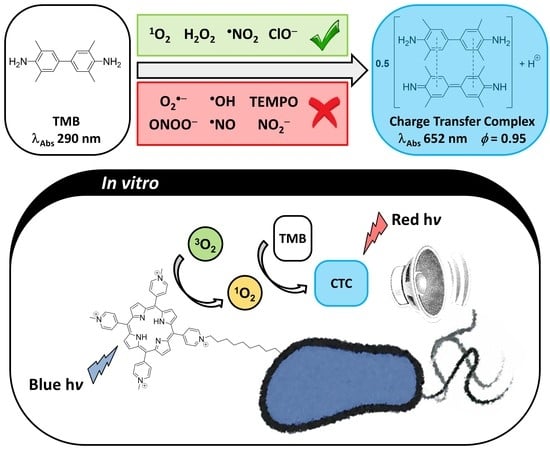
Tetramethylbenzidine: An Acoustogenic Photoacoustic Probe for Reactive Oxygen Species Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Spectroscopic Measurements
2.3. Statistical Analysis
2.4. Sources of the ROS and RNS Tested
2.5. Bacterial Cell Cultures
2.5.1. Escherichia coli Cells with the Endogenous PS MiniSOGs
2.5.2. Escherichia coli Cells with the Exogenous PS MDPyTMPyP
3. Results
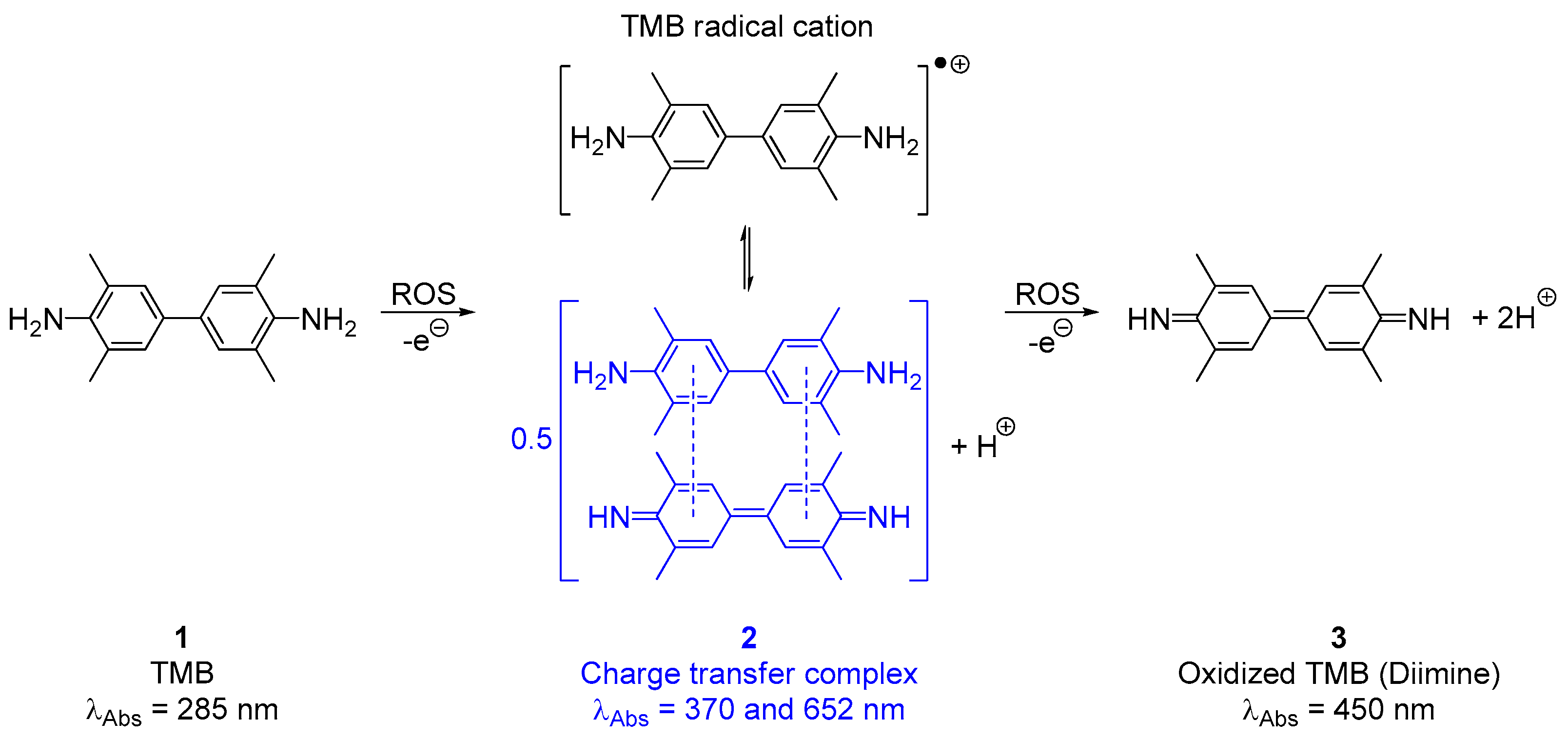
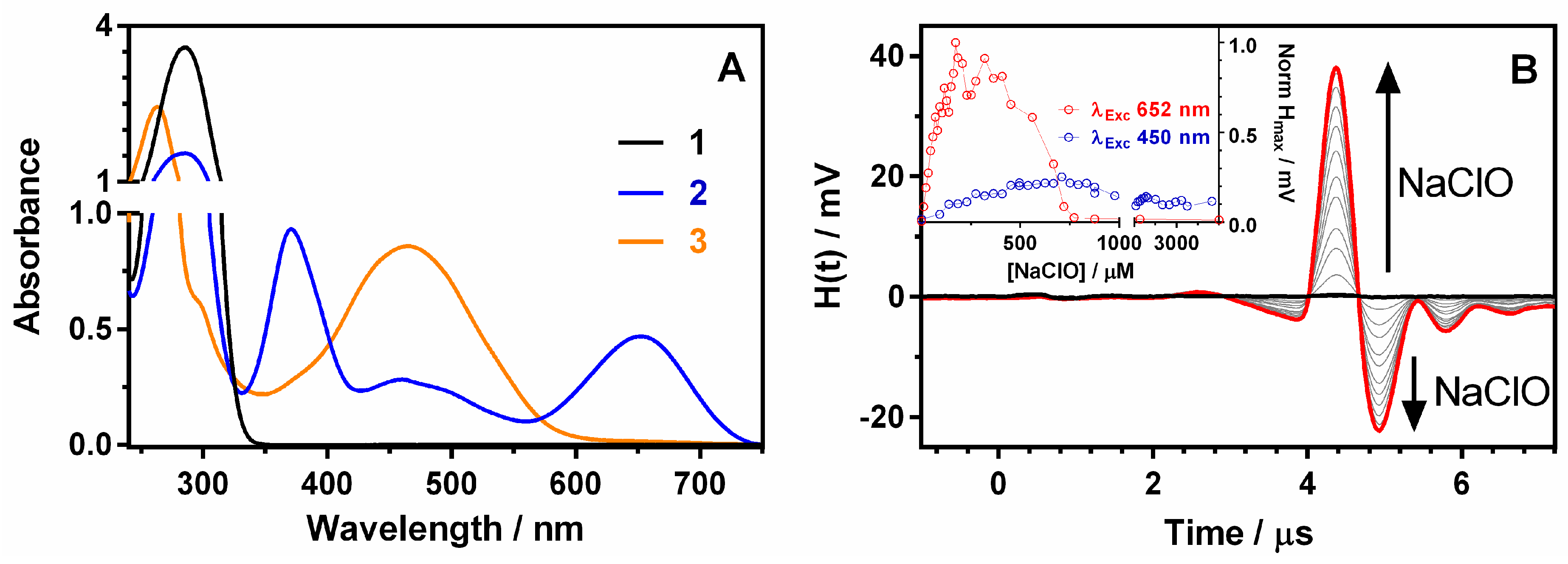
3.1. Photoacoustic and Spectroscopic Properties of TMB and Its Oxidation Products (2 and 3)
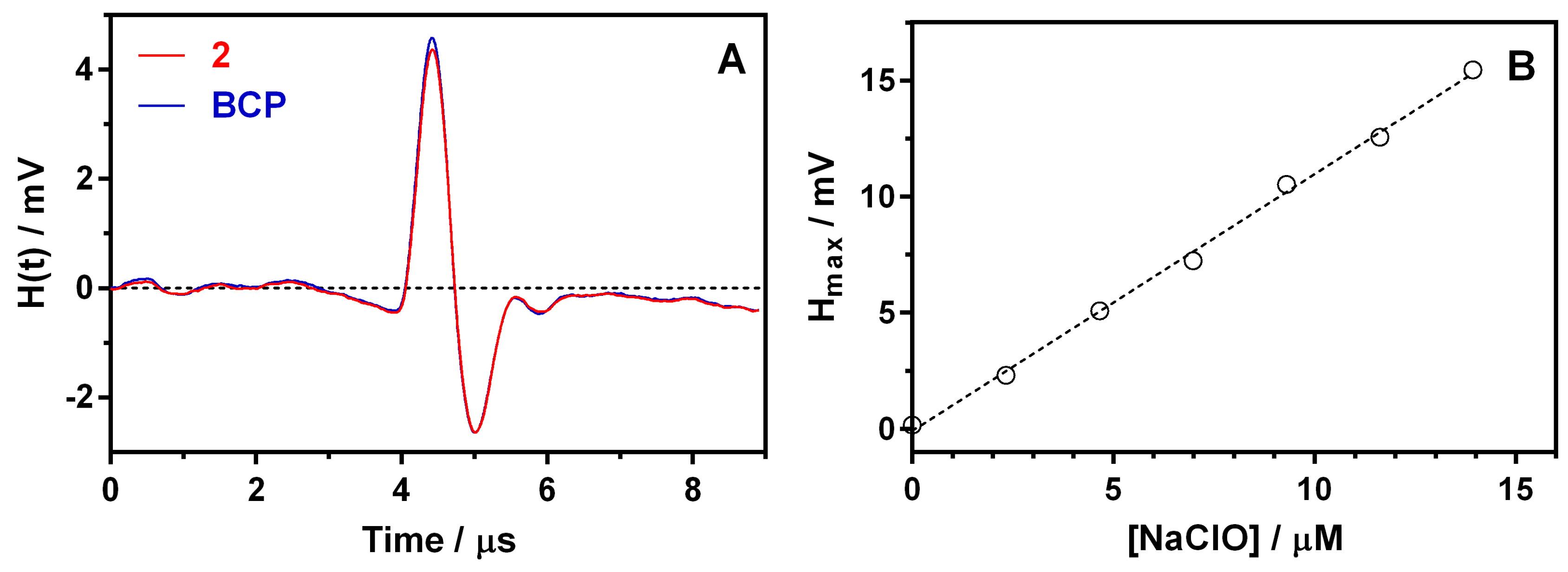
3.2. Factors Affecting the Photoacoustic Signal
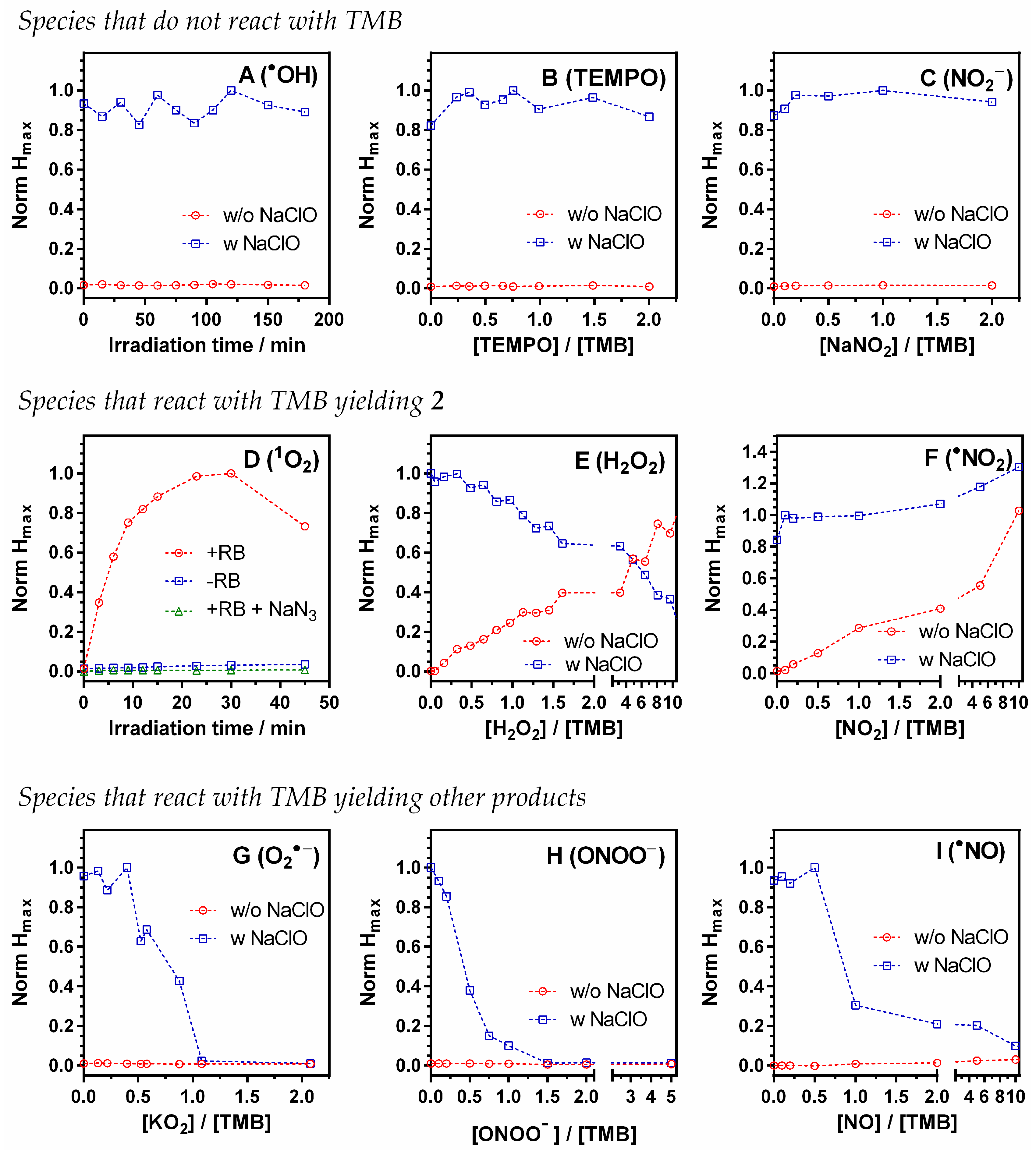
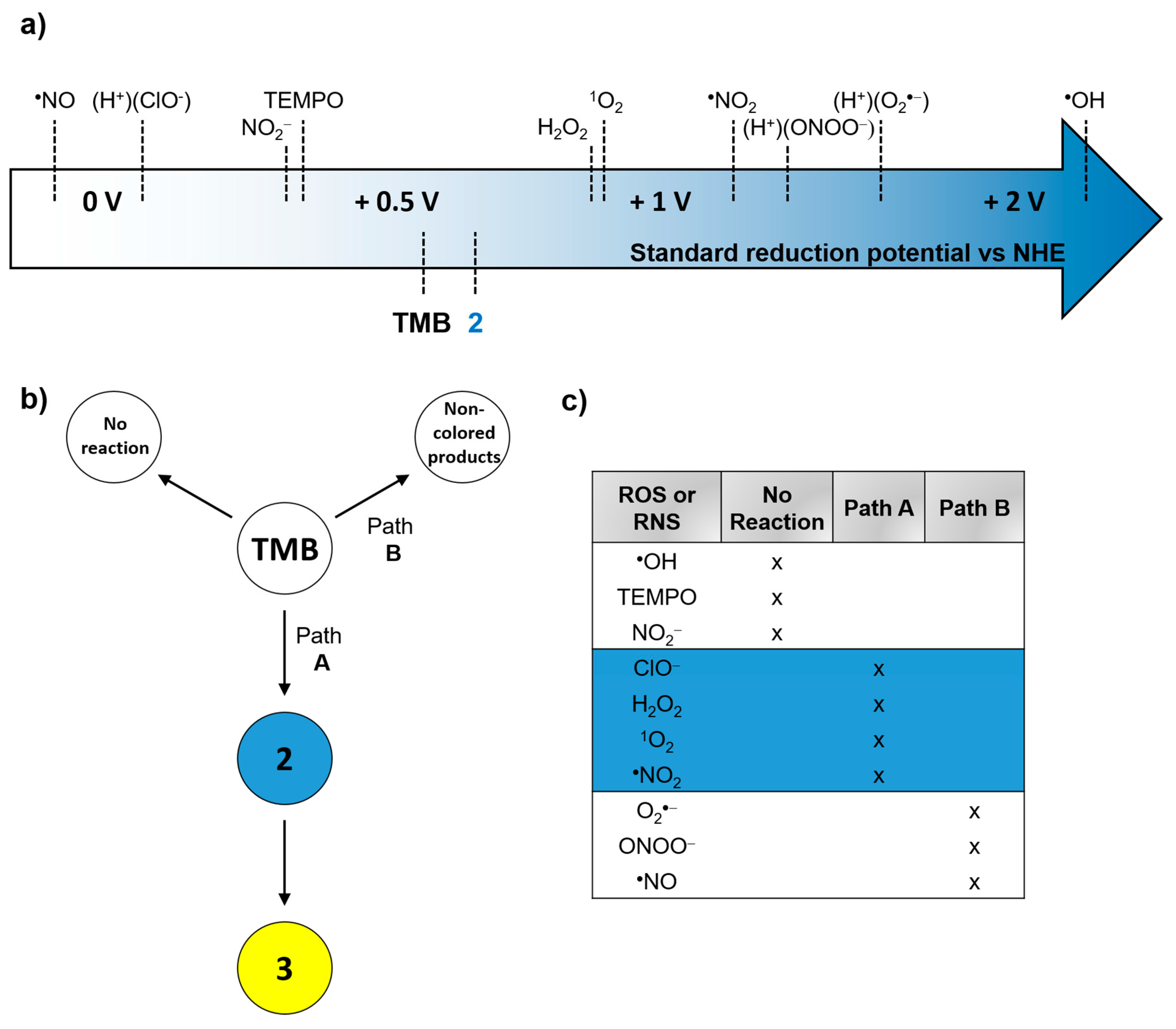
3.3. TMB Reactivity Towards the Tested ROS, RNS, and TEMPO
3.3.1. Species That Give no Photoacoustic Signal
3.3.2. Species That React with TMB Yielding 2
3.3.3. Species That React with TMB Yielding Non-Colored Products
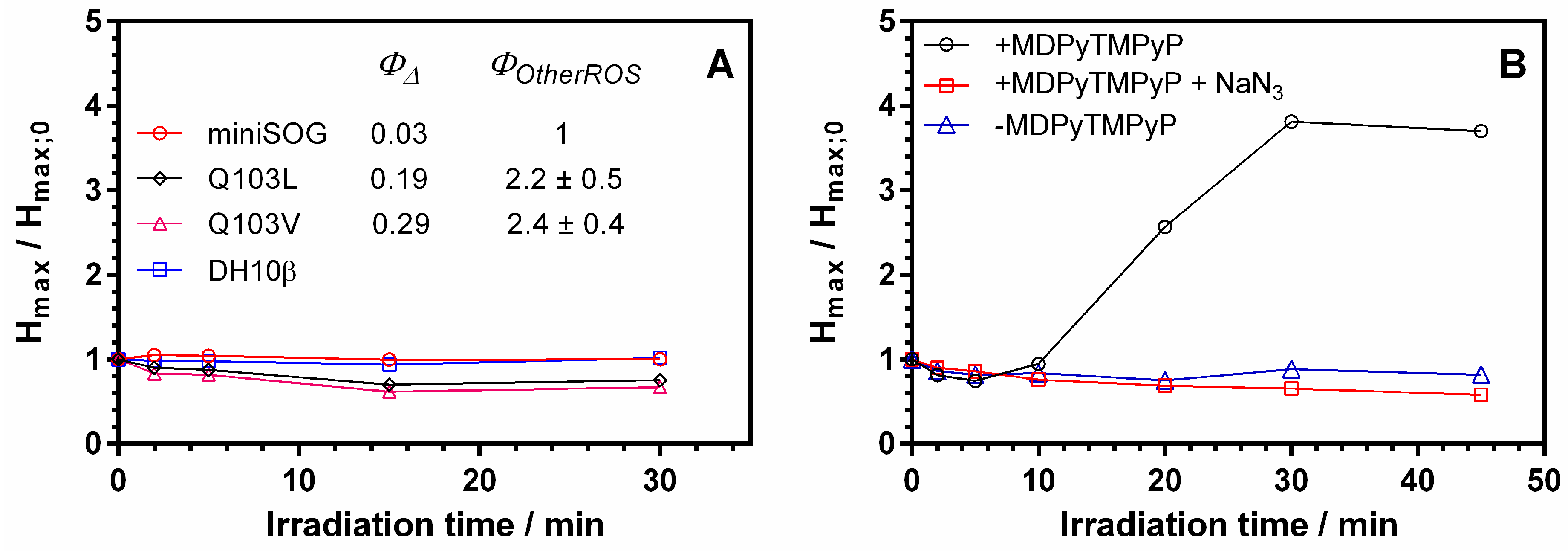
3.4. Detection of ROS in Biological Media
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krumova, K.; Cosa, G. Overview of Reactive Oxygen Species. In Singlet Oxygen: Applications in Biosciences and Nanosciences, 1st ed.; Nonell, S., Flors, C., Eds.; RSC: London, UK, 2016; Volume 1, pp. 1–21. [Google Scholar]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.P.; McAndrew, J.; Sellak, H.; White, C.R.; Jo, H.; Freeman, B.A.; Darley-Usmar, V.M. Biological aspects of reactive nitrogen species. Biochim. Biophys. Acta 1999, 1411, 385–400. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Barroso, J.B. Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Jiménez-Banzo, A.; Ragàs, X.; Kapusta, P.; Nonell, S. Time-resolved methods in biophysics. 7. Photon counting vs. analog time-resolved singlet oxygen phosphorescence detection. Photochem. Photobiol. Sci. 2008, 7, 1003–1010. [Google Scholar] [CrossRef]
- Yu, J.; Chen, J.; Li, C.; Wang, X.; Zhang, B.; Ding, H. ESR signal of superoxide radical anion adsorbed on TiO2 generated at room temperature. J. Phys. Chem. B 2004, 108, 2781–2783. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Bresolí-Obach, R.; Torra, J.; Zanocco, R.P.; Zanocco, A.L.; Nonell, S. Singlet oxygen quantum yield determination using chemical acceptors. In Methods in Molecular Biology: Reactive oxygen species methods and protocols, 1st ed.; Espada, J., Ed.; Springer: Berlin/Heidelberg, Germany; Humana: New York, NY, USA, 2021; Volume 2202, pp. 165–188. [Google Scholar]
- Sharma, S.K.; Hamblin, M.R. The use of fluorescent probes to detect ROS in photodynamic therapy. In Methods in Molecular Biology: Reactive Oxygen Species Methods and Protocols, 1st ed.; Espada, J., Ed.; Springer: Berlin/Heidelberg, Germany; Humana: New York, NY, USA, 2021; Volume 2202, pp. 215–229. [Google Scholar]
- Dawson, J.B.; Barker, D.J.; Ellis, D.J.; Grassam, E.; Cotterill, J.A.; Fisher, G.W.; Feather, J.W. A theoretical and experimental study of light absorption and scattering by in vivo skin. Phys. Med. Biol. 1980, 25, 695–709. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef] [Green Version]
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, 3, 305–324. [Google Scholar] [CrossRef] [Green Version]
- Braslavsky, S.E.; Heibel, G.E. Time-resolved photothermal and photoacoustic methods applied to photoinduced processes in solution. Chem. Rev. 1992, 92, 1381–1410. [Google Scholar] [CrossRef]
- Gensch, T.; Viappiani, C. Time-resolved photothermal methods: Accessing time-resolved thermodynamics of photoinduced processes in chemistry and biology. Photochem. Photobiol. Sci. 2003, 2, 699–721. [Google Scholar] [CrossRef]
- Miao, Q.; Pu, K. Emerging designs of activatable photoacoustic probes for molecular imaging. Bioconjug. Chem. 2016, 27, 2808–2823. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Chen, J.; Zeng, J.; Cheng, Z.; Liu, Z. A self-assembled albumin-based nanoprobe for in vivo ratiometric photoacoustic pH imaging. Adv. Mater. 2015, 27, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, H.J.; Hedhli, J.; Kim, T.W.; Khalili, K.; Dobrucki, L.W.; Chan, J. A bioreducible N-oxide based probe for photoacoustic imaging of hypoxia. Nat. Commun. 2017, 8, 1794. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.; Seeger, M.; Jiang, Y.; Mishra, A.; Sigmund, F.; Stelz, A.; Lauri, A.; Symvoulidis, P.; Rolbiesky, H.; Preller, M.; et al. Calcium sensor for photoacoustic imaging. J. Am. Chem. Soc. 2018, 140, 2718–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Zheng, J.; Zhang, T.; Xing, D. Ratiometric photoacoustic nanoprobes for monitoring and imaging of hydrogen sulfide in vivo. Nanoscale 2018, 10, 13462–13470. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 2014, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Zhou, E.Y.; Knox, H.J.; Reinhardt, C.J.; Partipilo, G.; Nilges, M.J.; Chan, J. Near-infrared photoactivatable nitric oxide donors with integrated photoacoustic monitoring. J. Am. Chem. Soc. 2018, 140, 11686–11697. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Y.; Yin, C.; Fan, Q.; Zhang, W.; Song, J.; Yu, G.; Tang, W.; Fan, W.; Yung, B.C.; et al. Activatable semiconducting theranostics: Simultaneous generation and ratiometric photoacoustic imaging of reactive oxygen species in vivo. Adv. Mater. 2018, 30, 1707509. [Google Scholar] [CrossRef]
- Zheng, J.; Zeng, Q.; Zhang, R.; Xing, D.; Zhang, T. Dynamic-Reversible photoacoustic probe for continuous ratiometric sensing and imaging of redox status in vivo. J. Am. Chem. Soc. 2019, 141, 19226–19230. [Google Scholar] [CrossRef]
- Hatamimoslehabadi, M.; Bellinger, S.; La, J.; Ahmad, E.; Frenette, M.; Yelleswarapu, C.; Rochford, J. Correlation of photophysical properties with the photoacoustic emission for a selection of established chromophores. J. Phys. Chem. C 2017, 121, 24168–24178. [Google Scholar] [CrossRef]
- Boguta, A.; Wróbel, D. Fluorescein and phenolphthalein—Correlation of fluorescence and photoelectric properties. J. Fluoresc. 2001, 11, 129–137. [Google Scholar] [CrossRef]
- Qi, J.; Li, J.; Liu, R.; Li, Q.; Zhang, H.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, D.; Ding, D.; Tang, B.Z. Boosting fluorescence-photoacoustic-Raman properties in one fluorophore for precise cancer surgery. J. Chem. 2019, 5, 2657–2677. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, C.Y.; Chang, C.C.; Peterson, R.; Maswadi, S.; Glickman, R.D.; Chang, H.C.; Yong-Ye, J. Photoacoustic emission from fluorescent nanodiamonds enhanced with gold nanoparticles. Biomed. Opt. Express 2012, 3, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Planas, O.; Faust, A.; Vogl, T.; Hermann, S.; Schäfers, M.; Nonell, S.; Strassert, C.A. Towards optimized naphthalocyanines as sonochromes for photoacoustic imaging in vivo. Photoacoustics 2018, 9, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Scaletti, F.; Truong, L.; Caplan, R.; Cao, A.; Bouchard, R.; Truskett, T.M.; Sokolov, K.V.; Johnston, K.P. Indocyanine green J aggregates in polymersomes for near-infrared photoacoustic imaging. ACS Appl. Mater. Interfaces 2019, 11, 46437–46450. [Google Scholar]
- Frenette, M.; Hatamimoslehabadi, M.; Bellinger-Buckley, S.; Laoui, S.; La, J.; Bag, S.; Mallidi, S.; Hasan, T.; Bouma, B.; Yelleswarapu, C.; et al. Shining light on the dark side of imaging: Excited-state absorption enhancement of a bis-styryl BODIPY photoacoustic contrast agent. J. Am. Chem. Soc. 2014, 136, 15853–15856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, H.J.; Chan, J. Acoustogenic probes: A new frontier in photoacoustic imaging. Acc. Chem. Res. 2018, 51, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Josephy, D.; Eling, T.; Mason, R. The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′- Tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J. Biol. Chem. 1982, 257, 3669–3675. [Google Scholar]
- Guo, Y.; Ma, Q.; Cao, F.; Zhao, Q.; Ji, X. Colorimetric detection of hypochlorite in tap water based on the oxidation of 3,3′,5,5′-tetramethylbenzidine. Anal. Methods 2015, 7, 4055–4058. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Xu, S.; Chen, J.; Zeng, Y.; Wu, P.; Hou, X. Photocatalytic oxidation of TMB with the double-stranded DNA–SYBR Green I complex for label-free and universal colorimetric bioassay. Chem. Commun. 2015, 51, 14465–14468. [Google Scholar] [CrossRef]
- Volpe, G.; Draisci, R.; Palleschi, G.; Compagnone, D. 3,3′,5,5′-Tetramethylbenzidine as electrochemical substrate for horseradish peroxidase based enzyme immunoassays. A comparative study. Analyst 1998, 123, 1303–1307. [Google Scholar] [CrossRef]
- Marquez, L.A.; Dunford, H.B. Mechanism of the oxidation of 3,5,3‘,5‘-tetramethylbenzidine by myeloperoxidase determined by transient- and steady-state kinetics. Biochemistry 1997, 36, 9349–9355. [Google Scholar] [CrossRef]
- Terazima, M.; Azumi, T. A time-resolved photoacoustic method with pulsed laser excitation in the condensed phase: The relation between signal intensity and decay-rate constant. Bull. Chem. Soc. Jpn. 1990, 63, 741–745. [Google Scholar] [CrossRef]
- Abbruzzetti, S.; Viappiani, C.; Murgida, D.H.; Erra-Balsells, R.; Bilmes, G.M. Non-toxic, water-soluble photocalorimetric reference compounds for UV and visible excitation. Chem. Phys. Lett. 1999, 304, 167–172. [Google Scholar] [CrossRef]
- Jankowski, J.J.; Kieber, D.J.; Mopper, K. Nitrate and Nitrite Ultraviolet Actinometers. Photochem. Photobiol. 1999, 70, 319–328. [Google Scholar] [CrossRef]
- Barreto, J.C.; Smith, G.S.; Strobel, N.H.; McQuillin, P.A.; Miller, T.A. Terephthalic acid: A dosimeter for the detection of hydroxyl radicals in vitro. Life Sci. 1994, 56, PL89–PL96. [Google Scholar] [CrossRef]
- Neckers, D.C. Rose Bengal. J. Photochem. Photobiol. A Chem. 1989, 47, 1–29. [Google Scholar] [CrossRef]
- Soares, A.C.; Leite, R.; Tatsuo, M.A.; Duarte, I.D. Activation of ATP-sensitive K+ channels: Mechanism of peripheral antinociceptive action of the nitric oxide donor, sodium nitroprusside. Eur. J. Pharmacol. 2000, 400, 67–71. [Google Scholar] [CrossRef]
- Kim, M.; Ko, S.-K.; Kim, H.; Shin, I.; Tae, J. Rhodamine cyclic hydrazide as a fluorescent probe for the detection of hydroxyl radicals. Chem. Commun. 2013, 49, 7959–7961. [Google Scholar] [CrossRef]
- Lin, L.; Xiao, D.; Yuan, H.; Choi, M.M.F.; Chan, W. A passive sampler for determination of nitrogen dioxide in ambient air. J. Chem. Educ. 2005, 82, 1231–1233. [Google Scholar] [CrossRef]
- Mahouche-Chergui, S.; Gam-Derouich, S.; Mangeney, C.; Chehimi, M.M. Aryl diazonium salts: A new class of coupling agents for bonding polymers, biomacromolecules, and nanoparticles to surfaces. Chem. Soc. Rev. 2011, 40, 4143–4166. [Google Scholar] [CrossRef] [PubMed]
- Berks, B.C.; Ferguson, S.J.; Moir, J.W.B.; Richardson, D.J. Enzymes and associated electron transport systems that catalyze the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1995, 1232, 97–173. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, M.V.; Luong, J.H.T. A water-soluble tetramethylbenzidine-2-hydroxypropyl-β-cyclodextrin inclusion complex as an efficient mediator for oxidoreductases. Electroanalysis 1996, 8, 223–228. [Google Scholar] [CrossRef]
- Nutting, J.E.; Rafiee, M.; Stahl, S.S. Tetramethylpiperidine N-Oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: Electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 2018, 118, 4834–4885. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- Khan, A.U.; Kasha, M. Singlet molecular oxygen evolution upon simple acidification of aqueous hypochlorite: Application to studies on the deleterious health effects of chlorinated drinking water. Proc. Natl. Acad. Sci. USA 1994, 91, 12362–12364. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Y.; Cline, C.S.; Koker, E.B.; Carmichael, H.H.; Chignell, C.F.; Bilski, P. Quenching of singlet molecular oxygen (1O2) by azide anion in solvent mixtures. Photochem. Photobiol. 2001, 74, 760–764. [Google Scholar] [CrossRef]
- Dixon, J.K. The absorption coefficient of nitrogen dioxide in the visible spectrum. J. Chem. Phys. 1940, 8, 157–160. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [Green Version]
- Frimer, A.A.; Aljadeff, G.; Ziv, J. Reaction of (arylmethyl)amines with superoxide anion radical in aprotic media. Insights into cytokinin senescence inhibition. J. Org. Chem. 1983, 48, 1700–1705. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Marietta, M.A. Superoxide-promoted oxidation reactions of aniline and N-methylaniline in dimethyl sulfoxide. J. Org. Chem. 1985, 50, 694–696. [Google Scholar] [CrossRef]
- Masuda, M.; Mower, H.F.; Pignatelli, B.; Celan, I.; Friesen, M.D.; Nishino, H.; Ohshima, H. Formation of N-nitrosamines and N-nitramines by the reaction of secondary amines with peroxynitrite and other reactive nitrogen species: comparison with nitrotyrosine formation. Chem. Res. Toxicol. 2000, 13, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Lev-Ram, V.; Deerinck, T.J.; Qi, Y.; Ramko, E.B.; Davidson, M.W.; Jin, Y.; Ellisman, M.H.; Tsien, R.Y. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011, 9, e1001041. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-González, R.; Cortajarena, A.L.; Mejias, S.H.; Agut, M.; Nonell, S.; Flors, C. Singlet oxygen generation by the genetically encoded tag miniSOG. J. Am. Chem. Soc. 2013, 135, 9564–9567. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pulido, A.; Cortajarena, A.L.; Torra, J.; Ruiz-González, R.; Nonell, S.; Flors, C. Assessing the potential of photosensitizing flavoproteins as tags for correlative microscopy. Chem. Commun. 2016, 52, 8405–8408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragàs, X.; Agut, M.; Nonell, S. Singlet oxygen in Escherichia coli: New insights for antimicrobial photodynamic therapy. Free Radic. Biol. Med. 2010, 49, 770–776. [Google Scholar] [CrossRef]
- Bresolí-Obach, R.; Busto-Moner, L.; Muller, C.; Reina, M.; Nonell, S. NanoDCFH-DA: A silica-based nanostructured fluorogenic probe for the detection of reactive oxygen species. Photochem. Photobiol. 2018, 94, 1143–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, M.; Kurz, T.; Brunk, U.T.; Nilsson, S.E.; Frennesson, C.I. What does the commonly used DCF test for oxidative stress really show? Biochem. J. 2010, 428, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Weston, M.; Patterson, M.S. Singlet oxygen dosimetry in biological media. In Singlet Oxygen: Applications in Biosciences and Nanosciences, 1st ed.; Nonell, S., Flors, C., Eds.; RSC: London, UK, 2016; Volume 2, pp. 151–168. [Google Scholar]
- Pogue, B.W.; Elliott, J.T.; Kanick, S.C.; Davis, S.C.; Samkoe, K.S.; Maytin, E.V.; Pereira, S.P.; Hasan, T. Revisiting photodynamic therapy dosimetry: Reductionist & surrogate approaches to facilitate clinical success. Phys. Med. Biol. 2016, 61, R57–R89. [Google Scholar]
- Singlet Oxygen Sensor Green Reagent Protoco. Available online: https://www.thermofisher.com/order/catalog/product/S36002#/S36002 (accessed on 11 October 2020).
- Ruiz-González, R.; Bresolí-Obach, R.; Gulías, O.; Agut, M.; Savoie, H.; Boyle, R.W.; Nonell, S.; Giuntini, F. NanoSOSG: A nanostructured fluorescent probe for the detection of intracellular singlet oxygen. Angew. Chem. Int. Ed. 2017, 56, 2885–2888. [Google Scholar] [CrossRef]
| Type of Probe | Acoustogenic |
|---|---|
| λmax / nm | 652 |
| ϕ a | 0.95 ± 0.10 |
| LOD b (ClO−) / μM | 0.6 |
| LOQ c (ClO−) / μM | 1.4 |
| Responsivity | ClO−, 1O2, H2O2, •NO2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bresolí-Obach, R.; Frattini, M.; Abbruzzetti, S.; Viappiani, C.; Agut, M.; Nonell, S. Tetramethylbenzidine: An Acoustogenic Photoacoustic Probe for Reactive Oxygen Species Detection. Sensors 2020, 20, 5952. https://doi.org/10.3390/s20205952
Bresolí-Obach R, Frattini M, Abbruzzetti S, Viappiani C, Agut M, Nonell S. Tetramethylbenzidine: An Acoustogenic Photoacoustic Probe for Reactive Oxygen Species Detection. Sensors. 2020; 20(20):5952. https://doi.org/10.3390/s20205952
Chicago/Turabian StyleBresolí-Obach, Roger, Marcello Frattini, Stefania Abbruzzetti, Cristiano Viappiani, Montserrat Agut, and Santi Nonell. 2020. "Tetramethylbenzidine: An Acoustogenic Photoacoustic Probe for Reactive Oxygen Species Detection" Sensors 20, no. 20: 5952. https://doi.org/10.3390/s20205952