Quantifying Reliable Walking Activity with a Wearable Device in Aged Residential Care: How Many Days Are Enough?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Instrumentation
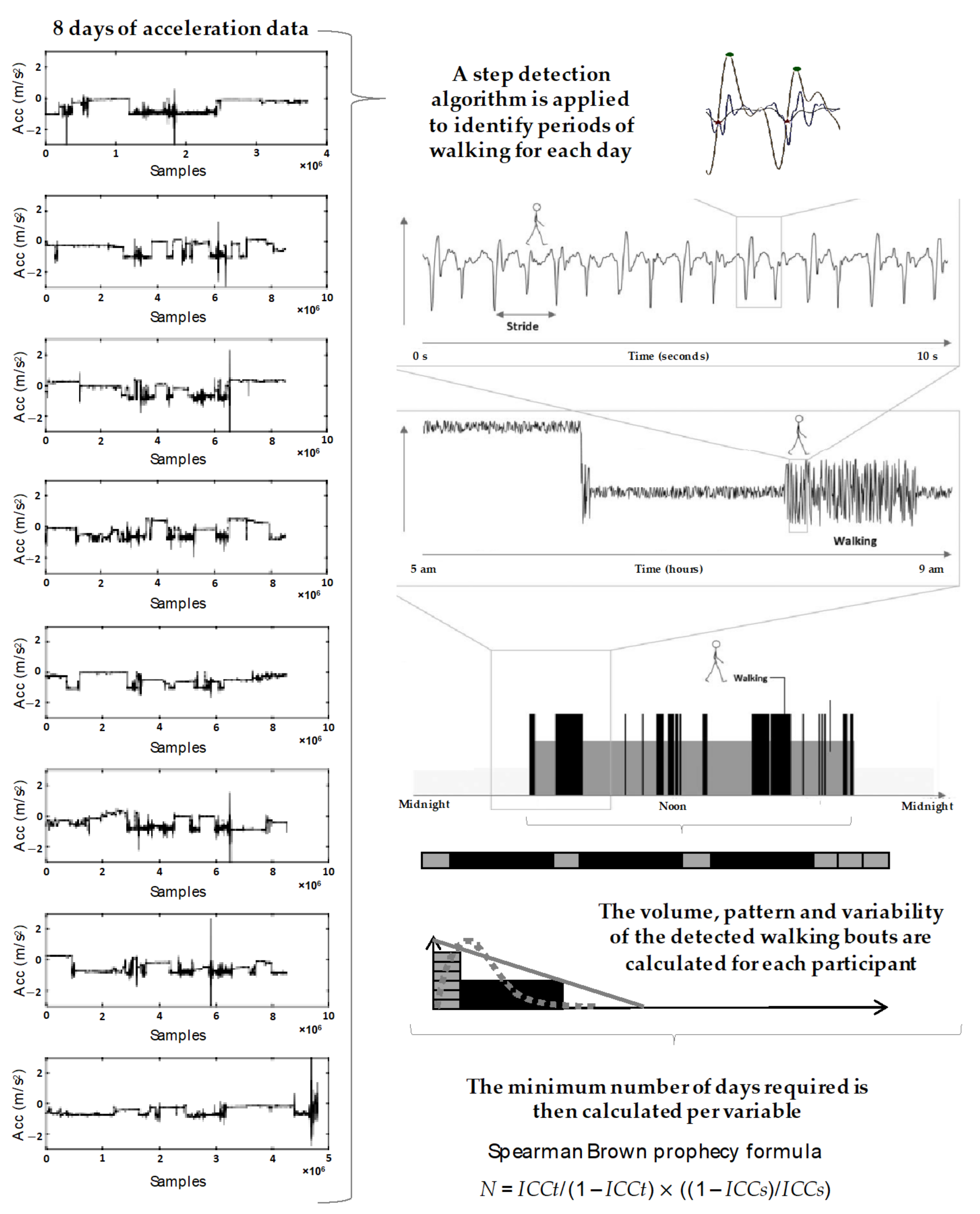
2.4. Wearable Data Analysis
2.5. Data Considerations
2.6. Statistical Analysis
3. Results
3.1. Number of Days Required
3.2. Data Collection: Success Rate, Compliance, and Reason for Data Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Schooten, K.S.; Rispens, S.M.; Elders, P.J.M.; Lips, P.; Van Dieën, J.H.; Pijnappels, M. Assessing Physical Activity in Older Adults: Required Days of Trunk Accelerometer Measurements for Reliable Estimation. J. Aging Phys. Act. 2015, 23, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.E.; Forster, A.; Birch, K.M. Levels and Patterns of Daily Physical Activity and Sedentary Behavior Measured Objectively in Older Care Home Residents in the United Kingdom. J. Aging Phys. Act. 2015, 23, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Airlie, J.; Birch, K.; Cicero, R.; Cundill, B.; Ellwood, A.; Godfrey, M.; Graham, L.; Green, J.; Hulme, C.; et al. Research Exploring Physical Activity in Care Homes (REACH): Study protocol for a randomised controlled trial. Trials 2017, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Resnick, B.; Galik, E.; Boltz, M.; Vigne, E.; Holmes, S.; Fix, S.; Zhu, S. The impact of physical activity and function on falls in assisted living residents. J. Aging Phys. Act. 2019, 27, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.-M.; Ejupi, A.; Van Schooten, K.S.; Aziz, O.; Feldman, F.; Mackey, D.C.; Ashe, M.C.; Robinovitch, S.N. Association between Sedentary Behaviour and Physical, Cognitive, and Psychosocial Status among Older Adults in Assisted Living. BioMed Res. Int. 2017, 2017, 9160504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galik, E.; Resnick, B.; Hammersla, M.; Brightwater, J. Optimizing Function and Physical Activity among Nursing Home Residents with Dementia: Testing the Impact of Function-Focused Care. Gerontologist 2014, 54, 930–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, N.; Eakin, E.; Henwood, T.; Keogh, J.W.L.; Senior, H.E.; Gardiner, P.A.; Winkler, E.; Healy, G.N. Objectively Measured Activity Patterns among Adults in Residential Aged Care. Int. J. Environ. Res. Public Health 2013, 10, 6783–6798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Alphen, H.J.M.; Volkers, K.M.; Blankevoort, C.G.; Scherder, E.J.A.; Hortobágyi, T.; Van Heuvelen, M.J.G. Older Adults with Dementia Are Sedentary for Most of the Day. PLoS ONE 2016, 11, e0152457. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.M.; Klenk, J.; Maney, A.J.; Kerse, N.; MacDonald, B.M.; Maddison, R. Validation of a Body-Worn Accelerometer to Measure Activity Patterns in Octogenarians. Arch. Phys. Med. Rehabil. 2014, 95, 930–934. [Google Scholar] [CrossRef]
- Egerton, T.; Brauer, S.G. Temporal Characteristics of Habitual Physical Activity Periods Among Older Adults. J. Phys. Act. Health 2009, 6, 644–650. [Google Scholar] [CrossRef]
- Taylor, L.; Kerse, N.; Klenk, J.; Borotkanics, R.; Maddison, R. Exergames to improve the mobility of long-term care residents: A cluster randomized controlled trial. Games Health J. 2018, 7, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.E.; Hélio Júnior, J.; Meneguci, J.; Tribess, S.; Marocolo Júnior, M.; Stabelini Neto, A.; Virtuoso Júnior, J.S. Number of days required for reliably estimating physical activity and sedentary behaviour from accelerometer data in older adults. J. Sports Sci. 2018, 36, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.A.; Kemble, C.D.; Robinson, T.S.; Mahar, M.T. Daily Walking in Older Adults: Day-to-Day Variability and Criterion-Referenced Validity of Total Daily Step Counts. J. Phys. Act. Health 2007, 4, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Parsons, J.; Taylor, D.; Binns, E.; Lord, S.; Edlin, R.; Rochester, L.; Del Din, S.; Klenk, J.; Buckley, C.; et al. Evaluating the effects of an exercise program (Staying UpRight) for older adults in long-term care on rates of falls: Study protocol for a randomised controlled trial. Trials 2020, 21, 46. [Google Scholar] [CrossRef] [Green Version]
- Del Din, S.; Hickey, A.; Woodman, S.; Hiden, H.; Morris, R.; Watson, P.; Nazarpour, K.; Catt, M.; Rochester, L.; Godfrey, A. Accelerometer-based gait assessment: Pragmatic deployment on an international scale. In Proceedings of the 2016 IEEE Workshop on Statistical Signal Processing (SSP), Palma de Mallorca, Spain, 26–29 June 2016; pp. 1–5. [Google Scholar]
- Hiden, H.; Woodman, S.; Watson, P.; Cala, J. Developing cloud applications using the e-Science Central platform. Philos. Trans. R. Soc. A 2013, 371, 20120085. [Google Scholar] [CrossRef] [Green Version]
- Del Din, S.; Yarnall, A.J.; Barber, T.R.; Lo, C.; Crabbe, M.; Rolinski, M.; Baig, F.; Hu, M.T.; Rochester, L. Continuous Real-World Gait Monitoring in Idiopathic REM Sleep Behavior Disorder. J. Parkinsons Dis. 2020, 10, 283–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, S.; Galna, B.; Rochester, L. Moving forward on gait measurement: Toward a more refined approach. Mov. Disord. 2013, 28, 1534–1543. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Galna, B.; Lord, S.; Rochester, L. Free-living gait characteristics in ageing and Parkinson’s disease: Impact of environment and ambulatory bout length. J. Neuroeng. Rehabil. 2016, 13, 46. [Google Scholar] [CrossRef] [Green Version]
- Del Din, S.; Galna, B.; Godfrey, A.; Bekkers, E.M.J.; Pelosin, E.; Nieuwhof, F.; Mirelman, A.; Hausdorff, J.M.; Rochester, L. Analysis of Free-Living Gait in Older Adults With and Without Parkinson’s Disease and With and Without a History of Falls: Identifying Generic and Disease-Specific Characteristics. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 74, 500–506. [Google Scholar] [CrossRef]
- Mc Ardle, R.; Del Din, S.; Donaghy, P.; Galna, B.; Thomas, A.; Rochester, L. Factors That Influence Habitual Activity in Mild Cognitive Impairment and Dementia. Gerontology 2020, 66, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Del Din, S.; Lewis, E.G.; Gray, W.K.; Collin, H.; Kissima, J.; Rochester, L.; Dotchin, C.; Urasa, S.; Walker, R. Monitoring Walking Activity with Wearable Technology in Rural-dwelling Older Adults in Tanzania: A Feasibility Study Nested within a Frailty Prevalence Study. Exp. Aging Res. 2020, 46, 367–381. [Google Scholar] [CrossRef]
- Hickey, A.; Del Din, S.; Rochester, L.; Godfrey, A. Detecting free-living steps and walking bouts: Validating an algorithm for macro gait analysis. Physiol. Meas. 2017, 38, N1. [Google Scholar] [CrossRef]
- Chastin, S.F.M.; Baker, K.; Jones, D.; Burn, D.; Granat, M.H.; Rochester, L. The pattern of habitual sedentary behavior is different in advanced Parkinson’s disease. Mov. Disord. 2010, 25, 2114–2120. [Google Scholar] [CrossRef]
- Rochester, L.; Chastin, S.F.M.; Lord, S.; Baker, K.; Burn, D.J. Understanding the impact of deep brain stimulation on ambulatory activity in advanced Parkinson’s disease. J. Neurol. 2012, 259, 1081–1086. [Google Scholar] [CrossRef]
- De Bruin, E.D.; Najafi, B.; Murer, K.; Uebelhart, D.; Aminian, K. Quantification of everyday motor function in a geriatric population. J. Rehabil. Res. Dev. 2007, 44, 417–428. [Google Scholar] [CrossRef]
- Schwenk, M.; Hauer, K.; Zieschang, T.; Englert, S.; Mohler, J.; Najafi, B. Sensor-Derived Physical Activity Parameters Can Predict Future Falls in People with Dementia. Gerontology 2014, 60, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Brodie, M.A.D.; Coppens, M.J.M.; Lord, S.R.; Lovell, N.H.; Gschwind, Y.J.; Redmond, S.J.; Del Rosario, M.B.; Wang, K.; Sturnieks, D.L.; Persiani, M.; et al. Wearable pendant device monitoring using new wavelet-based methods shows daily life and laboratory gaits are different. Med. Biol. Eng. Comput. 2016, 54, 663–674. [Google Scholar] [CrossRef]
- Aadland, E.; Ylvisåker, E. Reliability of Objectively Measured Sedentary Time and Physical Activity in Adults. PLoS ONE 2015, 10, e0133296. [Google Scholar] [CrossRef] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Trost, S.G.; McIver, K.L.; Pate, R.R. Conducting Accelerometer-Based Activity Assessments in Field-Based Research. Med. Sci. Sports Exerc. 2005, 37, S531–S543. [Google Scholar] [CrossRef]
- Sasaki, J.E.; Da Silva, K.S.; Da Costa, B.G.G.; John, D. Measurement of physical activity using accelerometers. In Computer-Assisted and Web-Based Innovations in Psychology, Special Education, and Health; Academic Press: London, UK, 2016; pp. 33–60. [Google Scholar]
- Algase, D.L.; Beattie, E.R.A.; Leitsch, S.A.; Beel-Bates, C.A. Biomechanical activity devices to index wandering behavior in dementia. Am. J. Alzheimers Dis. Other Demen. 2003, 18, 85–92. [Google Scholar] [CrossRef]
- Resnick, B.; Galik, E.; Boltz, M.; Vigne, E.; Holmes, S.; Fix, S.; Zhu, S. Physical Activity and Function in Assisted Living Residents. West. J. Nurs. Res. 2018, 40, 1734–1748. [Google Scholar] [CrossRef]
- Storm, F.A.; Heller, B.W.; Mazzà, C. Step Detection and Activity Recognition Accuracy of Seven Physical Activity Monitors. PLoS ONE 2015, 10, e0118723. [Google Scholar] [CrossRef]
- Morbidoni, C.; Cucchiarelli, A.; Fioretti, S.; Di Nardo, F. A Deep Learning Approach to EMG-Based Classification of Gait Phases during Level Ground Walking. Electronics 2019, 8, 894. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.D.; Mun, K.R.; Jung, D.; Han, J.; Park, M.; Kim, J.; Kim, J. IMU-based spectrogram approach with deep convolutional neural networks for gait classification. In Proceedings of the 2020 IEEE International Conference on Consumer Electronics (ICCE), Las Vegas, NV, USA, 4–6 January 2020; pp. 1–6. [Google Scholar]


| Level of Care | Age (Years) | Weight (kg) | Height (m) | MoCA 1 (/30) | TUG 2 (s) |
|---|---|---|---|---|---|
| Whole group | 84.54 ± 7.21 | 68.59 ± 17.66 | 1.62 ± 0.11 | 13.05 ± 7.63 | 30.13 ± 18.93 |
| Intermediate | 84.97 ± 7.55 | 70.4 ± 18.55 | 1.62 ± 0.11 | 14.29 ± 7.03 | 27.47 ± 17.26 |
| High | 85.04 ± 6.71 | 67.22 ± 15.1 | 1.62 ± 0.10 | 14.77 ± 7.29 | 35.53 ± 19.48 |
| Dementia unit | 81.53 ± 7.07 | 65.36 ± 11.79 | 1.63 ± 0.10 | 3.74 ± 5.04 | 26.01 ± 15.59 |
| Outcome | Level of Care | Number of Participants | Single Day ICC (95% CI) | Days Needed to Achieve ICC of 0.8 | |
|---|---|---|---|---|---|
| Volume | Total walk time | Whole group | 196 | 0.71 (0.65–0.75) | 2 |
| Dementia Unit | 24 | 0.63 (0.46–0.77) | 3 | ||
| High | 68 | 0.70 (0.61–0.77) | 2 | ||
| Intermediate | 104 | 0.67 (0.59–0.74) | 2 | ||
| Total steps | Whole group | 196 | 0.70 (0.65–0.75) | 2 | |
| Dementia Unit | 24 | 0.65 (0.49–0.79) | 3 | ||
| High | 68 | 0.69 (0.60–0.77) | 2 | ||
| Intermediate | 104 | 0.65 (0.57–0.72) | 3 | ||
| Total bouts | Whole group | 196 | 0.77 (0.73–0.81) | 2 | |
| Dementia Unit | 24 | 0.67 (0.51–0.80) | 2 | ||
| High | 68 | 0.74 (0.66–0.81) | 2 | ||
| Intermediate | 104 | 0.75 (0.68–0.80) | 2 | ||
| Pattern | Mean bout length | Whole group | 196 | 0.72 (0.68–0.77) | 2 |
| Dementia Unit | 24 | 0.76 (0.62–0.86) | 2 | ||
| High | 68 | 0.53 (0.43–0.64) | 4 | ||
| Intermediate | 104 | 0.56 (0.48–0.65) | 4 | ||
| Alpha | Whole group | 196 | 0.46 (0.40–0.53) | 5 | |
| Dementia Unit | 24 | 0.75 (0.60–0.85) | 2 | ||
| High | 68 | 0.43 (0.32–0.54) | 6 | ||
| Intermediate | 104 | 0.37 (0.29–0.47) | 7 | ||
| Variability | Variability | Whole group | 196 | 0.63 (0.57–0.68) | 3 |
| Dementia Unit | 24 | 0.80 (0.68–0.89) | 1 | ||
| High | 68 | 0.53 (0.42–0.63) | 4 | ||
| Intermediate | 104 | 0.49 (0.40–0.57) | 5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckley, C.; Cavadino, A.; Del Din, S.; Lord, S.; Taylor, L.; Rochester, L.; Kerse, N. Quantifying Reliable Walking Activity with a Wearable Device in Aged Residential Care: How Many Days Are Enough? Sensors 2020, 20, 6314. https://doi.org/10.3390/s20216314
Buckley C, Cavadino A, Del Din S, Lord S, Taylor L, Rochester L, Kerse N. Quantifying Reliable Walking Activity with a Wearable Device in Aged Residential Care: How Many Days Are Enough? Sensors. 2020; 20(21):6314. https://doi.org/10.3390/s20216314
Chicago/Turabian StyleBuckley, Christopher, Alana Cavadino, Silvia Del Din, Sue Lord, Lynne Taylor, Lynn Rochester, and Ngaire Kerse. 2020. "Quantifying Reliable Walking Activity with a Wearable Device in Aged Residential Care: How Many Days Are Enough?" Sensors 20, no. 21: 6314. https://doi.org/10.3390/s20216314







