrated lab-on-chip biosensing systems based on magnetic particle actuation—A comprehensive review. Lab Chip 2014, 14, 1966–1986. [Google Scholar] [CrossRef] [Green Version]
- Ravi, N.; Rizzi, G.; Chang, S.E.; Cheung, P.; Utz, P.J.; Wang, S.X. Quantification of cDNA on GMR biosensor array towards point-of-care gene expression analysis. Biosens. Bioelectron. 2019, 130, 338–343. [Google Scholar] [CrossRef]
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.J. A biosensor based on magnetoresistance technology1This paper was awarded the Biosensors & Bioelectronics Award for the most original contribution to the Congress.1. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar]
- Nabaei, V.; Chandrawati, R.; Heidari, H. Magnetic biosensors: Modelling and simulation. Biosens. Bioelectron. 2018, 103, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Llandro, J.; Palfreyman, J.J.; Ionescu, A.; Barnes, C.H. Magnetic biosensor technologies for medical applications: A review. Med Biol. Eng. Comput. 2010, 48, 977–998. [Google Scholar] [CrossRef] [PubMed]
- Heim, D.E.; Fontana, R.E.; Tsang, C.; Speriosu, V.S.; Gurney, B.A.; Williams, M.L. Design and operation of spin valve sensors. IEEE Trans. Magn. 1994, 30, 316–321. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, G. Advances in Giant Magnetoresistance Biosensors with Magnetic Nanoparticle Tags: Review and Outlook. IEEE Trans. Magn. 2008, 44, 1687–1702. [Google Scholar] [CrossRef]
- Silva, A.V.; Leitao, D.C.; Valadeiro, J.; Amaral, J.; Freitas, P.P.; Cardoso, S. Linearization strategies for high sensitivity magnetoresistive sensors. Eur. Phys. J. Appl. Phys. 2015, 72, 10601. [Google Scholar] [CrossRef] [Green Version]
- Krishnapriya, S.; Komaragiri, R.; Suja, K.J. Fabrication, characterization, and modelling of a novel via-less single metal level magnetic microcoil sensor for biosensing applications. Sens. Actuators A Phys. 2019, 290, 190–197. [Google Scholar]
- Haun, J.B.; Yoon, T.-J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. WIREs Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Besse, P.-A.; Boero, G.; Demierre, M.; Pott, V.; Popovic, R. Detection of a single magnetic microbead using a miniaturized silicon Hall sensor. Appl. Phys. Lett. 2002, 80, 4199–4201. [Google Scholar] [CrossRef]
- Cao, A.; Zhang, X.; Li, Z.; Leng, Q.; Wen, L.; Zhao, W. A Christmas-Tree-Like Magnetic Field Sensor Based on Domain Wall Depinning in a Notched Nanowire. IEEE Magn. Lett. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.; Pu, H. Magnetic impedance biosensor: A review. Biosens. Bioelectron. 2017, 90, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Béguin, F.; Nabily, S.; Gieraltowski, J.; Turzo, A.; Querellou, S.; Salaun, P.Y. Cytotoxicity and GMI bio-sensor detection of maghemite nanoparticles internalized into cells. J. Magn. Magn. Mater. 2009, 321, 192–197. [Google Scholar] [CrossRef]
- García-Arribas, A.; Martínez, F.; Fernández, E.; Ozaeta, I.; Kurlyandskaya, G.V.; Svalov, A.V.; Berganzo, J.; Barandiaran, J.M. GMI detection of magnetic-particle concentration in continuous flow. Sens. Actuators A Phys. 2011, 172, 103–108. [Google Scholar] [CrossRef]
- Oh, S.; Jadhav, M.; Lim, J.; Reddy, V.; Kim, C. An organic substrate based magnetoresistive sensor for rapid bacteria detection. Biosens. Bioelectron. 2013, 41, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.K. Bioinspired magnetoreception and navigation using magnetic signatures as waypoints. Bioinspir. Biomim. 2018, 13, 046003. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.Q.; Terki, F.; Kamara, S.; Kim, K.; Charar, S.; Kim, C. Planar Hall ring sensor for ultra-low magnetic moment sensing. J. Appl. Phys. 2015, 117, 154505. [Google Scholar] [CrossRef]
- Freitas, P.P.; Ferreira, H.A.; Graham, D.L.; Clarke, L.A.; Amaral, M.D.; Martins, V.; Fonseca, L.; Cabral, J.S. Chapter 7—Magnetoresistive DNA chips. In Magnetoelectronics; Johnson, M., Ed.; Academic Press: San Diego, CA, USA, 2004; pp. 331–386. [Google Scholar]
- Jeon, T.; Lee, J.H.; Talantsev, A.; Kim, C.G. Planar Hall Resistance Sensor with Improved Thermal Stability. IEEE Magn. Lett. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Li, F.; Kodzius, R.; Gooneratne, C.P.; Foulds, I.G.; Kosel, J. Magneto-mechanical trapping systems for biological target detection. Microchim. Acta 2014, 181, 1743–1748. [Google Scholar] [CrossRef]
- Tamanaha, C.R.; Mulvaney, S.P.; Rife, J.C.; Whitman, L.J. Magnetic labeling, detection, and system integration. Biosens. Bioelectron. 2008, 24, 1–13. [Google Scholar] [CrossRef]
- Hall, D.A.; Wang, S.X.; Murmann, B.; Gaster, R.S. Portable Biomarker Detection with Magnetic Nanotags. In Proceedings of the IEEE International Symposium on Circuits and Systems, Paris, France, 30 May–2 June 2010; pp. 1779–1782. [Google Scholar]
- Hung, T.Q.; Oh, S.; Sinha, B.; Jeong, J.-R.; Kim, D.-Y.; Kim, C. High field-sensitivity planar Hall sensor based on NiFe/Cu/IrMn trilayer structure. J. Appl. Phys. 2010, 107, 09E715. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Rama, E.C.; González-García, M.B.; Costa-García, A. Competitive electrochemical immunosensor for amyloid-beta 1-42 detection based on gold nanostructurated Screen-Printed Carbon Electrodes. Sens. Actuators B Chem. 2014, 201, 567–571. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, K.-S.; Kim, W.M.; Sohn, Y.-S. Detection of Amyloid-β42 Using a Waveguide-Coupled Bimetallic Surface Plasmon Resonance Sensor Chip in the Intensity Measurement Mode. PLoS ONE 2014, 9, e98992. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.N.; Xu, D.; Ho, S.L.; He, D.; Wong, M.S.; Li, H.W. Highly sensitive quantification of Alzheimer’s disease biomarkers by aptamer-assisted amplification. Theranostics 2019, 9, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Deng, P.; Que, L. Rapid multiplexed detection of beta-amyloid and total-tau as biomarkers for Alzheimer’s disease in cerebrospinal fluid. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Anandakumar, S.; Oh, S.; Kim, C. Micro-magnetometry for susceptibility measurement of superparamagnetic single bead. Sens. Actuators A Phys. 2012, 182, 34–40. [Google Scholar] [CrossRef]
- Kamara, S.; Tran, Q.-H.; Davesne, V.; Félix, G.; Salmon, L.; Kim, K.; Kim, C.; Bousseksou, A.; Terki, F. Magnetic Susceptibility Study of Sub-Pico-emu Sample Using a Micromagnetometer: An Investigation through Bistable Spin-Crossover Materials. Adv. Mater. 2017, 29, 1703073. [Google Scholar] [CrossRef]
- Hansen, T.B.G.; Damsgaard, C.D.; Dalslet, B.T.; Hansen, M.F. Theoretical study of in-plane response of magnetic field sensor to magnetic beads magnetized by the sensor self-field. J. Appl. Phys. 2010, 107, 124511. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.; Seidler, P.M.; Kurland, N.E.; Yadavalli, V.K. Investigations of Chemical Modifications of Amino-Terminated Organic Films on Silicon Substrates and Controlled Protein Immobilization. Langmuir 2010, 26, 2599–2608. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Torati, S.R.; Talantsev, A.; Jeon, C.; Lee, S.; Kim, C. Performance Validation of a Planar Hall Resistance Biosensor through Beta-Amyloid Biomarker. Sensors 2020, 20, 434. https://doi.org/10.3390/s20020434
Kim S, Torati SR, Talantsev A, Jeon C, Lee S, Kim C. Performance Validation of a Planar Hall Resistance Biosensor through Beta-Amyloid Biomarker. Sensors. 2020; 20(2):434. https://doi.org/10.3390/s20020434
Chicago/Turabian StyleKim, SungJoon, Sri Ramulu Torati, Artem Talantsev, ChangYeop Jeon, SungBae Lee, and CheolGi Kim. 2020. "Performance Validation of a Planar Hall Resistance Biosensor through Beta-Amyloid Biomarker" Sensors 20, no. 2: 434. https://doi.org/10.3390/s20020434




