Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Instrumentation
2.2. Synthesis of carbon nanodots (C-dots)
2.3. Synthesis of carbon nanodots and copper oxide nanocomposite (CuO-C-dots)
2.4. Preparation of the CuO-C-dots Nanocomposite Modified Electrode
2.5. Electrochemical Measurement of Glucose
3. Results and Discussion
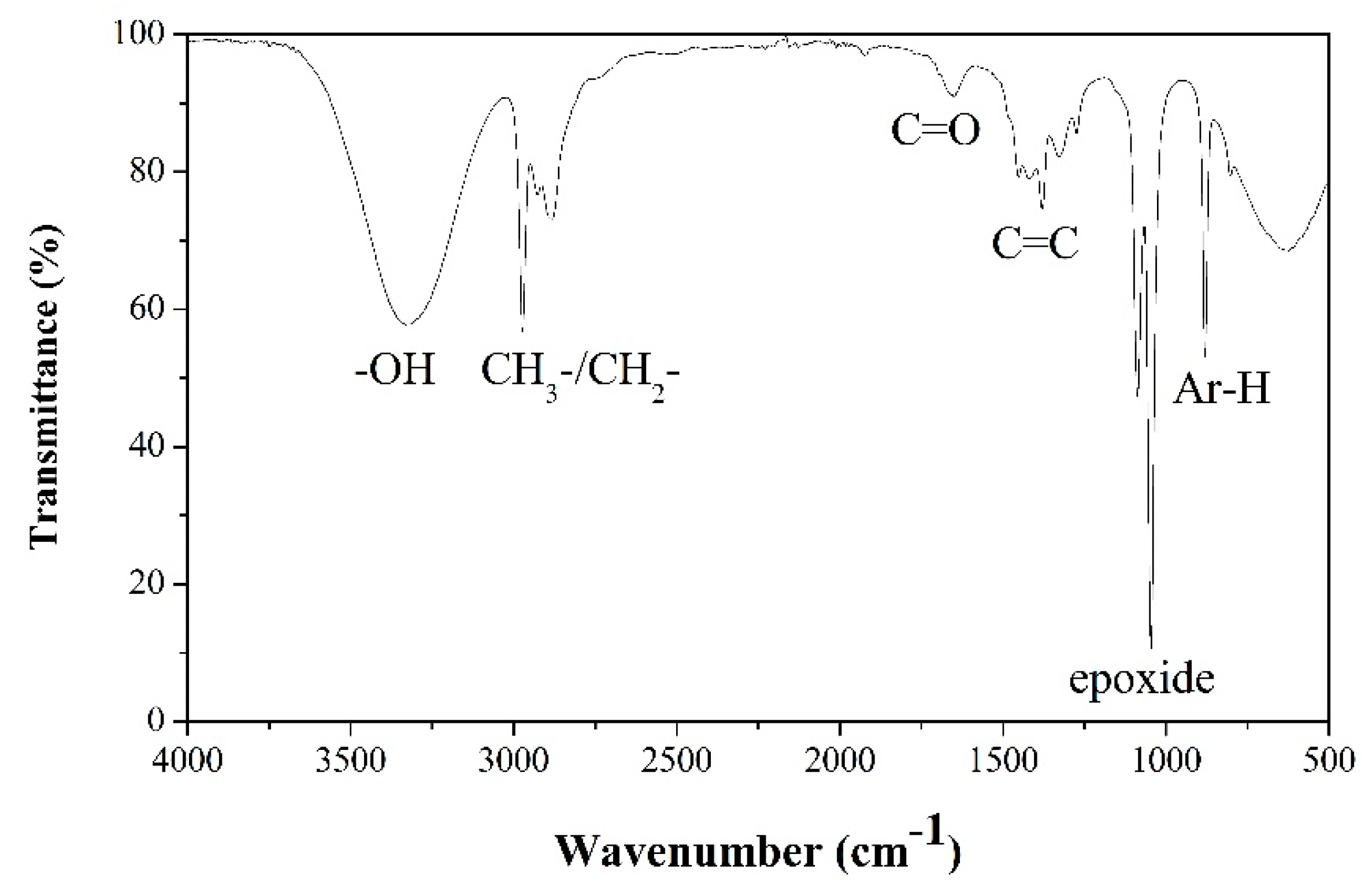
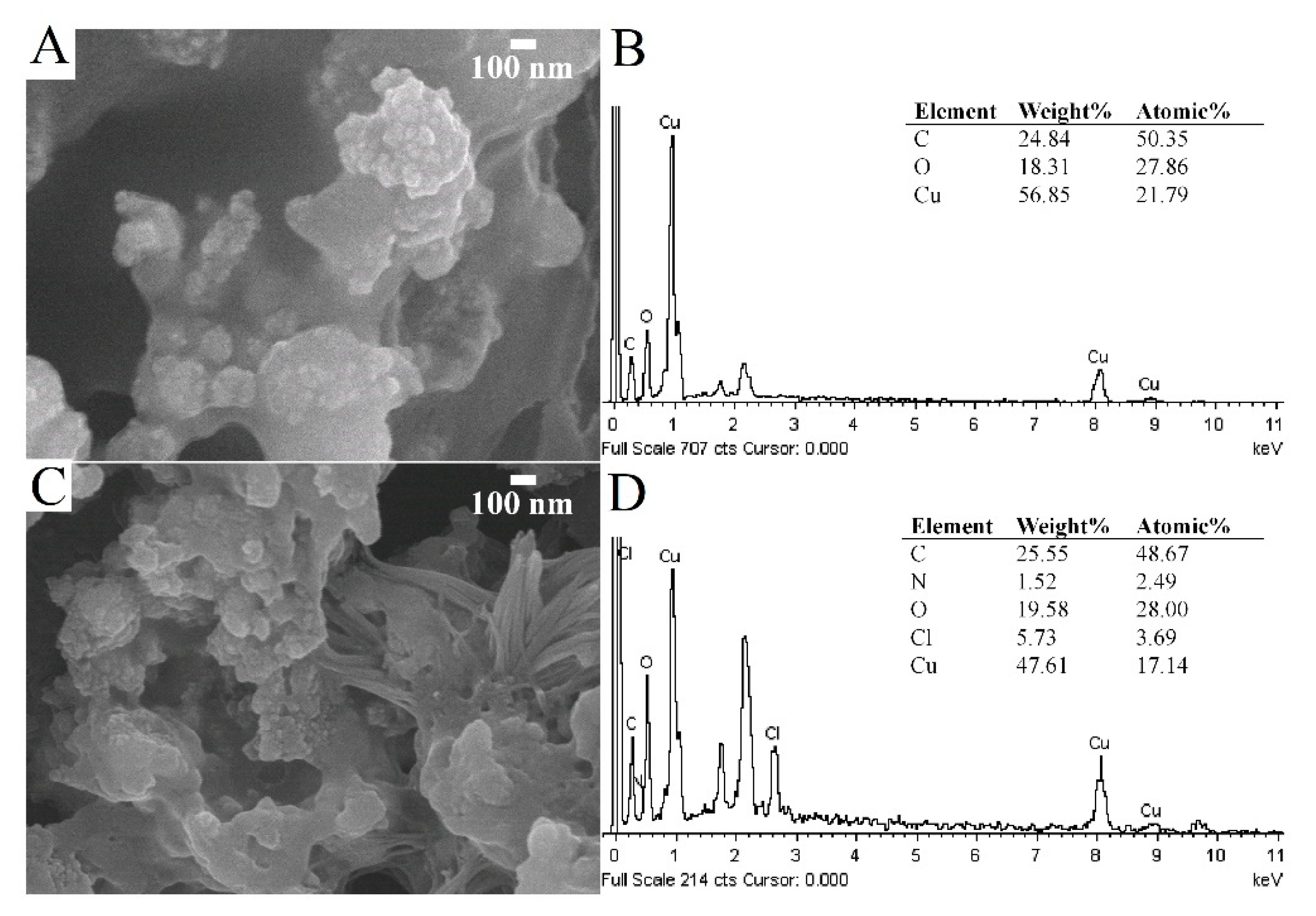
3.1. Characterizations of C-dots and CuO-C-dots
3.2. Electrochemical Characterization of the Proposed Electrode
3.3. Effect of CuO-C-dots Modified on Screen-printed Carbon Electrode
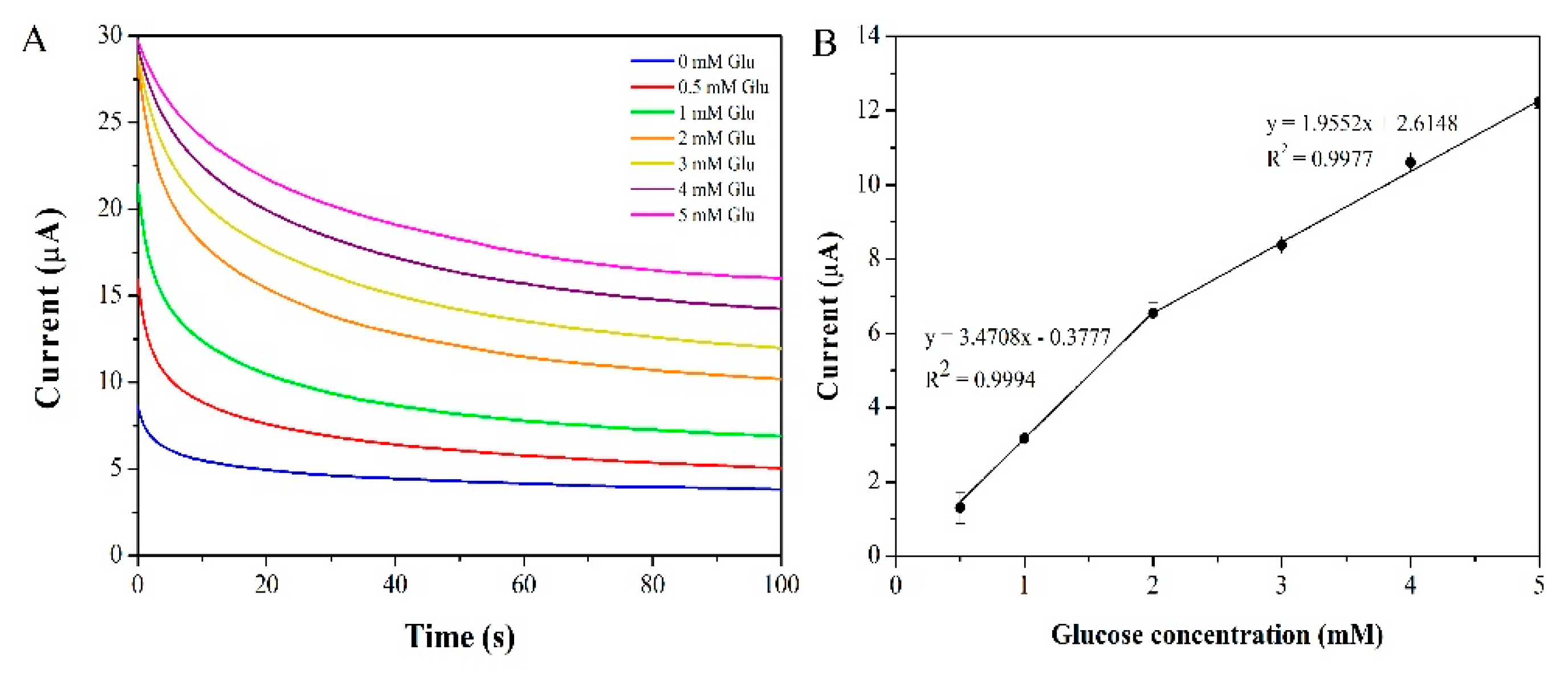
3.4. Amperometric Detection of Glucose by the Proposed Electrode
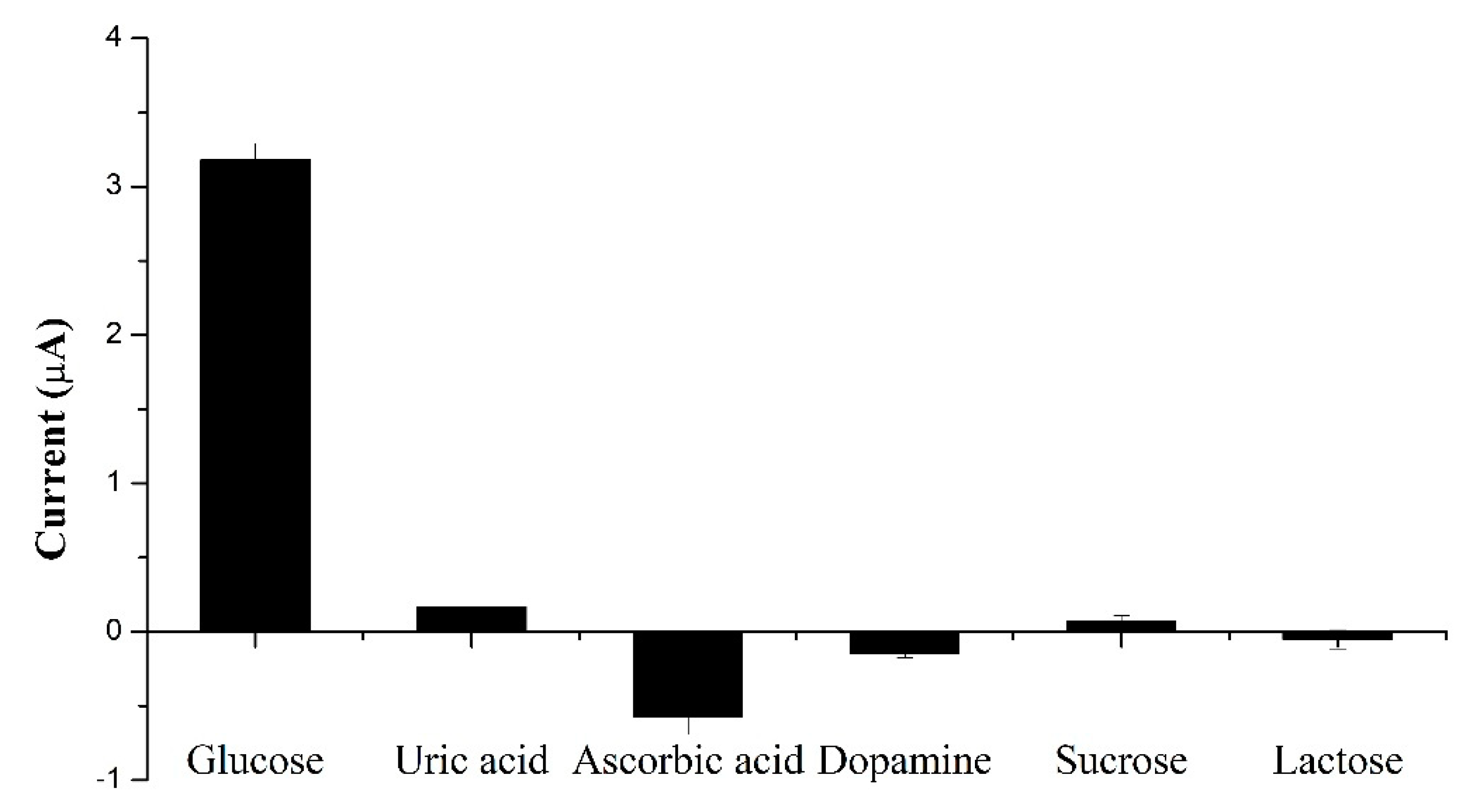
3.5. Selectivity and Stability of the Glucose Sensor
3.6. Applicability of the Prepared Sensor for Glucose Measurement in Real Time
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blood Sugar Level Ranges. Available online: https://www.diabetes.co.uk/diabetes_care/blood-sugar-level-ranges.html (accessed on 15 November 2019).
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Makaram, P.; Owens, D.; Aceros, J. Trends in nanomaterial-based non-invasive diabetes sensing technologies. Diagnostics 2014, 4, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalan, A.I.; Muthuchamy, N.; Lee, K.P. A novel bismuth oxychloride-graphene hybrid nanosheets based non-enzymatic photoelectrochemical glucose sensing platform for high performances. Biosens. Bioelectron. 2017, 89, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.I.; Muthuchamy, N.; Komathi, S.; Lee, K.P. A novel multicomponent redox polymer nanobead based high performance non-enzymatic glucose sensor. Biosens. Bioelectron. 2016, 84, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Muthuchamy, N.; Gopalan, A.; Lee, K.-P. Highly selective non-enzymatic electrochemical sensor based on a titanium dioxide nanowire–poly(3-aminophenyl boronic acid)–gold nanoparticle ternary nanocomposite. RSC Adv. 2018, 8, 2138–2147. [Google Scholar] [CrossRef] [Green Version]
- Sai-Anand, G.; Gopalan, A.-I.; Kang, S.-W.; Komathi, S.; Lee, K.-P. One pot synthesis of new gold nanoparticles dispersed poly (2-aminophenyl boronic acid) composites for fabricating an affinity based electrochemical detection of glucose. Sci. Adv. Mater. 2014, 6, 1356–1364. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Komathi, S.; Sai Anand, G.; Lee, K.P. Nanodiamond based sponges with entrapped enzyme: A novel electrochemical probe for hydrogen peroxide. Biosens. Bioelectron. 2013, 46, 136–141. [Google Scholar] [CrossRef]
- Manesh, K.M.; Santhosh, P.; Uthayakumar, S.; Gopalan, A.I.; Lee, K.P. One-pot construction of mediatorless bi-enzymatic glucose biosensor based on organic-inorganic hybrid. Biosens. Bioelectron. 2010, 25, 1579–1586. [Google Scholar] [CrossRef]
- Santhosh, P.; Manesh, K.M.; Uthayakumar, S.; Komathi, S.; Gopalan, A.I.; Lee, K.P. Fabrication of enzymatic glucose biosensor based on palladium nanoparticles dispersed onto poly(3,4-ethylenedioxythiophene) nanofibers. Bioelectrochemistry 2009, 75, 61–66. [Google Scholar] [CrossRef]
- Anand, G.S.; Gopalan, A.I.; Kang, S.-W.; Lee, K.-P. Development of a surface plasmon assisted label-free calorimetric method for sensitive detection of mercury based on functionalized gold nanorods. J. Anal. Atom. Spectrom. 2013, 28, 488. [Google Scholar] [CrossRef]
- Haldorai, Y.; Hwang, S.K.; Gopalan, A.I.; Huh, Y.S.; Han, Y.K.; Voit, W.; Sai-Anand, G.; Lee, K.P. Direct electrochemistry of cytochrome c immobilized on titanium nitride/multi-walled carbon nanotube composite for amperometric nitrite biosensor. Biosens. Bioelectron. 2016, 79, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, T.; Gopalan, A.I.; Lee, K.-P.; Park, S.-Y. Glucose sensing, photocatalytic and antibacterial properties of graphene–ZnO nanoparticle hybrids. Carbon 2012, 50, 2994–3000. [Google Scholar] [CrossRef]
- Ragupathy, D.; Gopalan, A.I.; Lee, K.-P. Synergistic contributions of multiwall carbon nanotubes and gold nanoparticles in a chitosan–ionic liquid matrix towards improved performance for a glucose sensor. Electrochem. Commun. 2009, 11, 397–401. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Sivanesan, A.; Benzigar, M.R.; Singh, G.; Gopalan, A.-I.; Baskar, A.V.; Ilbeygi, H.; Ramadass, K.; Kambala, V.; Vinu, A. Recent Progress on the Sensing of Pathogenic Bacteria Using Advanced Nanostructures. Bull. Chem. Soc. Jpn. 2019, 92, 216–244. [Google Scholar] [CrossRef] [Green Version]
- Komathi, S.; Gopalan, A.I.; Kim, S.-K.; Anand, G.S.; Lee, K.-P. Fabrication of horseradish peroxidase immobilized poly(N-[3-(trimethoxy silyl)propyl]aniline) gold nanorods film modified electrode and electrochemical hydrogen peroxide sensing. Electrochim. Acta 2013, 92, 71–78. [Google Scholar] [CrossRef]
- Thunkhamrak, C.; Chuntib, P.; Ounnunkad, K.; Banet, P.; Aubert, P.H.; Saianand, G.; Gopalan, A.I.; Jakmunee, J. Highly sensitive voltammetric immunosensor for the detection of prostate specific antigen based on silver nanoprobe assisted graphene oxide modified screen printed carbon electrode. Talanta 2020, 208, 120389. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Sai-Anand, G.; Gopalan, A.-I.; Lee, H.-G.; Yeo, H.K.; Kang, S.-W.; Lee, K.-P. Direct electrochemistry of cytochrome c with three-dimensional nanoarchitectured multicomponent composite electrode and nitrite biosensing. Sens. Actuators B Chem. 2016, 228, 737–747. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Cai, Z.; Oyama, M.; Chen, X. Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim. Acta 2014, 181, 689–705. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, Y. Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: Opportunities and challenges. RSC Adv. 2016, 6, 84893–84905. [Google Scholar] [CrossRef]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Amala, G.; Gowtham, S.M. Recent advancements, key challenges and solutions in non-enzymatic electrochemical glucose sensors based on graphene platforms. RSC Adv. 2017, 7, 36949–36976. [Google Scholar]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent Advances in Electrochemical Non-Enzymatic Glucose Sensors—A Review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Jothi, L.; Jayakumar, N.; Jaganathan, S.K.; Nageswaran, G. Ultrasensitive and selective non-enzymatic electrochemical glucose sensor based on hybrid material of graphene nanosheets/graphene nanoribbons/nickel nanoparticle. Mater. Res. Bull. 2018, 98, 300–307. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Q.; Yin, W.; Jiang, T.; Zhao, D.; Jiang, L. A novel nonenzymatic glucose sensor based on functionalized PDDA-graphene/CuO nanocomposites. Sens. Actuators B 2017, 253, 1087–1095. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, H.; Cui, Z.; Qin, D.; Gong, J.; Nie, Q. Amorphous Ni (OH)2/CQDs microspheres for highly sensitive non-enzymatic glucose detection prepared via CQDs induced aggregation process. Appl. Surf. Sci. 2017, 420, 323–330. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Farhadi, S. Development of non-enzymatic glucose sensor based on efficient loading Ag nanoparticles on functionalized carbon nanotubes. Sens. Actuators B Chem. 2016, 225, 354–362. [Google Scholar] [CrossRef]
- Scandurra, A.; Ruffino, F.; Sanzaro, S.; Grimaldi, M.G. Laser and thermal dewetting of gold layer onto graphene paper for non-enzymatic electrochemical detection of glucose and fructose. Sens. Actuators B Chem. 2019, 301, 127113. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Alizadeh, Z.; Veisi, H.; Hasheminejad, E. Non-enzymatic voltammetric glucose sensor made of ternary NiO/Fe3O4-SH/para-amino hippuric acid nanocomposite. J. Electroanal. Chem. 2018, 810, 69–77. [Google Scholar] [CrossRef]
- Saianand, G.; Gopalan, A.I.; Lee, J.C.; Sathish, C.I.; Gopalakrishnan, K.; Unni, G.E.; Shanbhag, D.; Dasireddy, V.; Yi, J.; Xi, S.; et al. Mixed Copper/Copper-Oxide Anchored Mesoporous Fullerene Nanohybrids as Superior Electrocatalysts toward Oxygen Reduction Reaction. Small 2019, e1903937. [Google Scholar] [CrossRef] [PubMed]
- Bui, Q.-T.; Yu, I.-K.; Gopalan, A.I.; Saianand, G.; Kim, W.; Choi, S.-H. Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Chen, L.; Jia, J. Three-Dimensional Copper Foam Supported CuO Nanowire Arrays: An Efficient Non-enzymatic Glucose Sensor. Electrochim. Acta 2017, 235, 519–526. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, X.; Liu, T.; Zhao, H.; Lan, M. Encapsulating Cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sens. Actuators B 2016, 227, 583–590. [Google Scholar] [CrossRef]
- Wang, X.; Liu, E.; Zhang, X. Non-enzymatic glucose biosensor based on copper oxide-reduced graphene oxide nanocomposites synthesized from water-isopropanol solution. Electrochim. Acta 2014, 130, 253–260. [Google Scholar] [CrossRef]
- Miao, P.; Han, K.; Tang, Y.; Wang, B.; Lin, T.; Cheng, W. Recent advances in carbon nanodots: Synthesis, properties and biomedical applications. Nanoscale 2015, 7, 1586–1595. [Google Scholar] [CrossRef]
- Yulong, Y.; Xinsheng, P. Recent advances in carbon-based dots for electroanalysis. Analyst 2016, 141, 2619–2628. [Google Scholar] [CrossRef]
- Tuerhong, M.; Yang, X.U.; Xue-Bo, Y. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, Y.; Niu, F.; Gooding, J.J.; Liu, J. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hai, X.; Xia, C.; Chen, X.-W.; Wang, J.-H. Growth of CuO nanoneedles on graphene quantum dots as peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 248, 374–384. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.-y.; Zhang, Z.-y. Graphitized carbon dots emitting strong green photoluminescence. J. Mater. Chem. C 2013, 1, 4902. [Google Scholar] [CrossRef]
- Wu, J.; Wang, P.; Wang, F.; Fang, Y. Investigation of the Microstructures of Graphene Quantum Dots (GQDs) by Surface-Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoun, S.B. Nanostructured carbon electrode modified with N-doped graphene quantum dots–chitosan nanocomposite: A sensitive electrochemical dopamine sensor. R. Soc. Open Sci. 2017, 4, 171199. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical synthesis of carbon nanodots directly from alcohols. Chem. A Eur. J. 2014, 20, 4993–4999. [Google Scholar] [CrossRef]
- Wei, H.; Sun, J.J.; Guo, L.; Li, X.; Chen, G.N. Highly enhanced electrocatalytic oxidation of glucose and shikimic acid at a disposable electrically heated oxide covered copper electrode. Chem. Commun. (Camb.) 2009, 20, 2842–2844. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Guan, Q.M.; Wu, J.; Li, H.N.; Yan, J.J. Enhanced direct electrochemistry of glucose oxidase and biosensing for glucose via synergy effect of graphene and CdS nanocrystals. Biosens. Bioelectron. 2011, 26, 2252–2257. [Google Scholar] [CrossRef]
- Hossain, M.F.; Park, J.Y. Fabrication of sensitive enzymatic biosensor based on multi-layered reduced graphene oxide added PtAu nanoparticles-modified hybrid electrode. PLoS ONE 2017, 12, e0173553. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Chang, G.; Su, J.; Cao, L.; Huang, Q.; Zhang, Y.; Xia, T.; He, Y. Single-step electrochemical deposition of high performance Au-graphene nanocomposites for nonenzymatic glucose sensing. Sens. Actuators B 2015, 220, 331–339. [Google Scholar] [CrossRef]
- Yin, H.; Cui, Z.; Wang, L.; Nie, Q. In situ reduction of the Cu/Cu2O/carbon spheres composite for enzymaticless glucose sensors. Sens. Actuators B 2016, 222, 1018–1023. [Google Scholar] [CrossRef]
- Ling, M.; Pengcheng, Z.; Jiyun, C.; Dandan, C.; Ying, Q.; Ning, G.; Genhua, Z.; Rongjing, C. Non-enzymatic sensing of glucose and hydrogen peroxide using a glassy carbon electrode modified with a nanocomposite consisting of nanoporous copper, carbon black and nafion. Microchim. Acta 2016, 183, 1359–1365. [Google Scholar]
- Safavi, A.; Maleki, N.; Farjami, E. Fabrication of a glucose sensor based on a novel nanocomposite electrode. Biosens. Bioelectron. 2009, 24, 1655–1660. [Google Scholar] [CrossRef] [PubMed]




| Sensing Electrode | Detection Method | Potential (V) | Sensitivity (µA mM−1cm−2) | Linear Range (mM) | LOD (mM) | Ref. |
|---|---|---|---|---|---|---|
| GOx/CdS/Gr on GCE | CV | - | 1.76 | 2–16 | 0.7 | [51] |
| PDDA/Ch/GOx/PtAuNPs/PtZn on Pt | Amp | +0.60 | 17.85 | 0.01–8 | 0.001 | [52] |
| Au/GO on GCE | Amp | +0.0 | 5.20, 4.56 | 0.1–2, 2–16 | 0.025 | [53] |
| Cu/Cu2O/CSs on GCE | Amp | +0.65 | 63.8, 22.6 | 0.01–0.69, 1.19–3.69 | 0.005 | [54] |
| Nafion/NPC-CB on GCE | Amp | +0.64 | 33.75 | 0.006–3.369 | 0.002 | [55] |
| PDDA/CuO-C-dot on SPCE | Amp | +0.50 | 110, 62.3 | 0.5–2, 2–5 | 0.2 | This work |
| Sample | Spiked Glucose (mM) | Detected Glucose (mM) | %Recovery |
|---|---|---|---|
| 1 | 0.5 | 0.47 ± 0.05 | 94 |
| 2 | 1.0 | 0.88 ± 0.08 | 88 |
| 3 | 3.0 | 2.71 ± 0.26 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridara, T.; Upan, J.; Saianand, G.; Tuantranont, A.; Karuwan, C.; Jakmunee, J. Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors 2020, 20, 808. https://doi.org/10.3390/s20030808
Sridara T, Upan J, Saianand G, Tuantranont A, Karuwan C, Jakmunee J. Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors. 2020; 20(3):808. https://doi.org/10.3390/s20030808
Chicago/Turabian StyleSridara, Tharinee, Jantima Upan, Gopalan Saianand, Adisorn Tuantranont, Chanpen Karuwan, and Jaroon Jakmunee. 2020. "Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode" Sensors 20, no. 3: 808. https://doi.org/10.3390/s20030808
APA StyleSridara, T., Upan, J., Saianand, G., Tuantranont, A., Karuwan, C., & Jakmunee, J. (2020). Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors, 20(3), 808. https://doi.org/10.3390/s20030808







