Frontiers and Challenges in Electrochemical Corrosion Monitoring; Surface and Downhole Applications
Abstract
1. Introduction
2. Type of Corrosion
2.1. CO2 Corrosion (Sweet Corrosion)
2.2. H2S Corrosion (Sour Corrosion)
2.3. Oxygen Related Corrosion
2.4. Galvanic Corrosion
2.5. Crevice Corrosion
2.6. Erosion Corrosion
2.7. Microbial Corrosion
2.8. Naphthenic Acidic Corrosion
3. Methods of Corrosion Detection
4. Electrochemical Corrosion Monitoring of Various Substrates
4.1. Corrosion Sensor for Monitoring Corrosion in Natural Gas Transmission Pipeline, Atmosphere, CO2 and Marine Environment
4.2. Corrosion Sensor for Monitoring Corrosion in A Hot Environment
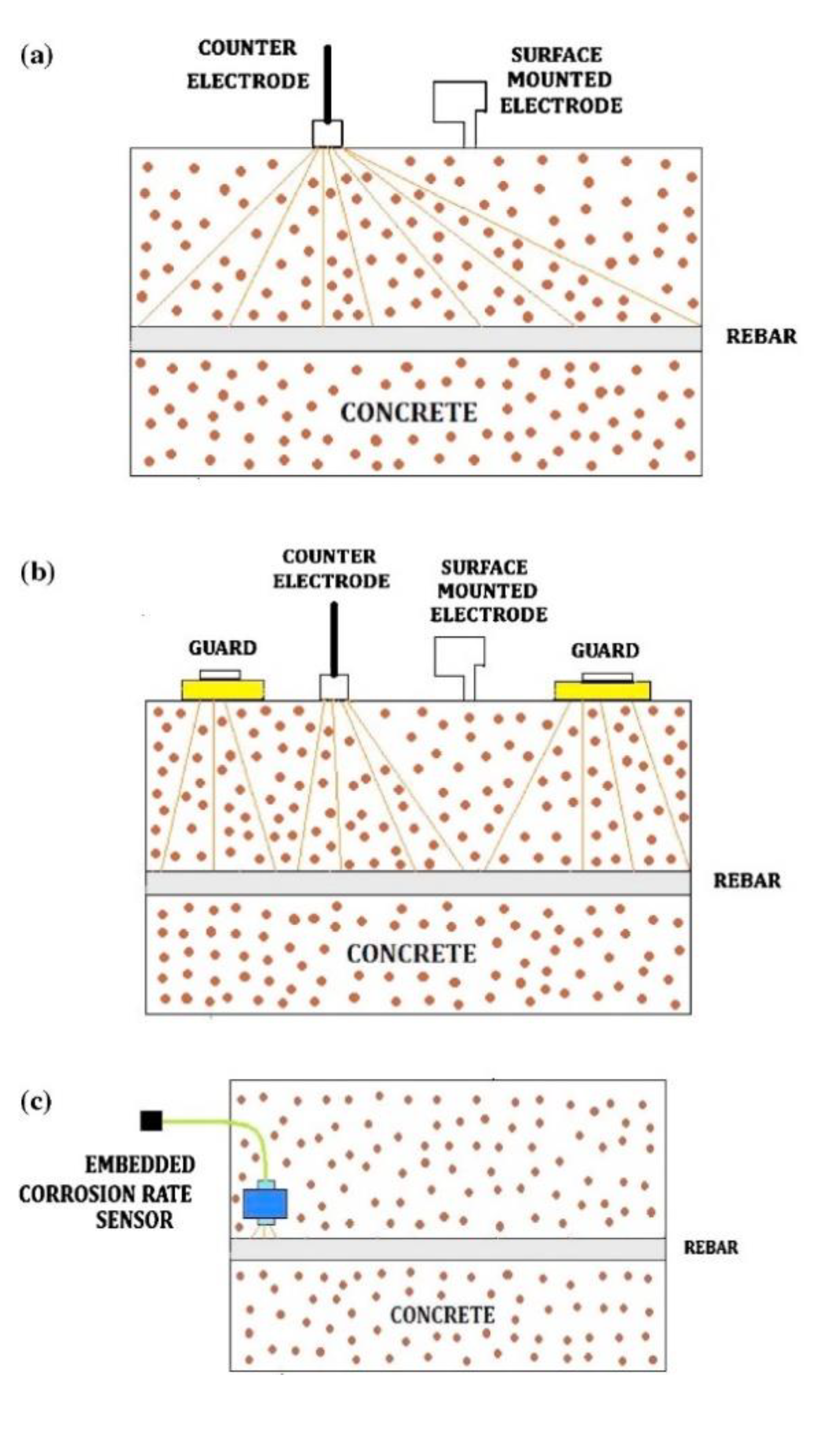
4.3. Corrosion Sensor for Monitoring Corrosion in Concrete
4.4. Corrosion Sensor orf Monitoring Corrosion in Another Environment
5. Existing Corrosion Monitoring Challenges in Surface and Downhole Conditions
5.1. Sub-Surface Temperature
5.2. Corrosive Chemical Environment
5.3. Distance
5.4. Data Acquisition
6. Outlook and Conclusions
- Development of effective and accurate corrosion sensor technology needs complete information in a downhole/surface/offshore environment, which causes various types of corrosions that can be retrieved by proper data, carry out modeling and so forth.
- Reliable and miniatured electrochemical sensors can be more effective in detecting the chemical environment, which results in corrosion, particularly in downhole and offshore conditions.
- Advanced wireless data acquisition tools can be integrated with electrochemical sensors to accomplish proper data collection, communication and alarm.
- More research on data mining and converting into real information related to corrosion under different environments.
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, L.H.; Kruger, J.; Parker, R.; Passaglia, E.; Reimann, E.; Ruff, A.; Yakowitz, H.; Berman, E. Economic Effects of Metallic Corrosion in the United States [Internet]. NBS Special Publication 511-1. 1978. Available online: https://www.osti.gov/biblio/6998680 (accessed on 3 October 2020).
- Ahmad, Z. (Ed.) Principles of Corrosion Engineering and Corrosion Control; Butterworth-Heinemann; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- NACE. Techniques for Monitoring Corrosion and Related Parameters in Field Ap2lications; NACE International: Houston, TX, USA, 1999. [Google Scholar]
- Mathiazhagan, A. Corrosion Management for Effective Mitigation of Corrosion in Ships Overview. In Proceedings of the 3rd International Conference on Information and Financial Engineering, Shanghai, China, 19–21 August 2011; IACSIT Press: Singapore, 2011. [Google Scholar]
- Rose, J.L.; Barshinger, J. Using Ultrasonic Guided Wave Mode Cutoff for Corrosion Detection and Classification. In Proceedings of the 1998 IEEE Ultrasonics Symposium. Proceedings (Cat. No. 98CH36102), Sendai, Japan, 5–8 October 1998; pp. 851–854. [Google Scholar]
- Pei, J.; Yousuf, M.; Degertekin, F.; Honein, B.; Khuri-Yakub, B. Lamb wave tomography and its application in pipe erosion/corrosion monitoring. J. Res. Nondestruct. Eval. 1996, 8, 189–197. [Google Scholar] [CrossRef]
- Shapiro, A.P.; Haskell, R.W. Method and Apparatus for Corrosion Sensing. U.S. Patent 6,628,111, 29 September 2003. [Google Scholar]
- Orazem, M. Underground Pipeline Corrosion; Woodhead Publishing, Sawston: Cambridge, UK, 2014. [Google Scholar]
- Wright, R.F.; Lu, P.; Devkota, J.; Lu, F.; Ziomek-Moroz, M.; Ohodnicki, P.R., Jr. Corrosion sensors for structural health monitoring of oil and natural gas infrastructure: A review. Sensors 2019, 19, 3964. [Google Scholar] [CrossRef] [PubMed]
- Strutt, J.; Nicholls, J.; Barbier, B. The prediction of corrosion by statistical analysis of corrosion profiles. Corros. Sci. 1985, 25, 305–315. [Google Scholar] [CrossRef]
- Yang, L.; Chiang, K.; Shukla, P.K.; Shiratori, N. Internal current effects on localized corrosion rate measurements using coupled multielectrode array sensors. Corrosion 2010, 66, 115005. [Google Scholar] [CrossRef]
- Roberge, P.R. Handbook of Corrosion Engineering; Mcgraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Kermani, M.; Smith, L. CO2 Corrosion Control in Oil and Gas Production Design Considerations; Institute of Materials: Leeds, UK, 1997; p. 688. [Google Scholar]
- Lusk, D.; Casserly, T.; Gupta, M.; Boinapally, K.; Cao, Y. Armoured against corrosion. Hydrocarb. Eng. 2008, 13, 11. [Google Scholar]
- National Association of Corrosion Engineers; American Petroleum Institute. Corrosion of Oil-and Gas-Well Equipment; Division of Production, American Petroleum Institute: Dallas, TX, USA, 1958. [Google Scholar]
- Brondel, D.; Edwards, R.; Hayman, A.; Hill, D.; Mehta, S.; Semerad, T. Corrosion in the oil industry. Oilfield Rev. 1994, 6, 4–18. [Google Scholar]
- Videm, K.; Dugstad, A. Film covered corrosion, film breakdown and pitting attack of carbon steels in aqueous CO2 environments. In K. Videm and A. Dugstad, Corrosion 88/186; Tx. Per Copy$ 4. 00; NACE International: Houston, TX, USA, 1988. [Google Scholar]
- Nalli, K. Corrosion and Its Mitigation in the Oil and Gas Industry. An Overview; PM-Pipeliner Report; Gewerbestrasse: Diepoldsau, Switzerland, 2010. [Google Scholar]
- Dugstad, A. The Importance of FeCO3 Supersaturation on the CO2 Corrosion of Carbon Steels, Corrosion; NACE: Houston, TX, USA, 1992. [Google Scholar]
- Gatzke, L.; Hausler, R.H. A Novel Correlation of Tubing Corrosion Rates in Deep, Hot Gas Wells with Water and Gas Production Rates. (Retroactive Coverage). Adv.CO2 Corros. 1984, 1, 87–102. [Google Scholar]
- Schmitt, G.; Horstemeier, M. Fundamental aspects of CO2 metal loss corrosion-Part II: Influence of different parameters on CO2 corrosion mechanisms. In Proceedings of the CORROSION 2006, San Diego, CA, USA, 12–16 March 2006; NACE International: Houston, TX, USA, 2006. [Google Scholar]
- Dunlop, A.; Hassell, H.; Rhodes, P. Fundamental considerations in sweet gas well corrosion. Corrosion 1983, 83, 461–4624. [Google Scholar]
- De Waard, C.; Lotz, U.; Milliams, D. Predictive model for CO2 corrosion engineering in wet natural gas pipelines. Corrosion 1991, 47, 976–985. [Google Scholar] [CrossRef]
- Nyborg, R. Overview of CO2 corrosion models for wells and pipelines. In Proceedings of the Corrosion 2002, Denver, Colorado, 7–11 April 2002; NACE International: Houston, TX, USA, 2002; p. 233. [Google Scholar]
- Kahyarian, A.; Brown, B.; Nešic, S. Technical Note: Electrochemistry of CO2 corrosion of mild steel: Effect of CO2 on cathodic currents. Corrosion 2018, 74, 851–859. [Google Scholar] [CrossRef]
- Kahyarian, A.; Achour, M.; Nesic, S. Mathematical modeling of uniform CO2 corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; Woodhead; Elsevier: Duxford, UK, 2017; pp. 805–849. [Google Scholar]
- Abid, K.; Gholami, R.; Choate, P.; Nagaratnam, B.H. A review on cement degradation under CO2-rich environment of sequestration projects. J. Nat. Gas Sci. Eng. 2015, 27, 1149–1157. [Google Scholar] [CrossRef]
- Bachu, S.; Bennion, D.B. Experimental assessment of brine and/or CO2 leakage through well cements at reservoir conditions. Int. J. Greenh. Gas Control 2009, 3, 494–501. [Google Scholar] [CrossRef]
- Jacquemet, N.; Pironon, J.; Lagneau, V.; Saint-Marc, J. Armouring of well cement in H2S–CO2 saturated brine by calcite coating–Experiments and numerical modelling. Appl. Geochem. 2012, 27, 782–795. [Google Scholar] [CrossRef]
- Ray, J.D.; Randall, B.; Parker, J. Use of reactive iron oxide to remove H2S from drilling fluid. J. Pet. Technol. 1979, 31, 797–801. [Google Scholar] [CrossRef]
- Chilingar, G.; Beeson, C. Surface Operations in Petroleum Production; Elsevier: New York, NY, USA, 1987; p. 1969. [Google Scholar]
- Martin, R.L. Corrosion Consequences of Oxygen Entry into Oilfield Brines; Corrosion’ 2002, Paper no. 270; NACE International: Denver, CO, USA, 2002. [Google Scholar]
- Snavely, E.S., Jr. Removal of Dissolved Oxygen from Water. US Patent 3,618,667, 19 November 1971. [Google Scholar]
- Marrese, M.; Guarino, V.; Ambrosio, L. Reference Module in Materials Science and Materials Engineering. In Microscopic Approach for Testing Mechanical Properties of Hard Tissues; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Martin, R. Use of Electrochemical Methods to Evaluate Corrosion Inhibitors under Laboratory and Field Condition; UMIST Conference of Electrochemical Techniques; IntechOpen Limited, 5 Princes Gate Court: London, UK, 1982; Volume 1982. [Google Scholar]
- Makhlouf, A.S.H.; Botello, M.A. Failure of the metallic structures due to microbiologically induced corrosion and the techniques for protection. In Handbook of Materials Failure Analysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–18. [Google Scholar]
- Hassani, S.; Roberts, K.; Shirazi, S.; Shadley, J.; Rybicki, E.; Joia, C. Flow loop study of NaCl concentration effect on erosion, corrosion, and erosion-corrosion of carbon steel in CO2-saturated systems. Corros. J. Sci. Eng. 2012, 68, 026001-1–026001-9. [Google Scholar] [CrossRef]
- Ajeel, S.A.; Ahmed, M.A. Study Synergy Effect on Erosion-Corrosion in Oil Pipes. Eng. Technol. J. 2008, 26, 1068–1080. [Google Scholar]
- Bertness, T. Reduction of failures caused by corrosion in pumping wells. API Dril Prod Pr. 1957, 37, 129–135. [Google Scholar]
- Ossai, C.I. Advances in asset management techniques: An overview of corrosion mechanisms and mitigation strategies for oil and gas pipelines. Isrn Corros. 2012, 570143. [Google Scholar] [CrossRef]
- Crawford, P.B. Possible reservoir damage from microbial enhanced oil recovery. In Proceedings of the 1982 International Conference on Microbial Enhancement of Oil Recovery, Afton, Oklahoma, 16–21 May 1982; pp. 76–79. [Google Scholar]
- Lazar, I.; Constantinescu, P. Field trials results of microbial enhanced oil recovery. Microbes Oil Recovery 1985, 1, 122–143. [Google Scholar]
- Singer, M. Microbial biosurfactants. Microbes Oil Recovery 1985, 1, 19–38. [Google Scholar]
- Silva, J.P.; de Senna, L.F.; do Lago, D.C.B.; da Silva, P.F., Jr.; Dias, E.G.; de Figueiredo, M.A.G.; Chiaro, S.S.X. Characterization of commercial ceramic adsorbents and its application on naphthenic acids removal of petroleum distillates. Mater. Res. 2007, 10, 219–225. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Yang, L. Techniques for Corrosion Monitoring; Woodhead Publishing, Boca Raton Boston: New York, NY, USA; Washington, DC, USA, 2008. [Google Scholar]
- Hilbert, L.R. Monitoring corrosion rates and localised corrosion in low conductivity water. Corros. Sci. 2006, 48, 3907–3923. [Google Scholar] [CrossRef]
- Cella, P.; Taylor, S. Electrical resistance changes as an alternate method for monitoring the corrosion of steel in concrete and mortar. Corrosion 2000, 56, 951–959. [Google Scholar] [CrossRef]
- Emmons, D.; Graham, G.; Holt, S.; Jordan, M.; Locardel, B. On-site, near-real-time monitoring of scale deposition. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999; Society of Petroleum Engineers: University Park, PA, USA, 1999. [Google Scholar]
- Jordan, M.M. Deployment of real-time scale deposition monitoring equipment to optimize chemical treatment for scale control during stimulation flowback. In Proceedings of the SPE International Oilfield Scale Conference, Aberdeen, UK, 28–29 May 2008; Society of Petroleum Engineers: University Park, PA, USA, 2008. [Google Scholar]
- Al-Matar, H.; Al-Ashhab, J.K.; Mokhtar, M.; Ridzauddin, S. Techniques used to monitor and remove strontium sulfate scale in UZ producing wells. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 5–8 November 2006; Society of Petroleum Engineers: University Park, PA, USA, 2006. [Google Scholar]
- Younes, H.; Al Ghaferi, A.; Saadat, I. Carbon nanostructure-based scale sensors using inkjet printing and casting techniques. In Advances in Carbon Nanostructures; IntechOpen Limited: London, UK, 2016. [Google Scholar]
- Wilson, A.; Vincent, P.; McMahon, P.; Muscat, R.; Hayes, J.; Solomon, M.; Barber, R.; McConnell, A. In A small, low-power, networked corrosion sensor suite. In Proceedings of the 2nd Asia-Pacific Workshop on Structural Health Monitoring, Corrosion, Melbourne, Australia, 2–4 December 2008. [Google Scholar]
- Broomfield, J.P. Corrosion of Steel in Concrete: Understanding, Investigation and Repair; E.&FN Spon Ltd.: London, UK, 1997. [Google Scholar]
- Gordon, G.A. Wireless Corrosion Sensor. U.S. Patent 11/834,255, 12 February 2009. [Google Scholar]
- Johnson, R.E.; Agarwala, V.S. The Use of Fluorescent Compounds and Complexes of Metals as Early Warning Detectors for Corrosion; NACE International: Houston, TX, USA, 1994. [Google Scholar]
- Johnson, R.E.; Agarwala, V.; Rajan, K.; Singh, A.; Durrett, M. Fluorescent Materials Utilized as Early Warning Sensors for Corrosion of Aluminum Alloy Surfaces; LeTourneau University: Longview, TX, USA, 1999. [Google Scholar]
- Sophocleous, M. Electrical resistivity sensing methods and implications. In Electrical Resistivity and Conductivity; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Figueira, R.B. Electrochemical Sensors for Monitoring the Corrosion Conditions of Reinforced Concrete Structures: A Review. Appl. Sci. 2017, 7, 1157. [Google Scholar] [CrossRef]
- Ropital, F. Environmental Degradation in Hydrocarbon Fuel Processing Plant: Issues and Mitigation. In Advances in Clean Hydrocarbon Fuel Processing; Woodhead Publishing: Philadephia, PA, USA, 2011; pp. 437–462. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Cottis, R.A. Electrochemical Noise for Corrosion Monitoring, in Techniques for Corrosion Monitoring; Woodhead Publishing, Boca Raton Boston: New York, NY, USA; Washington, DC, USA, 2008; pp. 86–110. [Google Scholar]
- Obot, I.B.; Onyeachu, I.B.; Zeino, A.; Umoren, S.A. Electrochemical noise (EN) technique: Review of recent practical applications to corrosion electrochemistry research. J. Adhes. Sci. Technol. 2019, 33, 1453–1496. [Google Scholar] [CrossRef]
- Fajardo, S.; García-Galvan, F.R.; Barranco, V.; Galvan, J.C.; Batlle, S.F. A critical review of the application of electrochemical techniques for studying corrosion of mg and mg alloys: Opportunities and challenges. In Magnesium Alloys—Selected Issue; Tański, T., Borek, W., Król, M., Eds.; Intech: Rijeka, Croatia, 2018; pp. 694–738. [Google Scholar]
- Covin, B.S., Jr.; Bullard, S.J.; Cramer, S.D.; Holcomb, G.R.; Ziomek-Moroz, M.; Kane, R.D.; Cayard, M.S.; Eden, D.C. Evaluation of the use of electrochemical noise corrosion sensors for natural gas transmission pipelines. In Proceedings of the Corrosion 2004, New Orleans, Louisiana, 28 March–1 April 2004; NACE International: Houston, TX, USA, 2004. [Google Scholar]
- Covino, B.S.; Cramer, S.D.; Bullard, S.J.; Holcomb, G.R.; Moroz, M.Z.; Kane, R.D.; Eden, D.C. Electrochemical Corrosion Rate Sensors for Detecting Internal Corrosion of Natural Gas Transmission Pipelines. In Proceedings of the Corrosion 2005, Houston, TX, USA, 3–7 April 2005; NACE International: Houston, TX, USA, 2005. [Google Scholar]
- Eden, D.A. Honeywell International Inc. Estimation of Localised Corrosion Penetration. U.S. Patent 7,520,975, 21 April 2009. [Google Scholar]
- Beck, J.; Hall, D.; Ziomek-Moroz, M.; Lvov, S. Membrane-coated electrochemical sensor for corrosion monitoring in natural gas pipelines. Sens. Transducers 2017, 214, 28. [Google Scholar]
- Shitanda, I.; Okumura, A.; Itagaki, M.; Watanabe, K.; Asano, Y. Screen-printed atmospheric corrosion monitoring sensor based on electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2009, 139, 292–297. [Google Scholar] [CrossRef]
- Nyrkova, L.; Polyakov, S.; Osadchuk, S.; Mel’nychuk, S.; Hapula, N. Determination of the rate of atmospheric corrosion of metal structures by the method of polarization resistance. Mater. Sci. 2012, 47, 683–688. [Google Scholar] [CrossRef]
- Xia, D.-H.; Ma, C.; Song, S.; Ma, L.; Wang, J.; Gao, Z.; Zhong, C.; Hu, W. Assessing atmospheric corrosion of metals by a novel electrochemical sensor combining with a thin insulating net using electrochemical noise technique. Sens. Actuators B Chem. 2017, 252, 353–358. [Google Scholar] [CrossRef]
- Ma, C.; Xia, D.-H.; ZHANG, Y.; Song, S.; Wang, J.; Gao, Z.; Hu, W. Sensing the instant corrosivity of haze using electrochemical probes by electrochemical noise technique. Electrochemistry 2017, 85, 784–789. [Google Scholar] [CrossRef]
- Aiba, A.; Fujii, N.; Hoshi, Y.; Shitanda, I.; Itagaki, M. Development of Printed-Type Corrosion Monitoring Sensor and Analysis of Electrochemical Impedance Response. J. Electrochem. Soc. 2018, 165, C743–C748. [Google Scholar] [CrossRef]
- Wang, S.; San, J.; Yu, J.; Lee, R.; Liu, N. A downhole CO2 sensor to monitor CO2 movement in situ for geologic carbon storage. Int. J. Greenh. Gas Control 2016, 55, 202–208. [Google Scholar] [CrossRef]
- Kahyarian, A.; Brown, B.; Nesic, S. Electrochemistry of CO2 corrosion of mild steel: Effect of CO2 on iron dissolution reaction. Corros. Sci. 2017, 129, 146–151. [Google Scholar] [CrossRef]
- Wright, R.F.; Brand, E.R.; Ziomek-Moroz, M.; Tylczak, J.H.; Ohodnicki, P.R., Jr. Effect of HCO3− on electrochemical kinetics of carbon steel corrosion in CO2-saturated brines. Electrochim. Acta 2018, 290, 626–638. [Google Scholar] [CrossRef]
- Zhao, J.; Xiong, D.; Gu, Y.; Zeng, Q.; Tian, B. A comparative study on the corrosion behaviors of X100 steel in simulated oilfield brines under the static and dynamic conditions. J. Pet. Sci. Eng. 2019, 173, 1109–1120. [Google Scholar] [CrossRef]
- Bierwagen, G.P.; Allahar, K.N.; Su, Q.; Gelling, V.J. Electrochemically characterizing the ac–dc–ac accelerated test method using embedded electrodes. Corros. Sci. 2009, 51, 95–101. [Google Scholar] [CrossRef]
- Klapper, H.S.; Laverde, D.; Vasquez, C. Evaluation of the corrosion of UNS G10200 steel in aerated brines under hydrodynamic conditions. Corros. Sci. 2008, 50, 2718–2723. [Google Scholar] [CrossRef]
- Zheng, H.; Liang, J.; Qin, Z.; Song, S.; Xu, L.; Gao, Z.; Hu, W.; Xia, D.-H. Identifying defect size in organic coatings by electrochemical noise, galvanostatic step and potentiostatic step techniques. J. Electroanal. Chem. 2020, 856, 113596. [Google Scholar] [CrossRef]
- An, Q.; Xin, Y.; Huo, K.; Cai, X.; Chu, P.K. Corrosion behavior of ZnO nanosheets on brass substrate in NaCl solutions. Mater. Chem. Phys. 2009, 115, 439–443. [Google Scholar] [CrossRef]
- Zhang, D.-L.; Wang, W.; Li, Y. An electrode array study of electrochemical inhomogeneity of zinc in zinc/steel couple during galvanic corrosion. Corros. Sci. 2010, 52, 1277–1284. [Google Scholar] [CrossRef]
- Carvalho, M.L.; Doma, J.; Sztyler, M.; Beech, I.; Cristiani, P. The study of marine corrosion of copper alloys in chlorinated condenser cooling circuits: The role of microbiological components. Bioelectrochemistry 2014, 97, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Neodo, S.; Wharton, J.; Cranny, A.; Harris, N.; Wood, R.; Stokes, K. Electrochemical detection of cupric ions with boron-doped diamond electrode for marine corrosion monitoring. Electrochim. Acta 2016, 202, 345–356. [Google Scholar] [CrossRef]
- Wu, J.; Yu, H.; Shi, X. Effectiveness of Products in Managing Metallic Corrosion Induced by Cyclic Deicer Exposure: Laboratory Study Using Multielectrode Array Sensors, Electrochemical Impedance, and Laser Profilometer. J. Mater. Civil Eng. 2015, 28, 04015186. [Google Scholar] [CrossRef]
- Covino, B.S., Jr.; Bullard, S.J.; Cramer, S.D.; Holcomb, G.R.; Ziomek-Moroz, M.; Cayard, M.S.; Eden, D.A. Electrochemical Corrosion Rate Probes for High Temperature Energy Applications; Albany Research Center (ARC): Albany, OR, USA, 2004. [Google Scholar]
- Covino, B.S., Jr.; Bullard, S.J.; Cramer, S.D.; Holcomb, G.R.; Ziomek-Moroz, M.; Kane, R.D.; Eden, D.C.; Eden, D.A. High temperature electrochemical corrosion rate probes for combustion environments. In Proceedings of the Corrosion 2004, New Orleans, Louisiana, 28 March–1 April 2004; NACE International: Houston, TX, USA, 2004. [Google Scholar]
- Chiang, K.; Yang, L.; Wei, R.; Coulter, K. Development of diamond-like carbon-coated electrodes for corrosion sensor applications at high temperatures. Thin Solid Film. 2008, 517, 1120–1124. [Google Scholar] [CrossRef]
- Mabbutt, S.; Simms, N.; Oakey, J. High temperature corrosion monitoring by electrochemical noise techniques. Corros. Eng. Sci. Technol. 2009, 44, 186–195. [Google Scholar] [CrossRef]
- Chiang, K.; Yang, L. High-temperature electrochemical sensor for online corrosion monitoring. Corrosion 2010, 66, 095002. [Google Scholar] [CrossRef]
- Aung, N.N.; Liu, X. High temperature electrochemical sensor for in situ monitoring of hot corrosion. Corros. Sci. 2012, 65, 1–4. [Google Scholar] [CrossRef]
- Aung, N.N.; Liu, X. Effect of SO2 in flue gas on coal ash hot corrosion of Inconel 740 alloy–A high temperature electrochemical sensor study. Corros. Sci. 2013, 76, 390–402. [Google Scholar] [CrossRef]
- Shi, J.-B.; Wang, J.-H.; Wang, K.; Xia, D.-H. Electrochemical Noise Study on the Corrosion Behavior of 304NG Stainless Steel in High Temperature Water. Electrochemistry 2014, 82, 647–653. [Google Scholar] [CrossRef]
- Muralidharan, S.; Saraswathy, V.; Madhavamayandi, A.; Thangavel, K.; Palaniswamy, N. Evaluation of embeddable potential sensor for corrosion monitoring in concrete structures. Electrochim. Acta 2008, 53, 7248–7254. [Google Scholar] [CrossRef]
- Colozza, N.; Sassolini, A.; Agosta, L.; Bonfanti, A.; Hermansson, K.; Arduini, F. A Paper-Based Potentiometric Sensor for Solid Samples:Corrosion Evaluation of Reinforcements Embedded in Concrete Structures as a Case Study. ChemElectroChem 2020, 7, 2274–2282. [Google Scholar] [CrossRef]
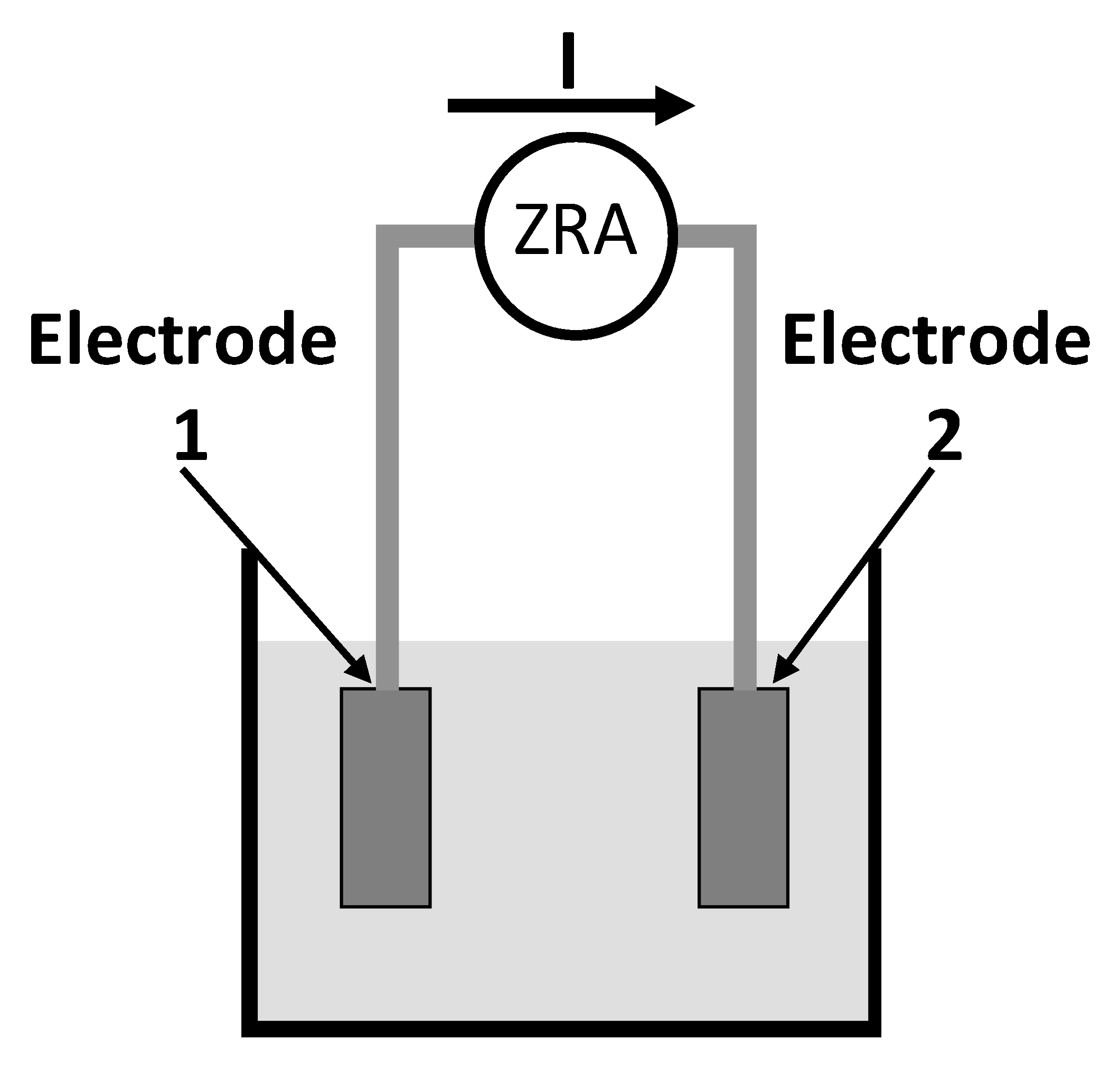
- Pereira, E.; Figueira, R.; Salta, M.M.; Da Fonseca, I.T. A galvanic sensor for monitoring the corrosion condition of the concrete reinforcing steel: Relationship between the galvanic and the corrosion currents. Sensors 2009, 9, 8391–8398. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Saraswathy, V.; Berchmans, L.J.; Thangavel, K.; Ann, K.Y. Nickel ferrite (NiFe2O4): A possible candidate material as reference electrode for corrosion monitoring of steel in concrete environments. Sens. Actuators B Chem. 2010, 145, 225–231. [Google Scholar] [CrossRef]
- Lu, S.; Ba, H.-J. Corrosion sensor for monitoring the service condition of chloride-contaminated cement mortar. Sensors 2010, 10, 4145–4158. [Google Scholar] [CrossRef]
- Qiao, G.; Sun, G.; Hong, Y.; Qiu, Y.; Ou, J. Remote corrosion monitoring of the RC structures using the electrochemical wireless energy-harvesting sensors and networks. NDT E Int. 2011, 44, 583–588. [Google Scholar] [CrossRef]
- Arup, H.; Klinghoffer, O.; Mietz, J. Long term performance of MnO2-reference electrodes in concrete. In Proceedings of the Corrosion97, New Orleans, Louisiana, 9–14 March 1997; NACE International: Houston, TX, USA, 1997. [Google Scholar]
- Muralidharan, S.; Ha, T.-H.; Bae, J.-H.; Ha, Y.-C.; Lee, H.-G.; Park, K.-W.; Kim, D.-K. Electrochemical studies on the solid embeddable reference sensors for corrosion monitoring in concrete structure. Mater. Lett. 2006, 60, 651–655. [Google Scholar] [CrossRef]
- Muralidharan, S.; Ha, T.H.; Bae, J.H.; Ha, Y.C.; Lee, H.G.; Kim, D.K. A promising potential embeddable sensor for corrosion monitoring application in concrete structures. Measurement 2007, 40, 600–606. [Google Scholar] [CrossRef]
- Muralidharan, S.; Saraswathy, V.; Thangavel, K.; Palaniswamy, N. Electrochemical studies on the performance characteristics of alkaline solid embeddable sensor for concrete environments. Sens. Actuators B Chem. 2008, 130, 864–870. [Google Scholar] [CrossRef]
- Castro, P.; Sagues, A.; Moreno, E.; Maldonado, L.; Genescá, J. Characterization of activated titanium solid reference electrodes for corrosion testing of steel in concrete. Corrosion 1996, 52, 609–617. [Google Scholar] [CrossRef]
- Duffó, G.; Farina, S.; Giordano, C. Characterization of solid embeddable reference electrodes for corrosion monitoring in reinforced concrete structures. Electrochim. Acta 2009, 54, 1010–1020. [Google Scholar] [CrossRef]
- Qiao, G.; Hong, Y.; Song, G.; Li, H.; Ou, J. Electrochemical characterization of the solid-state reference electrode based on NiFe2O4 film for the corrosion monitoring of RC structures. Sens. Actuators B Chem. 2012, 168, 172–177. [Google Scholar] [CrossRef]
- Karthick, S.; Muralidharan, S.; Saraswathy, V.; Thangavel, K. Long-term relative performance of embedded sensor and surface mounted electrode for corrosion monitoring of steel in concrete structures. Sens. Actuators B Chem. 2014, 192, 303–309. [Google Scholar] [CrossRef]
- Kursten, B.; Druyts, F.; Areias, L.; van Ingelgem, Y.; De Wilde, D.; Nieubourg, G.; Duffó, G.S.; Bataillon, C. Preliminary results of corrosion monitoring studies of carbon steel overpack exposed to supercontainer concrete buffer. Corros. Eng. Sci. Technol. 2014, 49, 485–491. [Google Scholar] [CrossRef]
- Sassolini, A.; Colozza, N.; Papa, E.; Hermansson, K.; Cacciotti, I.; Arduini, F. Screen-printed electrode as a cost-effective and miniaturized analytical tool for corrosion monitoring of reinforced concrete. Electrochem. Commun. 2019, 98, 69–72. [Google Scholar] [CrossRef]
- Davis, G.D.; Dacres, C.M.; Krebs, L.A. In-situ corrosion sensor for coating, testing and screening. Mater. Perform. 2000, 39, 2. [Google Scholar]
- Choi, Y.; Kim, J.; Yang, S. A Galvanic Sensor for Monitoring the Corrosion Damage of Buried Pipelines: Part 2—Correlation of Sensor Output to Actual Corrosion Damage of Pipeline in Soil and Tap Water Environments. Corrosion 2006, 62, 522–532. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Guan, H.; Mahajanam, S.; Wong, F. Active corrosion protection and corrosion sensing in chromate-free organic coatings. Prog. Org. Coat. 2003, 47, 174–182. [Google Scholar] [CrossRef]
- Kuhlmann, J.; Witte, F.; Heineman, W.R. Electrochemical sensing of dissolved hydrogen in aqueous solutions as a tool to monitor magnesium alloy corrosion. Electroanalysis 2013, 25, 1105–1110. [Google Scholar] [CrossRef]
- Elsener, B.; Alter, M.; Lombardo, T.; Ledergerber, M.; Wörle, M.; Cocco, F.; Fantauzzi, M.; Palomba, S.; Rossi, A. A non-destructive in-situ approach to monitor corrosion inside historical brass wind instruments. Microchem. J. 2016, 124, 757–764. [Google Scholar] [CrossRef]
- Barat, B.R.; Cano, E.; Letardi, P. Advances in the design of a gel-cell electrochemical sensor for corrosion measurements on metallic cultural heritage. Sens. Actuators B Chem. 2018, 261, 572–580. [Google Scholar] [CrossRef]
- Xia, D.; Song, S.; Gong, W.; Jiang, Y.; Gao, Z.; Wang, J. Detection of corrosion-induced metal release from tinplate cans using a novel electrochemical sensor and inductively coupled plasma mass spectrometer. J. Food Eng. 2012, 113, 11–18. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.-h.; Wang, H.-h.; Fu, C.-w.; Xia, D.-h.; Zheng, X.; Dang, L.-h.; Shi, J.-b. Corrosion detection of tinplate cans containing coffee using EIS/EN sensor. J. Cent. South Univ. 2014, 21, 76–82. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Su, M.; Xia, D.-H.; Wu, Z.; Qin, Z.; Xu, L.; Fan, H.-Q.; Hu, W. Development of an electrochemical sensor and measuring the shelf life of tinplate cans. Measurement 2019, 134, 500–508. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Zhao, X.-H.; Wang, Y.-G.; Mo, N. Development of an electrochemical in situ detection sensor for grounding grid corrosion. Corrosion 2010, 66, 076001. [Google Scholar] [CrossRef]
- Nazir, M.; Saeed, A.; Khan, Z.A. Electrochemical corrosion failure analysis of large complex engineering structures by using micro-LPR sensors. Sens. Actuators B Chem. 2018, 268, 232–244. [Google Scholar] [CrossRef]
- Caines, S.; Khan, F.; Zhang, Y.; Shirokoff, J. Simplified electrochemical potential noise method to predict corrosion and corrosion rate. J. Loss Prev. Process Ind. 2017, 47, 72–84. [Google Scholar] [CrossRef]
- Schmitt, G. Listen to corrosion at work—A newly developed versatile corrosion monitoring tool ready for plant application. Mater. Corros. 2007, 58, 924–939. [Google Scholar] [CrossRef]
- Craig, B. Deep oil and gas well construction. In Advanced Materials & Processes; ASM International: Cleveland, OH, USA, 2008. [Google Scholar]
- Richter, S.; Achour, M.; Addis, K.; Singer, M.; Nesic, S. Development and Application of a Downhole Corrosion Prediction Model. In Proceedings of the NACE International Corrosion Conference Proceedings, Vancouver, BC, Canada, 6–10 March 2016; NACE International: Houston, TX, USA, 2016; p. 1. [Google Scholar]
- Fang, H. Low Temperature and High Salt Concentration Effects on General CO2 Corrosion for Carbon Steel. Ph.D. Dissertation, Ohio University, Athens, OH, USA, 2006. [Google Scholar]
- Chandran, A.; Liu, Y.; Monteiro, O.; McClain Scott, T.; Liu, Z.; Krueger, S. Asset Optimization Using Downhole Corrosion Sensor for Electrical Submersible Pumps. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 13–16 November 2017; Society of Petroleum Engineers: University Park, PA, USA, 2017. [Google Scholar]
- Feng, R.; Beck, J.R.; Hall, D.M.; Buyuksagis, A.; Ziomek-Moroz, M.; Lvov, S.N. Effects of CO2 and H2S on Corrosion of Martensitic Steels in Brines at Low Temperature. Corrosion 2017, 74, 276–287. [Google Scholar] [CrossRef]
- Roberson, M.W.; Goodwin, S.; Donderici, B.; Wilson, G.A.; Rodney, P.F.; Shah, V.V.; Roddy, C.W.; Ravi, K.M. Methods and Apparatus for Evaluating Downhole Conditions through Fluid Sensing. U.S. Patent 9,879,519, 30 January 2018. [Google Scholar]
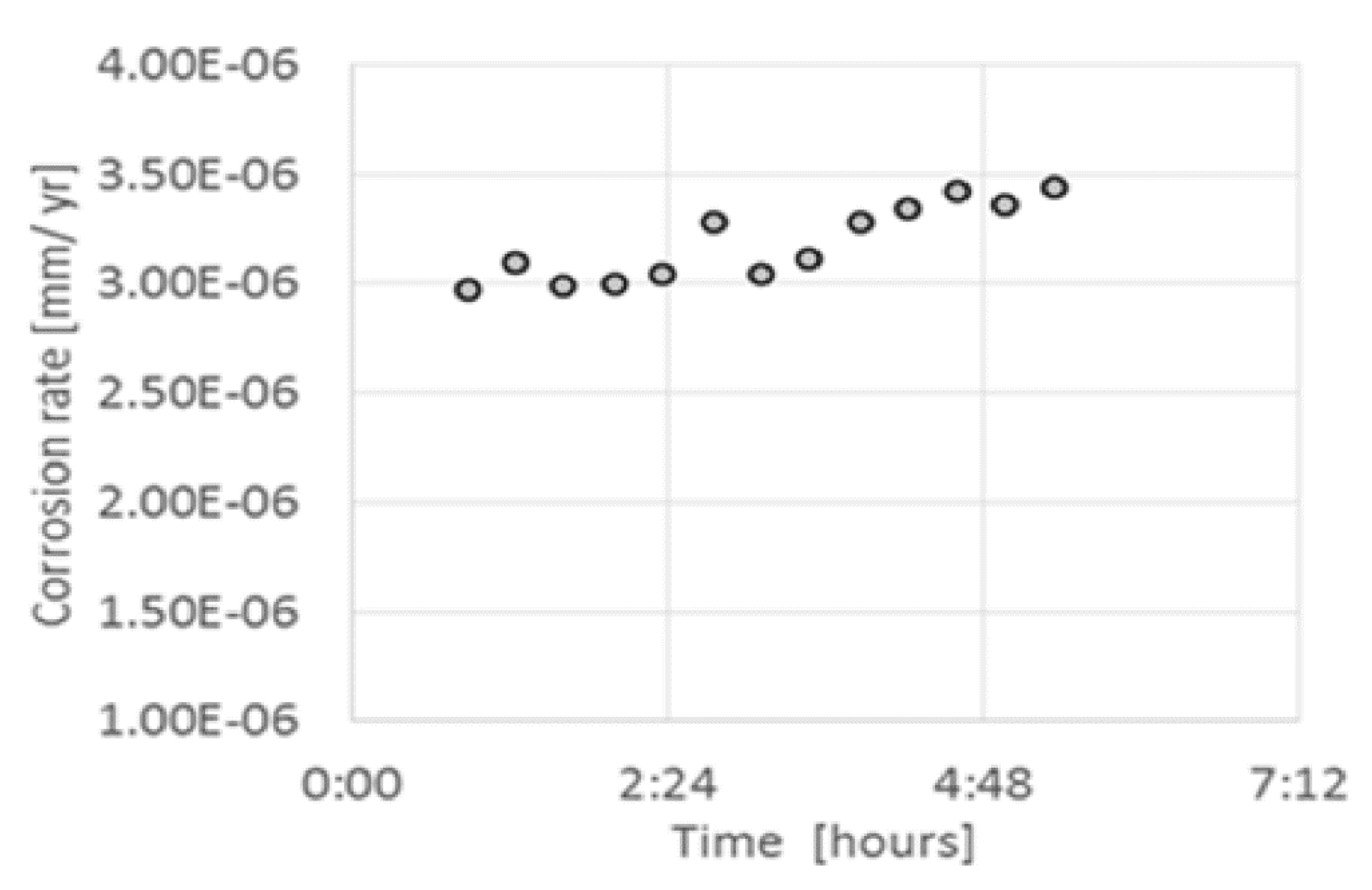
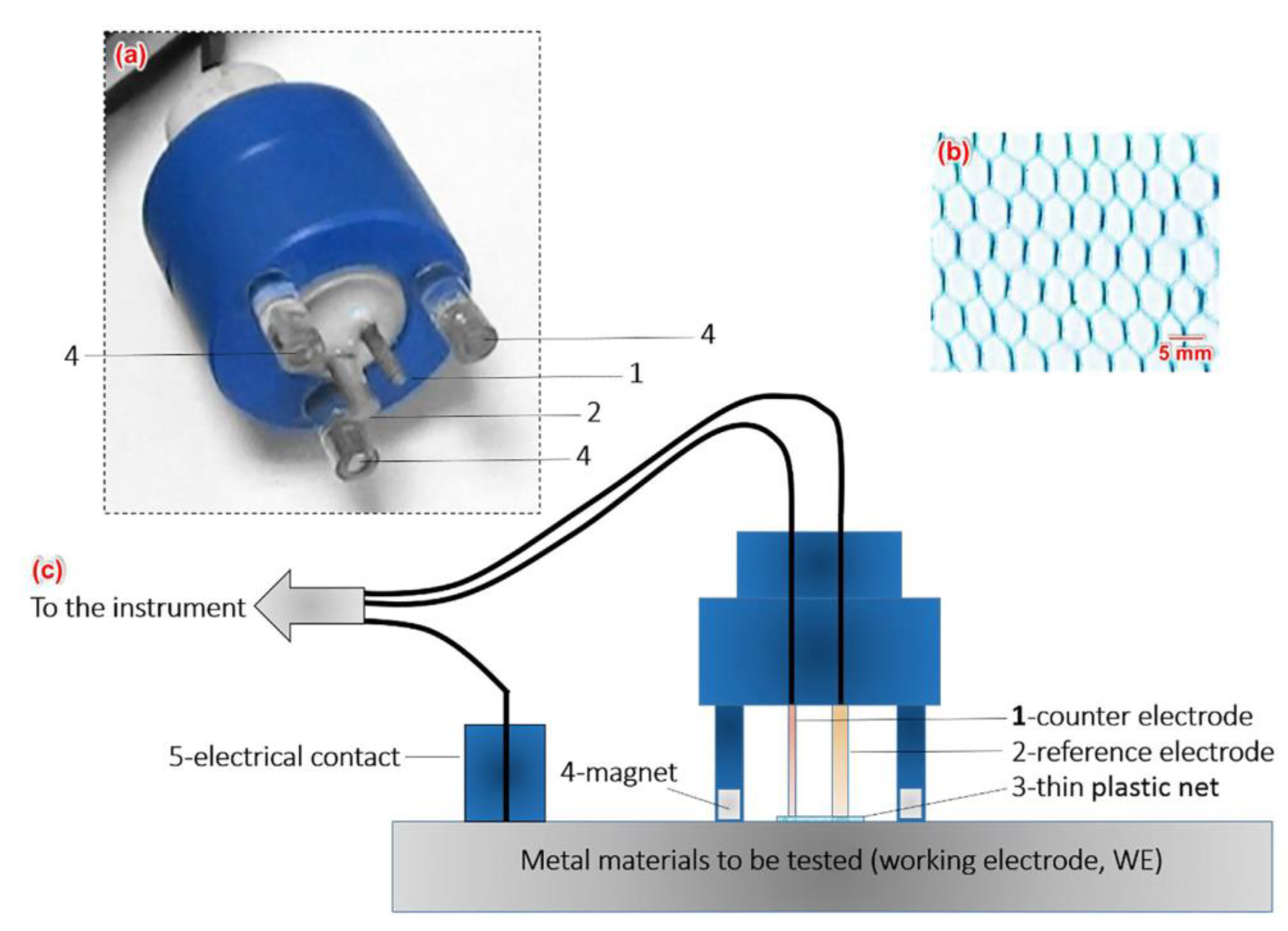
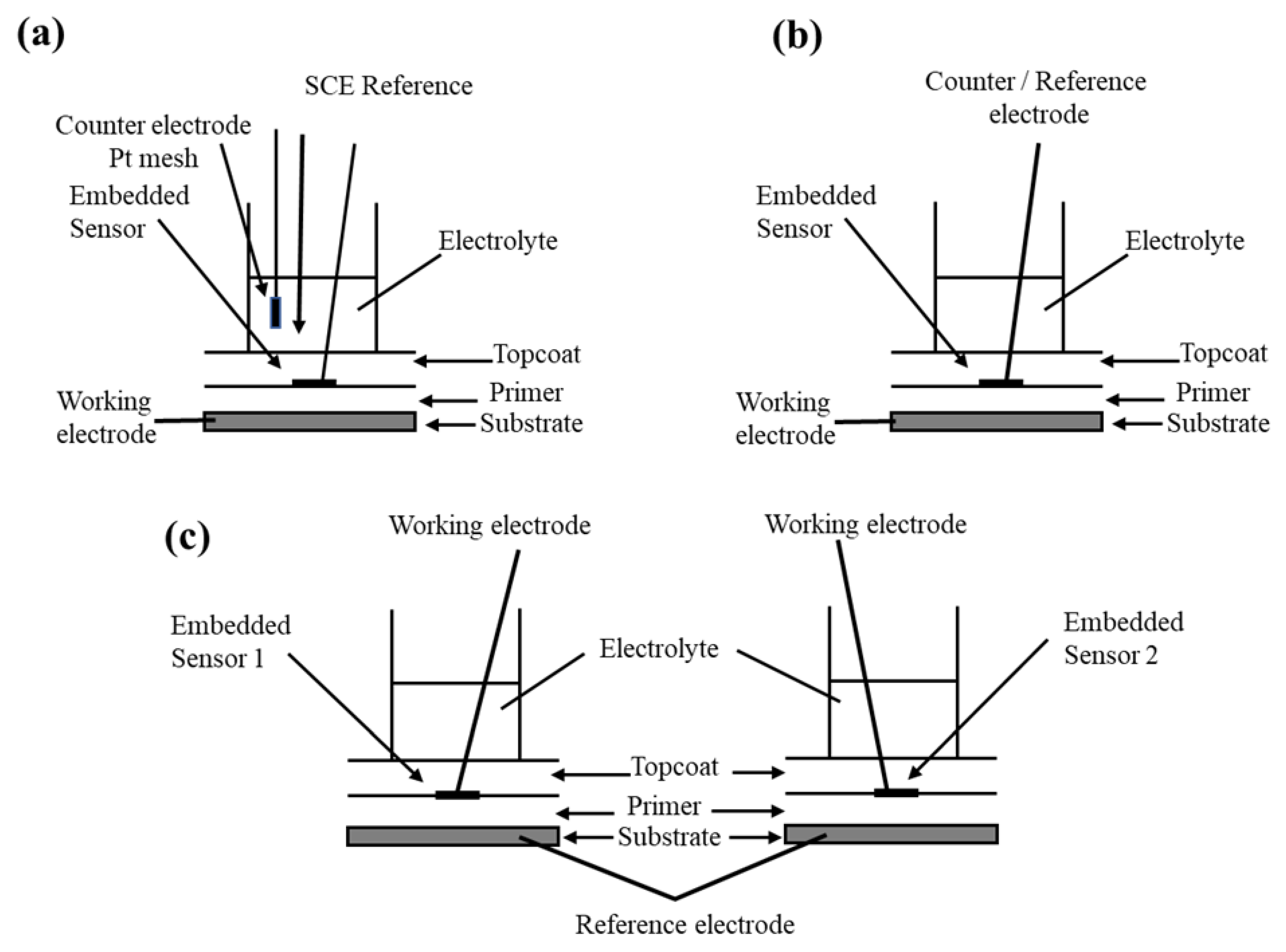

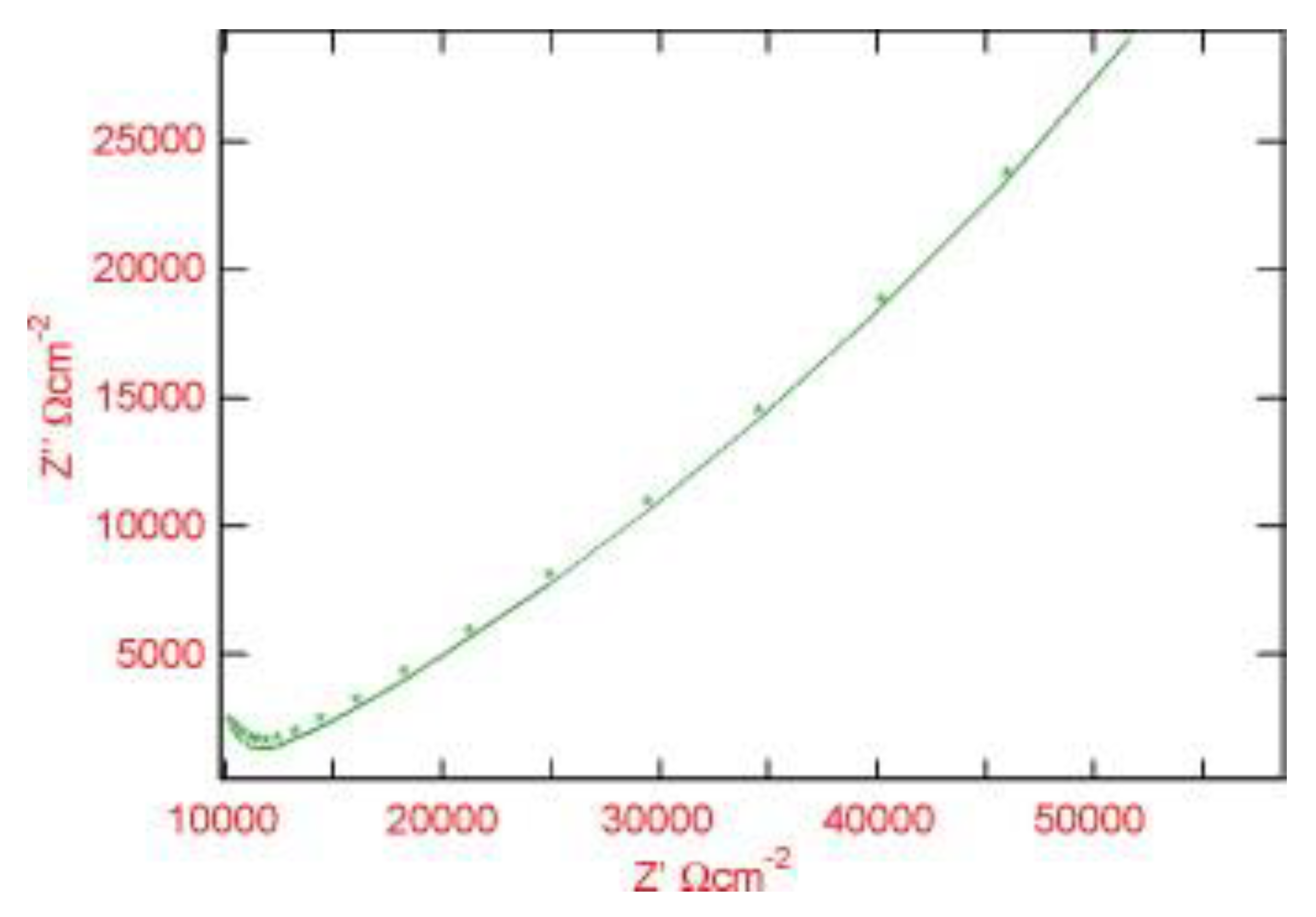


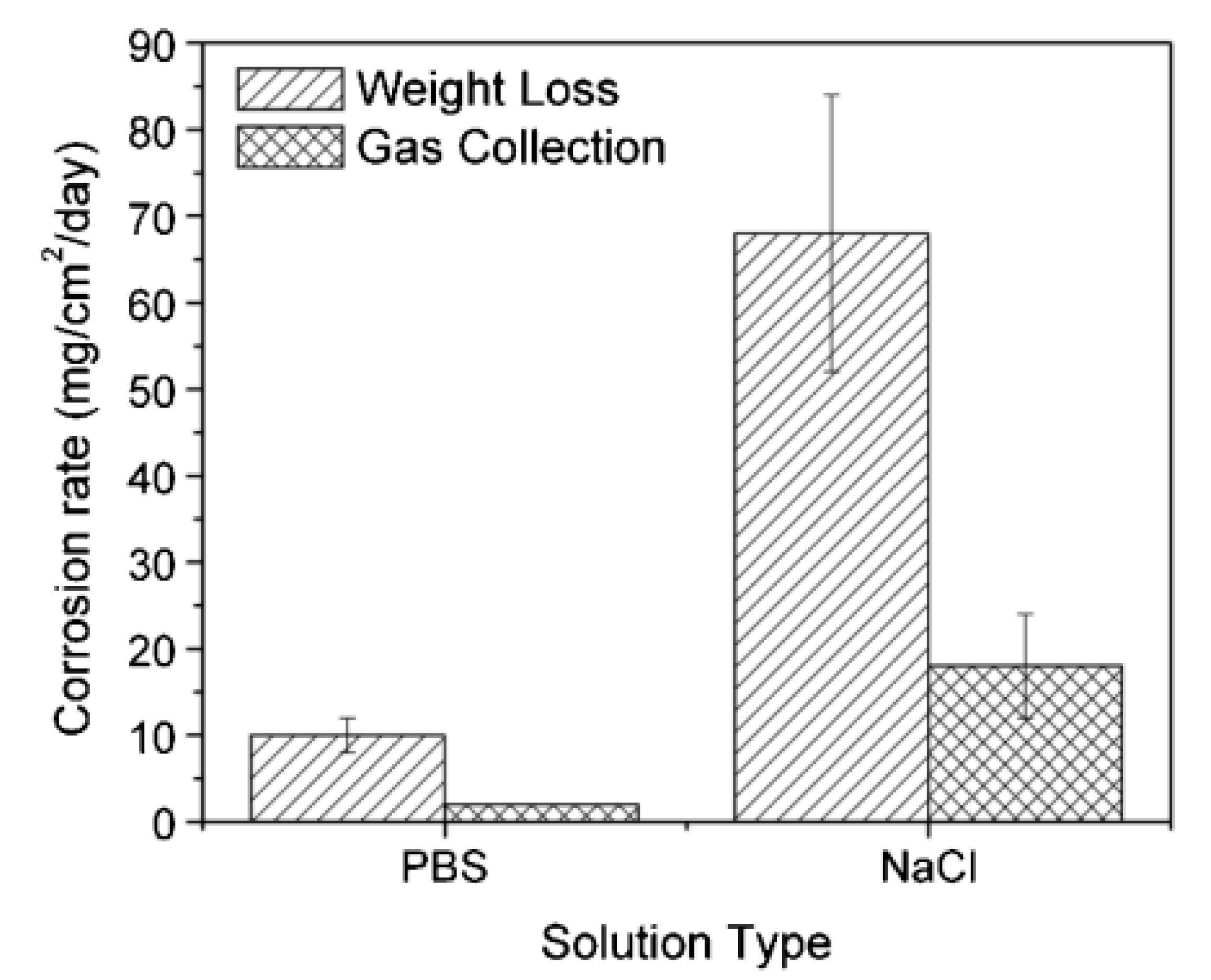
| T (°C) | w (rpm) | RpDC (k Ωcm2) | RpAC (k Ωcm2) | βC (mV) | βA (mV) | B (mV) | icorr (μA/cm2) |
|---|---|---|---|---|---|---|---|
| 25 | 0 | 0.9141 | 0.8123 | 24.30 | 43.98 | 6.81 | 7.44 |
| 500 | 0.2127 | 0.2085 | 54.49 | 146.79 | 17.28 | 81.23 | |
| 1250 | 0.1241 | 0.1245 | 54.12 | 123.35 | 163.35 | 131.79 | |
| 2000 | 0.1179 | 0.1057 | 56.76 | 111.54 | 16.36 | 138.72 | |
| 35 | 0 | 0.6502 | 0.6315 | 24.38 | 41.83 | 6.70 | 10.30 |
| 500 | 0.1388 | 0.1326 | 47.60 | 185.64 | 16.47 | 118.67 | |
| 1250 | 0.0759 | 0.0759 | 53.28 | 98.61 | 15.04 | 198.15 | |
| 2000 | 0.0691 | 0.0706 | 58.62 | 141.11 | 18.01 | 260.59 | |
| 45 | 0 | 0.6166 | 0.5921 | 24.20 | 47.68 | 6.98 | 11.32 |
| 500 | 0.1143 | 0.1257 | 47.83 | 160.91 | 16.03 | 140.25 | |
| 1250 | 0.0712 | 0.0715 | 54.01 | 127.90 | 16.51 | 231.89 | |
| 2000 | 0.0618 | 0.0575 | 52.96 | 153.04 | 17.11 | 276.80 |
| Working Electrode | Electrochemical Techniques | Sample | Corrosion Sensor/Behavior/Casing Agents/Others | Publication Year | Ref. |
|---|---|---|---|---|---|
| A106 pipeline steel in the form of flange probes | EN | Natural gas transmission pipelines | Corrosion | 2004 | [65] |
| A106 pipeline steel | EN, LPR, HDA | Natural gas transmission pipelines | Corrosion sensor | 2005 | [66] |
| Carbon steel electrode | LPR, EIS | Carbon steel in Natural Gas Pipelines | Corrosion | 2017 | [68] |
| - | EIS | circuit board | atmospheric corrosion monitoring | 2009 | [69] |
| Metal material to be tested | Rp | St.3 steel | Atmospheric corrosion | 2012 | [70] |
| Metal material to be tested | EN | atmospheric corrosion of metals | Corrosion | 2017 | [71] |
| Q235B and T91 steels | EN | atmospheric corrosivity of Q235B and T91 steels | Sensing the Instant Corrosivity of Haze | 2017 | [72] |
| Screen Printed-Type Ag | EIS | Screen printed type Ag | Corrosion monitoring | 2018 | [73] |
| Ir/IrOx | Potentiometric | CO2 detection in downhole (high temperature and high pressure) | CO2 sensor | 2016 | [74] |
| A disk-shaped API 5L X65 mild steel | Anodic polarization | CO2 corrosion of mild steel | Corrosion | 2017 | [75] |
| X65 carbon steel | LPR | X65 carbon steel (It is noted that X65 carbon steel is commonly used for transmission pipeline handling oil and natural gas) | Corrosion sensor | 2018 | [76] |
| X100 steel | EIS, OCP and PDP | X100 steel in simulated oilfield brines under the static and dynamic conditions | Corrosion behavior | 2019 | [77] |
| Substrate of interest | EIS/EN | Aircraft and vehicular structures Protected by organic coatings in 3.5% NaCl solution | corrosion | 2008 | [78] |
| UNS G10200 steel | LPR, EIS, PDP | Corrosion of UNS G10200 steel in aerated brines under hydrodynamic conditions | Corrosion | 2008 | [79] |
| Galvanized Q235 carbon steel | OCP, EN, EIS and potentiostatic step | Galvanized Q235 carbon steel with a size of 40 × 20 cm covered by a layer of high solid epoxy resin coating with an average thickness of ~500 μm. | Corrosion sensor | 2020 | [80] |
| ZnO nanosheets on a brass substrate | PDP and OCP | ZnO nanosheets on brass substrate | Corrosion behavior | 2009 | [81] |
| Electrode array composed of zinc and mild steel wire | OCP | Zinc/steel couple immersed in seawater | Electrochemical inhomogeneity of zinc in zinc/steel couple during galvanic corrosion | 2010 | [82] |
| - | LPR | Marine corrosion of copper alloys | Corrosion | 2014 | [83] |
| BDD | Voltammetry | Copper alloys in chloride background electrolyte | Corrosion monitoring | 2016 | [84] |
| 1008 carbon steel, 304 stainless steel and 1100 aluminum | Multielectrode array sensors | 1008 carbon steel, 304 stainless steel and 1100 aluminum in 2.3% NaCl and 3.0% MgCl2 | Corrosion sensor | 2016 | [85] |
| Working Electrode | Electrochemical Techniques | Sample | Corrosion Sensor/Behavior/Casing Agents/Others | Publication Year | Ref. |
|---|---|---|---|---|---|
| Mild Steel, 304L SS, 316L SS probes | EN, LPR and HDA | The probe in mixed gas environment (O2, N2, H2O and CO2) in high temperature (e.g., Inside of boiler/coal combustor) | Corrosion sensor | 2004 | [86] |
| Low carbon steel | EN, LPR and HDA | Steel either in an air/H2O or a mixed gas environment identical to a waste to energy (WTE) Environment | High-temperature corrosion | 2004 | [87] |
| Diamond-like carbon-coated Alloy 22 (Ni-22Cr-13Mo-3Fe-3W) electrodes | Based on the current measured from the most anodic electrode | DLC-coated Alloy 22 in a solution saturated with NaCl–NaNO3–KNO3 | corrosion | 2008 | [88] |
| Metal material (either superheater/reheater or waterwall) to be tested | EN | High-temperature corrosion monitoring | corrosion | 2009 | [89] |
| Diamond-like carbon-coated Alloy 22 (Ni-22Cr-13Mo-3Fe-3W) and Titanium Grade 7 (Ti-0.2Pd) electrodes | Anodic current (Multielectrode Array Sensors) | online, real-time corrosion monitoring of Diamond-like carbon-coated Alloy 22 (Ni-22Cr-13Mo-3Fe-3W) and Titanium Grade 7 (Ti-0.2Pd) at high temperatures. | corrosion | 2010 | [90] |
| Ni-based superalloy (inconel alloy 740) | EN, EIS and PDP | Ni-based superalloy in the presence of a synthetic coal ash and a synthetic flue gas containing sulfur dioxide | corrosion | 2012 | [91] |
| Inconel 740 alloy | EN, EIS, PDP | Inconel 740 superalloy | Corrosion sensor | 2013 | [92] |
| 304NG Stainless Steel | EN | 304NG Stainless Steel in high-temperature Water | Corrosion | 2014 | [93] |
| Solution | System | E1/2 (mV vs. SCE) | Rct (×104 Ω cm2) | Cdl (×10−5 Fcm−2) |
|---|---|---|---|---|
| SCS | S0 | 205 | 2.010 | 1.620 |
| S1 | 206 | 1.475 | 1.777 | |
| S2 | 201 | 1.424 | 1.729 | |
| S3 | 204 | 1.039 | 1.588 | |
| CPS | S0 | 200 | 1.585 | 1.966 |
| S1 | 201 | 1.432 | 1.440 | |
| S2 | 206 | 1.099 | 1.078 | |
| S3 | 206 | 0.937 | 1.537 | |
| CE | S0 | 204 | 1.311 | 1.189 |
| S1 | 208 | 1.260 | 1.949 | |
| S2 | 202 | 1.248 | 1.010 | |
| S3 | 200 | 0.867 | 1.355 |
| Sensor Number | OCP (mV vs. SCE) | Rct (Ω cm2) | Cdl (Fcm−2) |
|---|---|---|---|
| Saturated calcium hydroxide solution | |||
| S1 | −293 | 2.168 × 102 | 1.111 × 10−3 |
| S2 | −290 | 3.718 × 102 | 1.482 × 10−3 |
| S3 | −309 | 4.545 × 102 | 1.176 × 10−3 |
| S4 | −307 | 2.388 × 102 | 3.256 × 10−3 |
| S5 | −295 | 2.736 × 102 | 2.111 × 10−3 |
| S6 | −294 | 2.651 × 102 | 4.017 × 10−3 |
| Average value with standard deviation | −299.5 ± 9.5 | ||
| Concrete pore solution | |||
| S1 | −291 | 5.811 × 102 | 1.566 × 10−3 |
| S2 | −302 | 3.391 × 102 | 1.148 × 10−3 |
| S3 | −305 | 6.064 × 102 | 1.673 × 10−3 |
| S4 | −309 | 1.852 × 102 | 1.850 × 10−3 |
| S5 | −308 | 1.846 × 102 | 1.013 × 10−3 |
| S6 | −308 | 1.833 × 102 | 1.288 × 10−3 |
| Average value with standard deviation | −300.0 ± 9.0 | ||
| Cement extracts | |||
| S1 | −302 | 2.588 × 102 | 1.674 × 10−3 |
| S2 | −306 | 5.187 × 102 | 1.144 × 10−3 |
| S3 | −301 | 5.422 × 102 | 1.611 × 10−3 |
| S4 | −301 | 2.596 × 102 | 2.116 × 10−3 |
| S5 | −302 | 2.239 × 102 | 2.980 × 10−3 |
| S6 | −303 | 2.069 × 102 | 1.774 × 10−3 |
| Average value with standard deviation | −303.5 ± 2.0 |
| Working Electrode | Electrochemical Techniques | Sample | Corrosion Sensor/Behavior/Casing Agents/Others | Publication Year | Ref. |
|---|---|---|---|---|---|
| Steel | PDP | Corrosion monitoring in concrete structures | Corrosion | 2008 | [94] |
| Metallic bar | Potentiometric measurements | Reinforced concrete artefacts | Corrosion | 2020 | [95] |
| Carbon steel | Galvanic and potentiostatic pulse method | Concrete reinforcing steel in saturated Ca(OH)2 aqueous solutions | Corrosion sensor | 2009 | [96] |
| NiFe2O4 reference electrodes as the working electrode | PDP, EIS | steel in concrete environments | Corrosion | 2010 | [97] |
| Steel | LPR, EIS | Chloride-Contaminated Cement Mortar | Corrosion | 2010 | [98] |
| Q235 steel | Active monitoring techniques (AMTs) and passive monitoring techniques. AMT covers EIS, HA, transient galvanostatic/potentiostatic decay, potential dynamic scan, LPR and coulostatic method | Reinforcing concrete structures | Remote corrosion monitoring | 2011 | [99] |
| Solid-state reference electrode containing NiFe2O4 film | Polarization, EIS | RC structure | Corrosion sensor | 2012 | [106] |
| Steel | PDP, EIS | Corrosion monitoring of steel in concrete structures | Corrosion | 2014 | [107] |
| A carbon steel plate | LPR, ESI | carbon steel overpack exposed to super container concrete buffer | Corrosion | 2014 | [108] |
| Iron-reinforced concrete specimens | Potentiometric measurements | Iron reinforced concrete | Corrosion | 2019 | [109] |
| Working Electrode | Electrochemical Techniques | Sample | Corrosion Sensor/Behavior/Casing Agents/Others | Publication Year | Ref. |
|---|---|---|---|---|---|
| Materials of interest | Based on EIS | Coating materials deterioration and substrate corrosion | Corrosion | 2000 | [110] |
| Carbon steel | Galvanic | Steel Pipelines | Corrosion sensor | 2006 | [111] |
| Aluminum alloy substrate. | EIS | Aluminum alloy substrate | Corrosion Sensor | 2003 | [112] |
| Pt disc electrode | Potentiometric | Magnesium Alloy Corrosion in Aqueous Solutions | Magnesium alloy corrosion in aqueous solutions | 2013 | [113] |
| Tuning slides of different brass instruments | OCP and LPR | Historical brass wind instruments | Corrosion Sensor | 2016 | [114] |
| Stainless steel and Bronze | EIS | metallic cultural heritage | Corrosion Sensor | 2018 | [115] |
| Tinplate cans | EIS and EN | tinplate cans | Corrosion | 2012 | [116] |
| Tinplate cans | EIS/EN | tinplate cans containing coffee | Corrosion | 2014 | [117] |
| Tinplate cans | OCP, (EIS and potentiostatic step techniques | Four lacquered tinplate cans (65 mm diameter X 90 mm high) provided by the ORG Can making Company (China) were used as the investigated objectives | Corrosion sensor | 2019 | [118] |
| Carbon steel | Potential time curve | Grounding grid | Corrosion sensor | 2010 | [119] |
| Material of structure | LPR | large complex engineering structures | Electrochemical corrosion failure | 2018 | [120] |
| carbon steel electrodes | EN | Carbon steel | corrosion | 2017 | [121] |
| Materials of metal (e.g., steel, copper, magnesium, etc.) | EN | metals (e.g., steel, copper, magnesium) in diverse media | corrosion | 2007 | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Qurashi, A.; Badeghaish, W.; Noui-Mehidi, M.N.; Aziz, M.A. Frontiers and Challenges in Electrochemical Corrosion Monitoring; Surface and Downhole Applications. Sensors 2020, 20, 6583. https://doi.org/10.3390/s20226583
Khan A, Qurashi A, Badeghaish W, Noui-Mehidi MN, Aziz MA. Frontiers and Challenges in Electrochemical Corrosion Monitoring; Surface and Downhole Applications. Sensors. 2020; 20(22):6583. https://doi.org/10.3390/s20226583
Chicago/Turabian StyleKhan, Abuzar, Ahsanulhaq Qurashi, Wael Badeghaish, Mohamed N. Noui-Mehidi, and Md. Abdul Aziz. 2020. "Frontiers and Challenges in Electrochemical Corrosion Monitoring; Surface and Downhole Applications" Sensors 20, no. 22: 6583. https://doi.org/10.3390/s20226583
APA StyleKhan, A., Qurashi, A., Badeghaish, W., Noui-Mehidi, M. N., & Aziz, M. A. (2020). Frontiers and Challenges in Electrochemical Corrosion Monitoring; Surface and Downhole Applications. Sensors, 20(22), 6583. https://doi.org/10.3390/s20226583






