High-Temperature Hydrogen Sensing Performance of Ni-Doped TiO2 Prepared by Co-Precipitation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Undoped and Ni-Doped TiO2 Powders
2.2. Material Characterization
2.3. Gas Sensing Tests
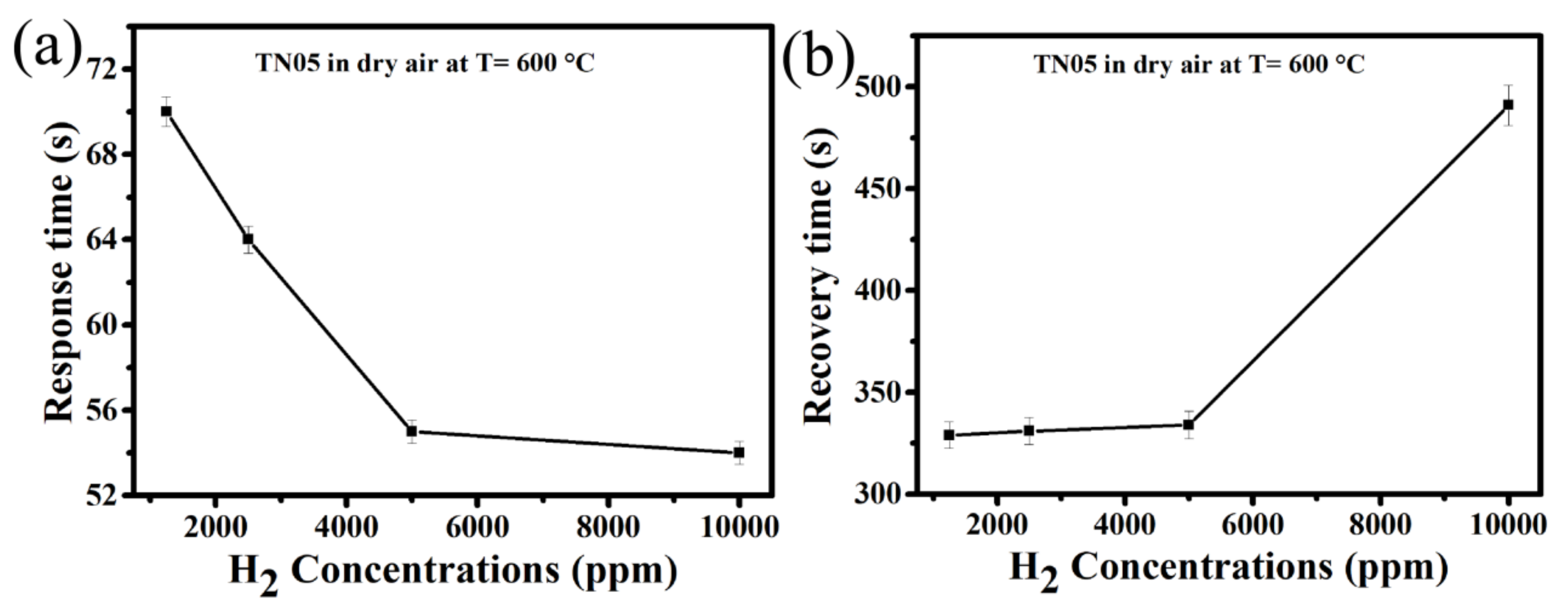
3. Results and Discussion
Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Sinigaglia, T.; Lewiski, F.; Santos Martins, M.E.; Mairesse Siluk, J.C. Production, storage, fuel stations of hydrogen and its utilization in automotive applications—A review. Int. J. Hydrogen Energy 2017, 42, 24597–24611. [Google Scholar] [CrossRef]
- Ramachandran, R.; Menon Raghu, K. An overview of industrial uses of hydrogen. Int. J. Hydrogen Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Hermawan, A.; Asakura, Y.; Kobayashi, M.; Kakihana, M.; Yin, S. High temperature hydrogen gas sensing property of GaN prepared from α-GaOOH. Sens. Actuators B Chem. 2018, 276, 388–396. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh Chauhan, P.; Bhattacharya, S. Hydrogen gas sensing methods, materials, and approach to achieve parts per billion level detection: A review. Int. J. Hydrogen Energy 2019, 44, 26076–26099. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Sheldon Shi, Q.; Zhang, H. High-temperature gas sensors for harsh environment applications: A review. CLEAN Soil Air Water 2019, 47, 1800491–1800506. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; Tedjieukeng Kamta, H.M.; Ngolui Lambi, J.; Lahem, D.; Eloy, P.; Debliquy, M.; Delcorte, A. A sub-ppm level formaldehyde gas sensor based on Zn-doped NiO prepared by a co-precipitation route. J. Alloy. Comp. 2018, 731, 1188–1196. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Su, C.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; Yang, Z. Sonochemical synthesis of hierarchical WO3 flower-like spheres for highly efficient triethylamine detection. Sens. Actuators B Chem. 2020, 306, 127536. [Google Scholar] [CrossRef]
- Cho, H.J.; Choi, S.J.; Kim, N.H.; Kim, I.D. Porosity controlled 3D SnO2 spheres via electrostatic spray: Selective acetone sensors. Sens. Actuators B Chem. 2020, 304, 127350. [Google Scholar] [CrossRef]
- Miao, J.; Chen, C.; Lin, J.Y.S. Humidity independent hydrogen sulfide sensing response achieved with monolayer film of CuO nanosheets. Sens. Actuators B Chem. 2020, 309, 127785. [Google Scholar] [CrossRef]
- Gao, R.; Gao, S.; Wang, P.; Xu, Y.; Zhang, X.; Cheng, X.; Zhou, X.; Major, Z.; Zhu, H.; Huo, L. Ionic liquid assisted synthesis of snowflake ZnO for detection of NOx and sensing mechanism. Sens. Actuators B Chem. 2020, 303, 127085. [Google Scholar] [CrossRef]
- Xie, L.; Li, Z.; Sun, L.; Dong, B.; Fatima, Q.; Wang, Z.; Yao, Z.; Haidry, A.A. Sol-gel synthesis of TiO2 with p-type response to hydrogen gas at elevated temperature. Front. Mater. 2019, 6, 96. [Google Scholar] [CrossRef]
- Liu, Y.X.; Parisi, J.; Sun, X.C.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Esmaeilzadeh, J.; Marzbanrad, E.; Zamani, C.; Raissi, B. Fabrication of undoped-TiO2 nanostructure-based NO2 high temperature gas sensor using low frequency AC electrophoretic deposition method. Sens Actuators B Chem. 2012, 161, 401–405. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; Saruhan, B. Synthesis of Co3+ doped TiO2 by co-precipitation route and its gas sensing properties. Front. Mater. 2019, 6, 252. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zhang, D.; Wang, Y.; Feng, C.; Zhou, J.; Liu, C.; Ruan, S. Xylene gas sensor based on Ni doped TiO2 bowl-like submicron particles with enhanced sensing performance. RSC Adv. 2015, 5, 28105–28110. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Haidry, A.A.; Saruhan, B. Nanotubular Cr-doped TiO2 for use as high-temperature NO2 gas sensor. Sens. Actuators B Chem. 2015, 217, 78–87. [Google Scholar] [CrossRef]
- Bayata, F.; Saruhan-Brings, B.; Ürgen, M. Hydrogen gas sensing properties of nanoporous Al-doped titania. Sens. Actuators B Chem. 2014, 204, 109–118. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C.; Wang, X. Ni-doped TiO2 nanotubes for wide-range hydrogen sensing. Nanoscale Res. Lett. 2014, 9, 118. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Wei, P.; Xia, X.; Huang, Z.; Homewood, K.; Gao, Y. Remarkably enhanced H2 response and detection range in Nb doped rutile/anatase heterophase junction TiO2 thin film hydrogen sensors. Sens. Actuators B Chem. 2019, 301, 127143. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N., Jr.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef] [Green Version]
- Govindhan, M.; Sidhureddy, B.; Chen, A. High-temperature hydrogen gas sensor based on three-dimensional hierarchical-nanostructured nickel−cobalt oxide. ACS Appl. Nano Mater. 2018, 1, 6005–6014. [Google Scholar] [CrossRef]
- Steinebach, H.; Kannan, S.; Rieth, L.; Solzbacher, F. H2 gas sensor performance of NiO at high temperatures in gas mixtures. Sens. Actuators B Chem. 2010, 151, 162–168. [Google Scholar] [CrossRef]
- Lu, G.; Miura, N.; Yamazoe, N. High-temperature hydrogen sensor based on stabilized zirconia and a metal oxide electrode. Sens. Actuators B Chem. 1996, 35, 130–135. [Google Scholar] [CrossRef]
- Patil, L.A.; Suryawanshi, D.N.; Pathan, I.G.; Patil, D.M. Nickel doped spray pyrolyzed nanostructured TiO2 thin films for LPG gas sensing. Sens. Actuators B Chem. 2013, 171, 514–521. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Chanda, A.; Rout, K.; Vasundhara, M.; Joshi, S.R.; Singh, J. Structural and magnetic study of undoped and cobalt doped TiO2 nanoparticles. RSC Adv. 2018, 8, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; li Bassi, A.; Bottani, C.E.; Ducati, C. Raman spectroscopy characterization of TiO2 rutile nanocrystals. Phys. Rev. B Condens. Matter. 2007, 75, 045416. [Google Scholar] [CrossRef]
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Ni-doped TiO2 materials for photocurrent and photocatalytic applications. Sci. World J. 2012, 2012, 127326. [Google Scholar]
- Chen, W.; Li, Q.; Xu, L.; Zeng, W. Gas sensing properties of ZnO-SnO2 nanostructures. J. Nanosci. Nanotechnol. 2015, 15, 1245–1252. [Google Scholar] [CrossRef]
- Van Toan, N.; Manh Hung, C.; van Duy, N.; Duc Hoa, N.; le Thanh, D.T.; van Hieu, N. Bilayer SnO2–WO3 nanofilms for enhanced NH3 gas sensing performance. Mater. Sci. Eng. B 2017, 224, 163–170. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; Saruhan, B. Influence of humidity on NO2-sensing and selectivity of spray-CVD grown ZnO thin film above 400 °C. Chemosensors 2019, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, C.C.; Luo, L.S. A rapid process for fabricating gas sensors. Sensors 2014, 14, 12219–12232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Deng, M.; Xia, X.; Gao, Y.; Shao, G. Fundamental basis for distinctive sensing of H2 in humid environment. Energy Environ. Mater. 2018, 1, 174–178. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C. Hydrogen sensing with Ni-doped TiO2 nanotubes. Sensors 2013, 13, 8393–8402. [Google Scholar] [CrossRef] [Green Version]
- Lyson-Sypiena, B.; Czapla, A.; Lubecka, M.; Gwizdz, P.; Schneider, K.; Zakrzewska, K.; Michalow, K.; Graule, T.; Reszka, A.; Rekas, M.; et al. Nanopowders of chromium doped TiO2 for gas sensors. Sens. Actuators B Chem. 2012, 175, 163–172. [Google Scholar] [CrossRef]
- Liu, H.; Ding, D.; Ning, C.; Li, Z. Wide-range hydrogen sensing with Nb-doped TiO2 nanotubes. Nanotechnology 2012, 23, 015502. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Ning, C. p-Type hydrogen sensing with Al-and V-doped TiO2 nanostructures. Nanoscale Res. Lett. 2013, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Lontio Fomekong, R.; Lahem, D.; Debliquy, M.; Yunus, S.; Lambi Ngolui, J.; Delcorte, A. Ni0.9Zn0.1O/ZnO nanocomposite prepared by malonate coprecipitation route for gas sensing. Sens. Actuators B Chem. 2016, 231, 520–528. [Google Scholar] [CrossRef]


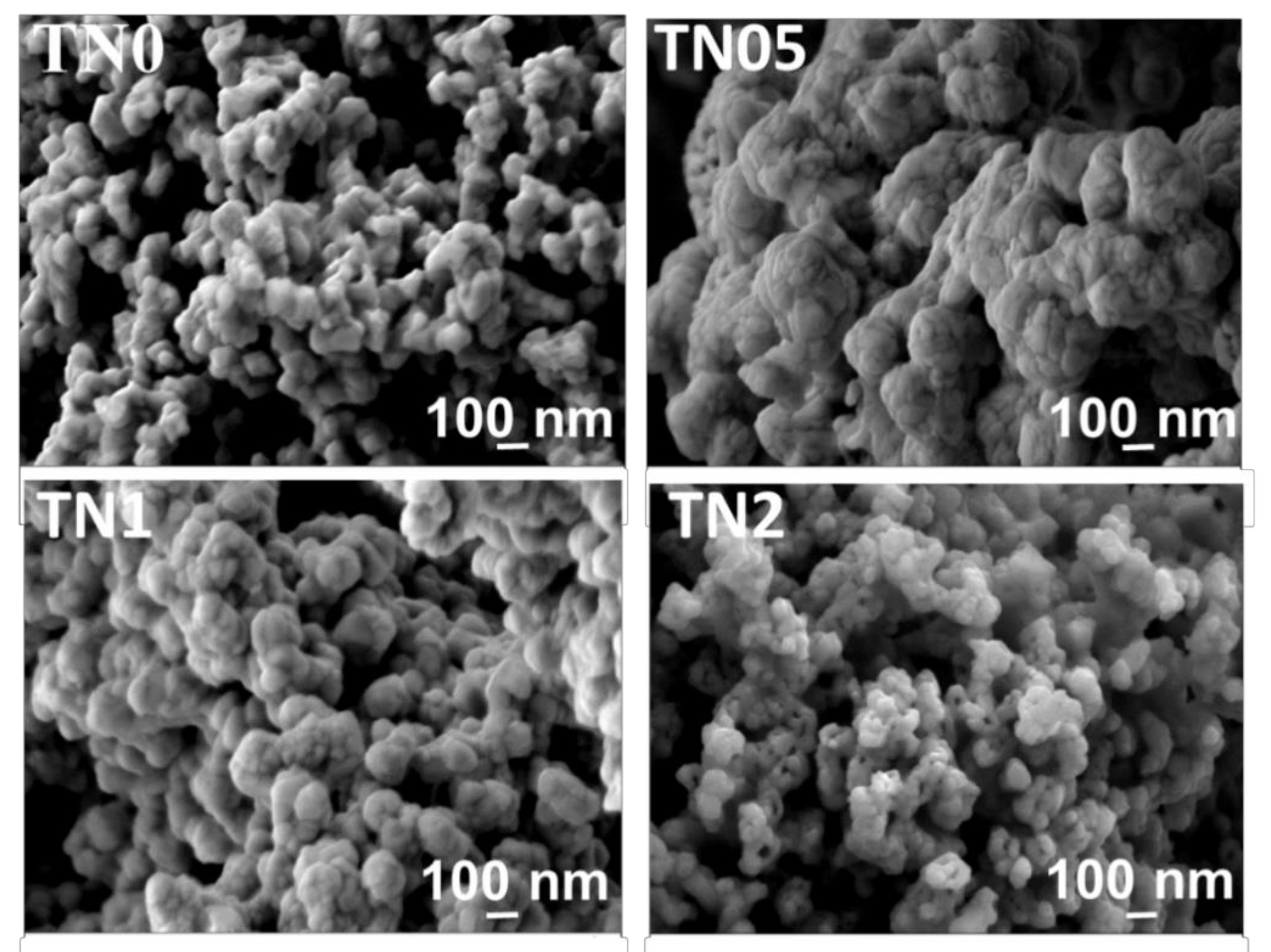







| Sample Name | Lattice Parameter of Anatase, a (Å) | Lattice Parameter of Rutile, a (Å) | Phase Composition (%) | BET (m2/g) |
|---|---|---|---|---|
| TN0 | 3.7874 ± 0.0001 | / | Anatase (98)/Rutile (2) | 7.8 |
| TN05 | 3.7839 ± 0.0001 | 4.5932 ± 0.0002 | Anatase (53)/Rutile (47) | 6.9 |
| TN1 | 3.7836 ± 0.0002 | 4.5928 ± 0.0002 | Anatase (21)/Rutile (79) | 6.4 |
| TN2 | / | 4.5939 ± 0.0002 | Anatase (1)/Rutile (99) | 10.7 |
| Samples | Ti (at.%) | Ni (at.%) | O (at.%) | Ni/Ti × 100 (%) Experimental | Ni/Ti × 100 (%) Expected |
|---|---|---|---|---|---|
| TN0 | 33.25 | / | 66.85 | 0 | 0 |
| TN05 | 33.12 | 0.16 | 66.73 | 0.48 | 0.5 |
| TN1 | 24.62 | 0.24 | 75.14 | 0.97 | 1 |
| TN2 | 21.76 | 0.43 | 77.81 | 1.98 | 2 |
| Material | Synthesis Method | Temperature (°C) | Gas Concentration (ppm) | Sensor Response | Reference |
|---|---|---|---|---|---|
| Ni-doped TiO2 nanotube | Anodic oxidation | 200 | 1000 | 13% | [43] |
| Ni-doped TiO2 nanotube | Electrochemical anodization | 200 | 5000 | 60% | [25] |
| Cr-doped TiO2 spherical | Flame spray synthesis | 350 | 3000 | 50% | [44] |
| Nb-doped TiO2 nanotube | anodization | RT | 1000 | 30% | [45] |
| Cr doped TiO2 irregular morphology | Sol-gel | 500 | 1000 | 99.8% | [15] |
| Al-doped TiO2 | Sputtering | 350 | 2500 | 99.2% | [24] |
| Al-V doped TiO2 Nanotube | Electrochemical anodization | 300 | 1000 | 50% | [46] |
| Nb-doped TiO2 nanorod | Hydrothermal | RT | 8000 | 98.9% | [26] |
| Ni-doped TiO2 nanoparticles | Co-precipitation | 600 | 5000 | 65% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomekong, R.L.; Kelm, K.; Saruhan, B. High-Temperature Hydrogen Sensing Performance of Ni-Doped TiO2 Prepared by Co-Precipitation Method. Sensors 2020, 20, 5992. https://doi.org/10.3390/s20215992
Fomekong RL, Kelm K, Saruhan B. High-Temperature Hydrogen Sensing Performance of Ni-Doped TiO2 Prepared by Co-Precipitation Method. Sensors. 2020; 20(21):5992. https://doi.org/10.3390/s20215992
Chicago/Turabian StyleFomekong, Roussin Lontio, Klemens Kelm, and Bilge Saruhan. 2020. "High-Temperature Hydrogen Sensing Performance of Ni-Doped TiO2 Prepared by Co-Precipitation Method" Sensors 20, no. 21: 5992. https://doi.org/10.3390/s20215992
APA StyleFomekong, R. L., Kelm, K., & Saruhan, B. (2020). High-Temperature Hydrogen Sensing Performance of Ni-Doped TiO2 Prepared by Co-Precipitation Method. Sensors, 20(21), 5992. https://doi.org/10.3390/s20215992







