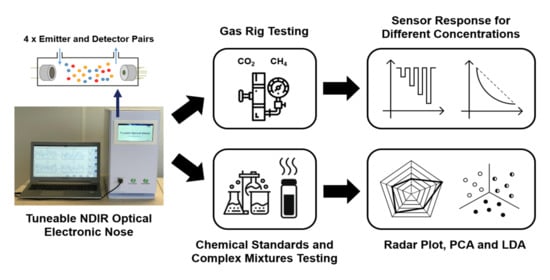
Development of a Tuneable NDIR Optical Electronic Nose
Abstract
:1. Introduction
2. Materials and Methods
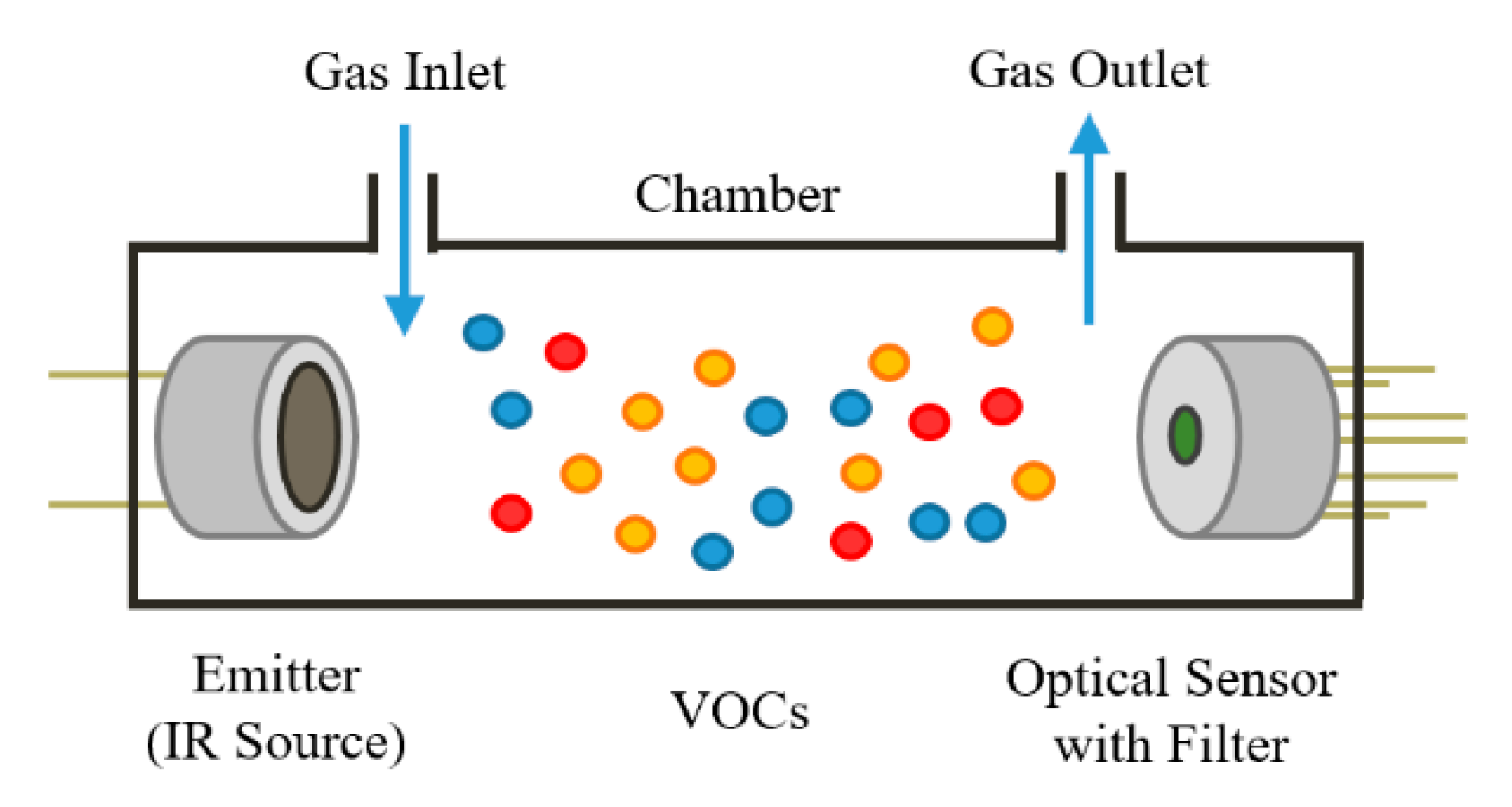
2.1. Optical Gas Sensors
2.2. Sensor Chamber Design and Heating
2.3. Electronic Design and Software
2.4. Mechanical Design
3. Results
3.1. Gas Rig Testing
3.2. Simple and Complex Chemical Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of electronic nose as a non-invasive technique for odor fingerprinting and detection of bacterial foodborne pathogens: A review. J. Food Sci. Technol. 2020, 57, 1977–1990. [Google Scholar] [CrossRef]
- Persaud, K. Electronic Noses and Tongues in the Food Industry. In Electronic Noses and Tongues in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–12. ISBN 978-0-12-800243-8. [Google Scholar]
- Wilson, A.D. Review of Electronic-nose Technologies and Algorithms to Detect Hazardous Chemicals in the Environment. Procedia Technol. 2012, 1, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.D.; Baietto, M. Advances in Electronic-Nose Technologies Developed for Biomedical Applications. Sensors 2011, 11, 1105–1176. [Google Scholar] [CrossRef] [PubMed]
- Mansurova, M.; Ebert, B.E.; Blank, L.M.; Ibáñez, A.J. A breath of information: The volatilome. Curr. Genet. 2018, 64, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Schubert, J. From highly sophisticated analytical techniques to life-saving diagnostics: Technical developments in breath analysis. TrAC Trends Anal. Chem. 2006, 25, 665–673. [Google Scholar] [CrossRef]
- Dospinescu, V.-M.; Tiele, A.; Covington, J.A. Sniffing Out Urinary Tract Infection—Diagnosis Based on Volatile Organic Compounds and Smell Profile. Biosensors 2020, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Madrolle, S.; Grangeat, P.; Jutten, C. A Linear-Quadratic Model for the Quantification of a Mixture of Two Diluted Gases with a Single Metal Oxide Sensor. Sensors 2018, 18, 1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persaud, K.C. Polymers for chemical sensing. Mater. Today 2005, 8, 38–44. [Google Scholar] [CrossRef]
- Berna, A. Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis. Sensors 2010, 10, 3882–3910. [Google Scholar] [CrossRef] [Green Version]
- Covington, J.; Westenbrink, E.; Ouaret, N.; Harbord, R.; Bailey, C.; O’Connell, N.; Cullis, J.; Williams, N.; Nwokolo, C.; Bardhan, K.; et al. Application of a Novel Tool for Diagnosing Bile Acid Diarrhoea. Sensors 2013, 13, 11899–11912. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Santos, J.; Lozano, J. Use of Electronic Noses for Diagnosis of Digestive and Respiratory Diseases through the Breath. Biosensors 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.D. Applications of Electronic-Nose Technologies for Noninvasive Early Detection of Plant, Animal and Human Diseases. Chemosensors 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Abidin, M.Z.; Asmat, A.; Hamidon, M.N. Comparative Study of Drift Compensation Methods for Environmental Gas Sensors. IOP Conf. Ser. Earth Environ. Sci. 2018, 117, 012031. [Google Scholar] [CrossRef]
- Wilson, A. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Wilson, A.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Korotcenkov, G. Comparative Analysis of Gas Sensors. In Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications Volume 1: Conventional Approaches; Integrated Analytical Systems; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-7164-6. [Google Scholar]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, T.; Yonemura, S.; Tani, A. Comparison of the Characteristics of Small Commercial NDIR CO2 Sensor Models and Development of a Portable CO2 Measurement Device. Sensors 2012, 12, 3641–3655. [Google Scholar] [CrossRef] [Green Version]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic Noses for Environmental Monitoring Applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef] [PubMed]
- Farraia, M.V.; Cavaleiro Rufo, J.; Paciência, I.; Mendes, F.; Delgado, L.; Moreira, A. The electronic nose technology in clinical diagnosis: A systematic review. Porto Biomed. J. 2019, 4, e42. [Google Scholar] [CrossRef]
- Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic Noses in Medical Diagnostics. Curr. Med. Chem. 2019, 26, 197–215. [Google Scholar] [CrossRef]
- García-Orellana, C.J.; Macías-Macías, M.; González-Velasco, H.M.; García-Manso, A.; Gallardo-Caballero, R. Low-Power and Low-Cost Environmental IoT Electronic Nose Using Initial Action Period Measurements. Sensors 2019, 19, 3183. [Google Scholar] [CrossRef] [Green Version]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic Nose as a Tool for Monitoring the Authenticity of Food. A Review. Food Anal. Methods 2017, 10, 1800–1816. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, S. Electronic Nose Instrumentation for Biomedical Applications. Ph.D. Thesis, University of Warwick, Coventry, UK, 2018. [Google Scholar]
- Pan, D.D.; Wu, Z.; Peng, T.; Zeng, X.Q.; Li, H. Volatile organic compounds profile during milk fermentation by Lactobacillus pentosus and correlations between volatiles flavor and carbohydrate metabolism. J. Dairy Sci. 2014, 97, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563. [Google Scholar] [CrossRef] [PubMed]
- Baetz, W.; Kroll, A.; Bonow, G. Mobile robots with active IR-optical sensing for remote gas detection and source localization. In Proceedings of the 2009 IEEE International Conference on Robotics and Automation, Kobe, Japan, 12–17 May 2009; pp. 2773–2778. [Google Scholar]
- Neumann, N. Tunable infrared detector with integrated micromachined Fabry-Perot filter. J. MicroNanolithogr. MEMS MOEMS 2008, 7, 021004. [Google Scholar] [CrossRef]
- Intelligent Opto Sensors. In Smart Sensors and MEMS; Yurish, S.Y.; Gomes, M.T.S.R. (Eds.) NATO Science Series; Springer: Dordrecht, The Netherlands, 2004; Volume 181, ISBN 978-1-4020-2928-8. [Google Scholar]
- Hariharan, P. The Fabry–Perot Interferometer. In Basics of Interferometry; Elsevier: Amsterdam, The Netherlands, 1992; pp. 37–43. ISBN 978-0-08-091861-7. [Google Scholar]
- InfraTec FPI Detectors—Pyroelectric Detectors with Spectrometer Functionality; InfraTec GmbH: Dresden, Germany, 2015; pp. 1–8.
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Persaud, K.C. Electronic gas and odour detectors that mimic chemoreception in animals. TrAC Trends Anal. Chem. 1992, 11, 61–67. [Google Scholar] [CrossRef]
- Honeycutt, W.T.; Ley, M.T.; Materer, N.F. Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors. Sensors 2019, 19, 3157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Huttmann, S.E.; Windisch, W.; Storre, J.H. Techniques for the Measurement and Monitoring of Carbon Dioxide in the Blood. Ann. Am. Thorac. Soc. 2014, 11, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Patra, A.; Kutty, V.; Venkatesan, T. Critical Review of Volatile Organic Compound Analysis in Breath and In Vitro Cell Culture for Detection of Lung Cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trefz, P.; Obermeier, J.; Lehbrink, R.; Schubert, J.K.; Miekisch, W.; Fischer, D.-C. Exhaled volatile substances in children suffering from type 1 diabetes mellitus: Results from a cross-sectional study. Sci. Rep. 2019, 9, 15707. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perdoni, F.; Infantino, V.; Faliva, M.A.; Peroni, G.; Iannello, G.; Nichetti, M.; Alalwan, T.A.; Perna, S.; Cocuzza, C. Volatile Organic Compounds as Biomarkers of Gastrointestinal Diseases and Nutritional Status. J. Anal. Methods Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wlodzimirow, K.A.; Abu-Hanna, A.; Schultz, M.J.; Maas, M.A.W.; Bos, L.D.J.; Sterk, P.J.; Knobel, H.H.; Soers, R.J.T.; Chamuleau, R.A.F.M. Exhaled breath analysis with electronic nose technology for detection of acute liver failure in rats. Biosens. Bioelectron. 2014, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Hauschild, A.-C.; Fijten, R.R.R.; Dallinga, J.W.; Baumbach, J.; van Schooten, F.J. Current breathomics—A review on data pre-processing techniques and machine learning in metabolomics breath analysis. J. Breath Res. 2014, 8, 027105. [Google Scholar] [CrossRef] [PubMed]
- Pénard-Morand, C.; Annesi-Maesano, I. Air pollution: From sources of emissions to health effects. Breathe 2004, 1, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Mečiarová, Ľ.; Vilčeková, S.; Krídlová Burdová, E.; Kiselák, J. Factors Effecting the Total Volatile Organic Compound (TVOC) Concentrations in Slovak Households. Int. J. Environ. Res. Public. Health 2017, 14, 1443. [Google Scholar] [CrossRef] [Green Version]
- Pobkrut, T.; Siyang, S.; Thepodum, T.; Kerdcharoen, T. The Applications of Portable Electronic Nose for Indoor and Outdoor Air Quality Assessments. In Proceedings of the 2019 IEEE International Conference on Consumer Electronics—Taiwan (ICCE-TW), Yilan, Taiwan, 20–22 May 2019; pp. 1–2. [Google Scholar]
- Coronel Teixeira, R.; Rodríguez, M.; Jiménez de Romero, N.; Bruins, M.; Gómez, R.; Yntema, J.B.; Chaparro Abente, G.; Gerritsen, J.W.; Wiegerinck, W.; Pérez Bejerano, D.; et al. The potential of a portable, point-of-care electronic nose to diagnose tuberculosis. J. Infect. 2017, 75, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Durán, C.; Reyes, A. Electronic Nose for Quality Control of Colombian Coffee through the Detection of Defects in “Cup Tests”. Sensors 2009, 10, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, Y.; Wang, T. Design of a six-gas NDIR gas sensor using an integrated optical gas chamber. Opt. Express 2020, 28, 11451. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.V. Medical Internet of Things and Big Data in Healthcare. Healthc. Inform. Res. 2016, 22, 156. [Google Scholar] [CrossRef]
- Tiele, A.; Wicaksono, A.; Ayyala, S.K.; Covington, J.A. Development of a Compact, IoT-Enabled Electronic Nose for Breath Analysis. Electronics 2020, 9, 84. [Google Scholar] [CrossRef] [Green Version]













| Manufacturer | Type | Optical Wavelength |
|---|---|---|
| InfraTec | LFP-3144C-337 | 3.1–4.4 μm |
| InfraTec | LFP-3850C-337 | 3.8–5.0 μm |
| InfraTec | LFP-5580C-337 | 5.5–8.0 μm |
| InfraTec | LFP-80105C-337 | 8.0–10.5 μm |
| Gas Chamber Length | Differential Voltage Sensor Response |
|---|---|
| 10 cm | 55.4 mV |
| 20 cm | 77.9 mV |
| 30 cm | 103.3 mV |
| 40 cm | 58.0 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esfahani, S.; Tiele, A.; Agbroko, S.O.; Covington, J.A. Development of a Tuneable NDIR Optical Electronic Nose. Sensors 2020, 20, 6875. https://doi.org/10.3390/s20236875
Esfahani S, Tiele A, Agbroko SO, Covington JA. Development of a Tuneable NDIR Optical Electronic Nose. Sensors. 2020; 20(23):6875. https://doi.org/10.3390/s20236875
Chicago/Turabian StyleEsfahani, Siavash, Akira Tiele, Samuel O. Agbroko, and James A. Covington. 2020. "Development of a Tuneable NDIR Optical Electronic Nose" Sensors 20, no. 23: 6875. https://doi.org/10.3390/s20236875