Anomaly Detection Using Electric Impedance Tomography Based on Real and Imaginary Images
Abstract
1. Introduction
2. Materials and Methods
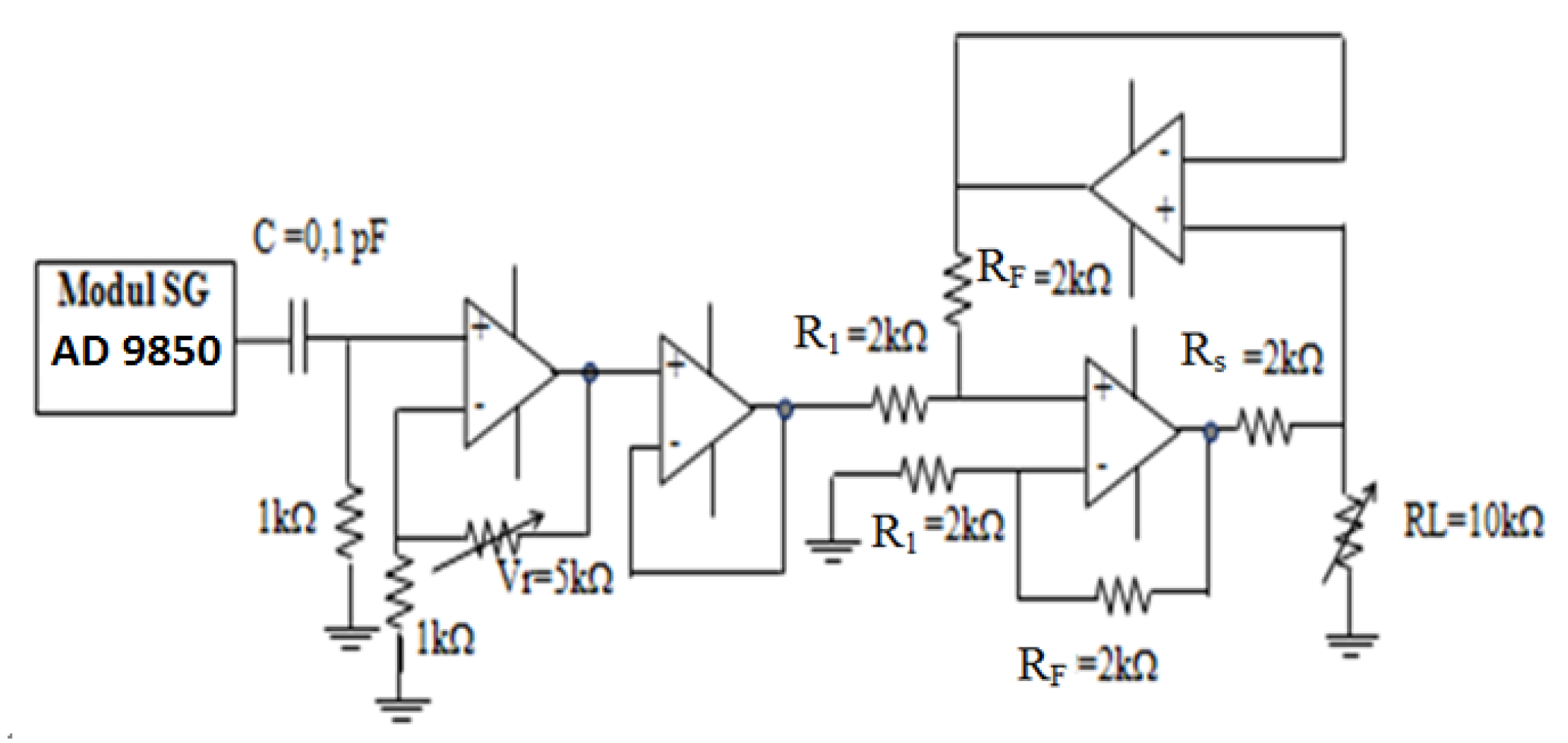
2.1. Voltage Controlled Current Source (VCCS)
2.2. Electric Mechanism of Current Injection and Voltage Measurement, Current to Voltage Circuit to Measure Phase Difference
2.2.1. Electronic mechanism for Current Injection
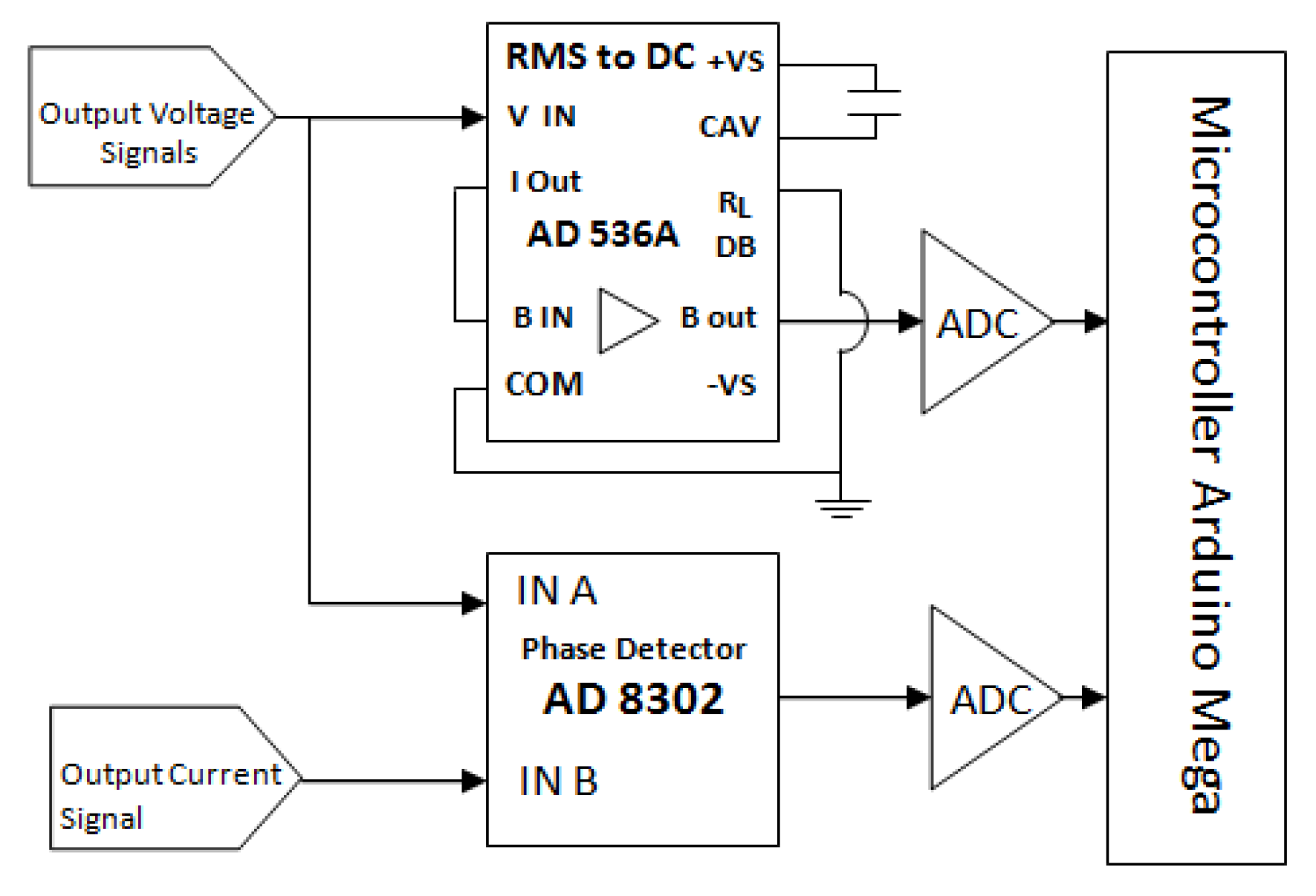
2.2.2. Electronic Mechanism of Voltage Measurement
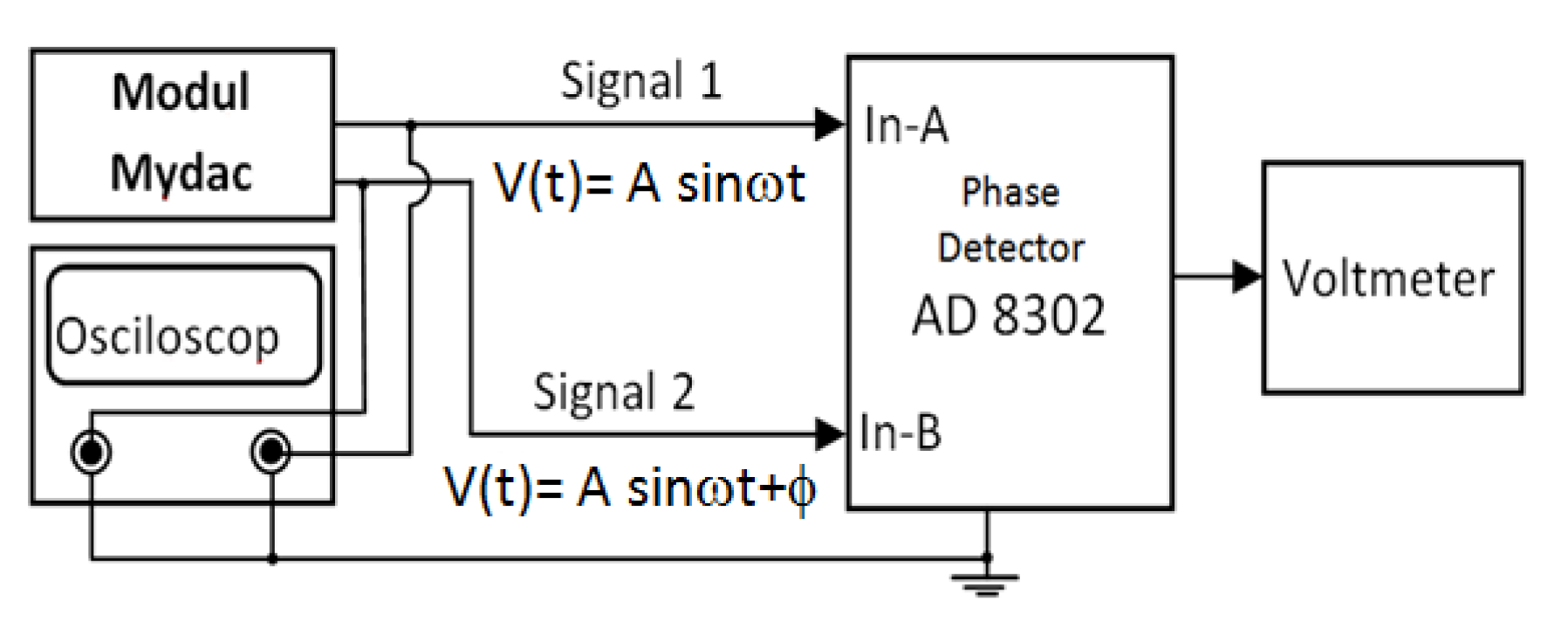
2.2.3. Measurement of Voltage and Phase Difference
2.3. Reconstruction
3. Results
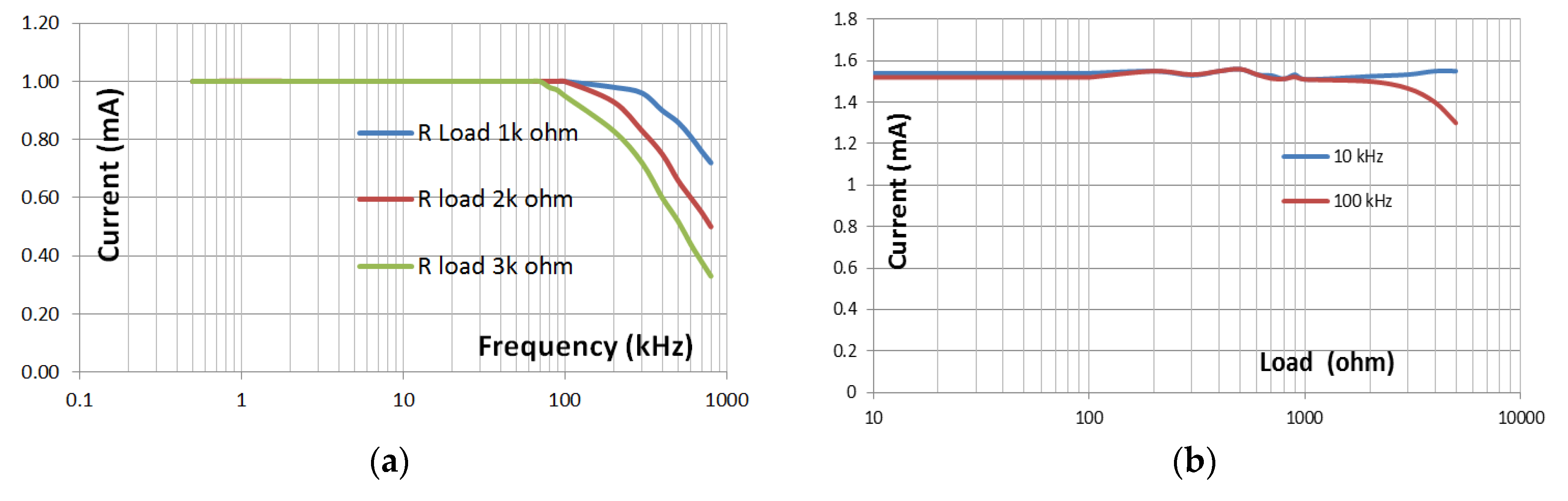
3.1. Voltage Controlled Current Source (VCCS)
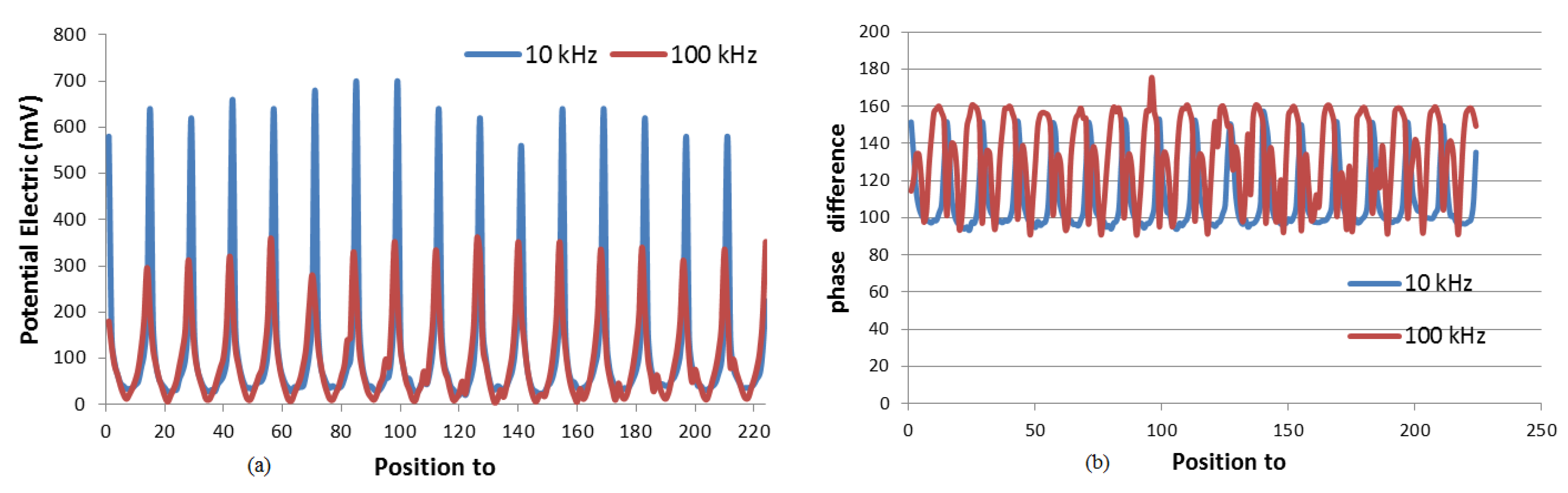
3.2. Voltage and Phase Difference Measurements
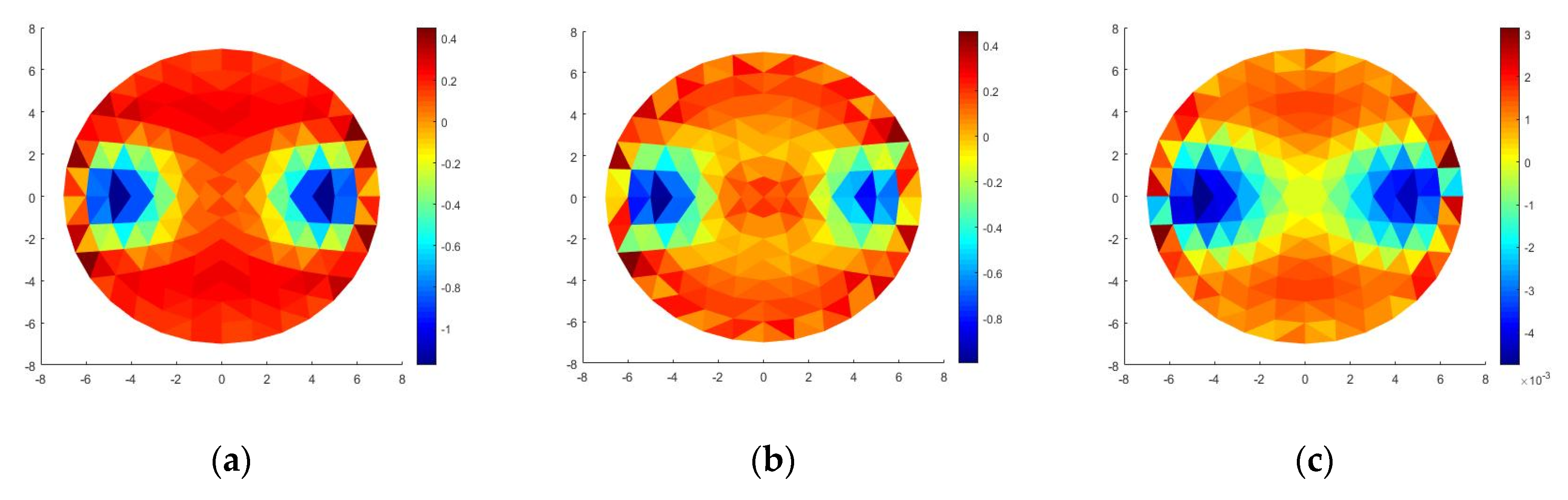
3.3. Simulation
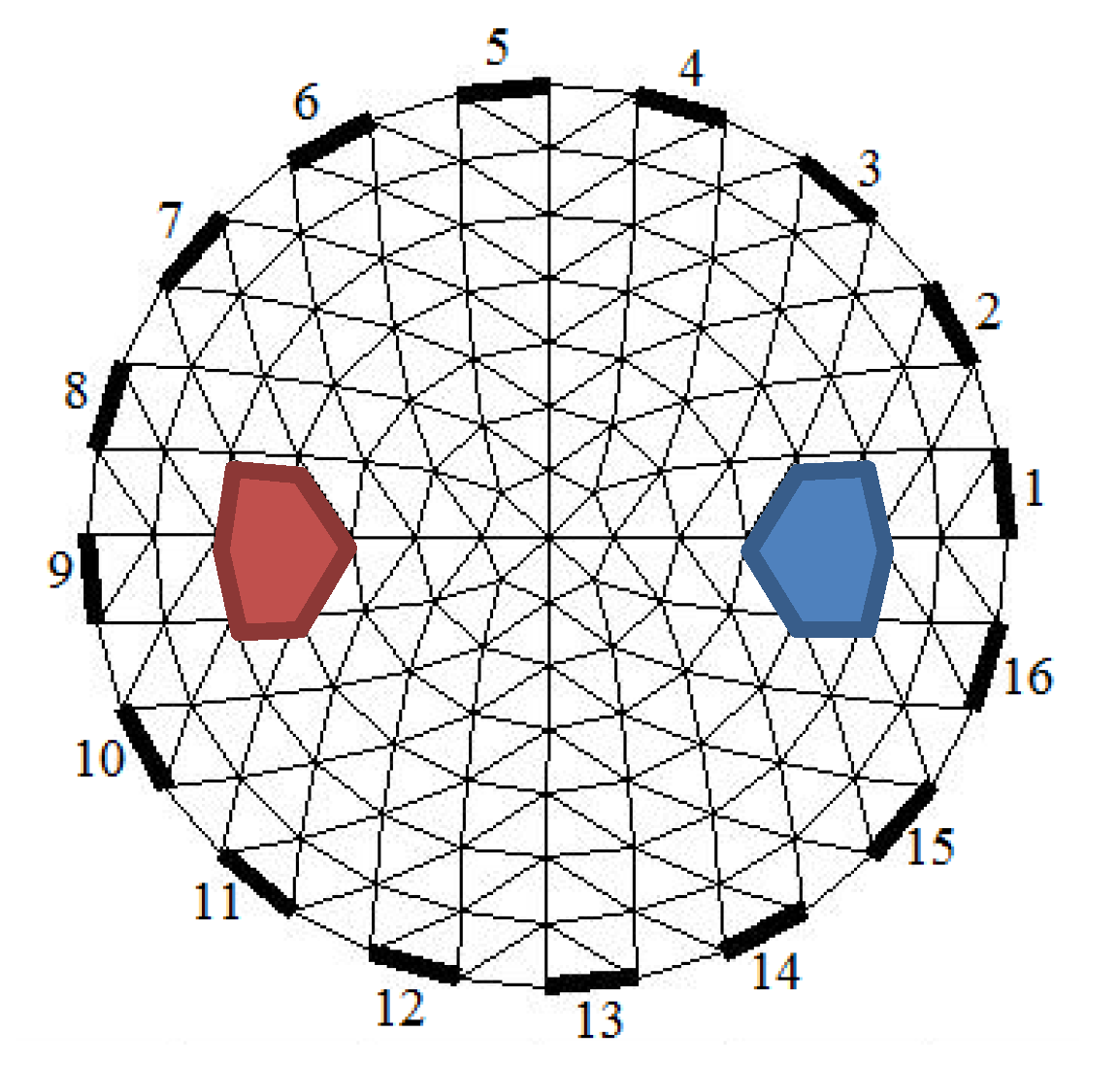
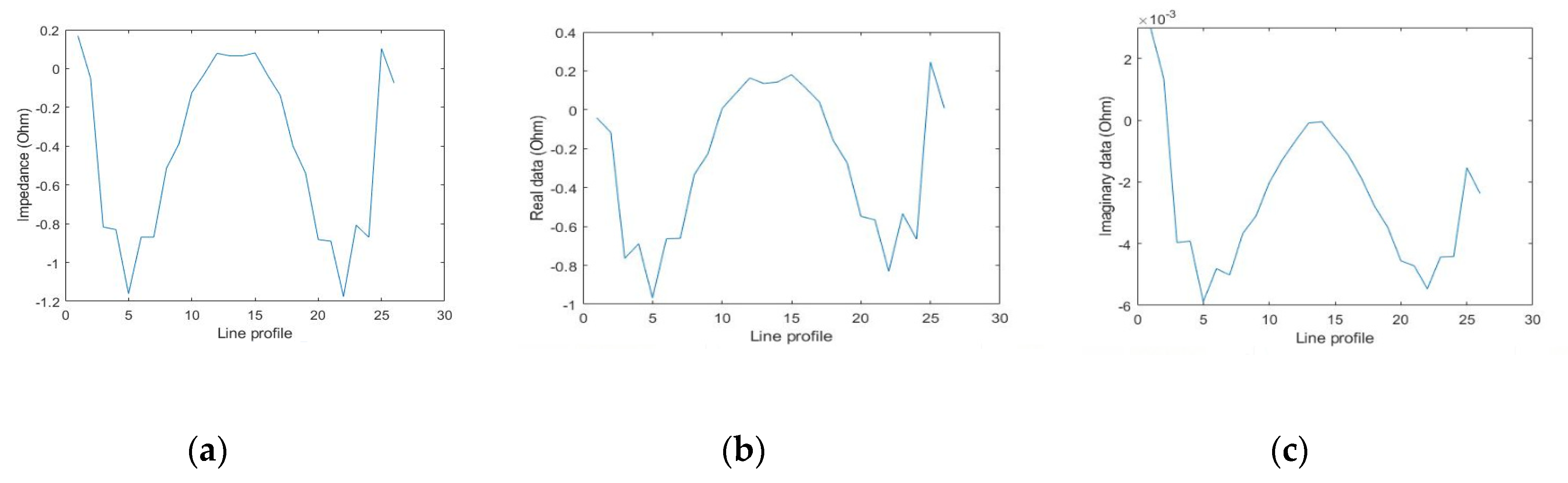
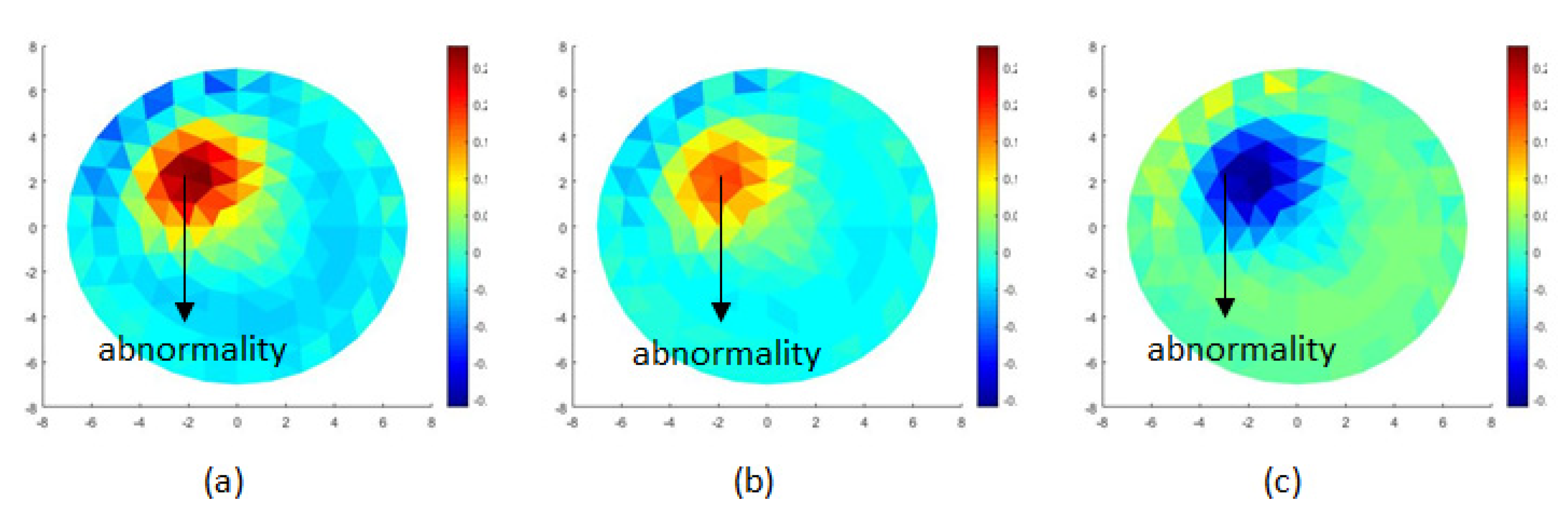
3.4. Image Scanning and Reconstruction
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, G.; He, B. Magnetoacoustic Imaging of Electrical Conductivity of Biological Tissues at a Spatial Resolution Better than 2 mm. PLoS ONE 2011, 6, e23421. [Google Scholar] [CrossRef] [PubMed]
- Affanni, A. Ruben Specogna, Francesco Trevisan Estimating the Volume of Unknown Inclusions in an Electrically Conducting Body with Voltage Measurements. Sensors 2019, 19, 637. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Nagaraju, J.; Lubineau, G. Electrical impedance spectroscopy (EIS)-based evaluation of biological tissue phantoms to study multifrequency electrical impedance tomography (Mf-EIT) systems. J. Vis. 2016, 19, 691–713. [Google Scholar] [CrossRef]
- Ain, K.; Kurniadi, D.; Suprijanto, S.; Santoso, O. Dual modality electrical impedance and ultrasound reflection tomography to improve image quality. J. Electr. Bioimpedance 2017, 8, 3–10. [Google Scholar] [CrossRef]
- Choi, M.H.; Kao, T.J.; Isaacson, D.; Saulnier, G.J.; Newell, J.C. A Reconstruction Algorithm for Breast Cancer Imaging With Electrical Impedance Tomography in Mammography Geometry. IEEE Trans. Biomed. Eng. 2007, 54, 700–710. [Google Scholar] [CrossRef]
- Pak, D.D.; Rozhkova, N.I.; Kireeva, M.N.; Ermoshchenkova*, M.V.; Nazarov, A.A.; Fomin, D.K.; Rubtsova, N.A. Diagnosis of Breast Cancer Using Electrical Impedance Tomography. Biomed. Eng. 2012, 46, 154–157. [Google Scholar] [CrossRef]
- Aristovich, K.Y.; Packham, B.C.; Koo, H.; dos Santos, G.S.; McEvoy, A.; Holder, D.S. Imaging fast electrical activity in the brain with electrical impedance tomography. NeuroImage 2016, 124, 204–213. [Google Scholar] [CrossRef]
- Yang, L.; Liu, W.; Chen, R.; Zhang, G.; Li, W.; Fu, F.; Dong, X. In Vivo Bioimpedance Spectroscopy Characterization of Healthy, Hemorrhagic and Ischemic Rabbit Brain within 10 Hz–1 MHz. Sensors 2017, 17, 791. [Google Scholar] [CrossRef]
- Visentin, F.; Fiorini, P.; Suzuki, K. A Deformable Smart Skin for Continuous Sensing Based on Electrical Impedance Tomography. Sensors 2016, 16, 1928. [Google Scholar] [CrossRef]
- Karsten, J.; MBohlmann, K.; Sedemund-Adib, B.; Wnent, J.; Paarmann, H.; Iblher, P.; Meier, T.; Heinze, H. Electrical impedance tomography may optimize ventilation in a postpartum woman with respiratory failure. Int. J. Obstet. Anesth. 2013, 22, 67–71. [Google Scholar] [CrossRef]
- Shetiye, P.C.; Ghatol, A.A.; Ghate, V.N.; Patil, S.R. Detection of Breast Cancer Using Electrical Impedance and RBF Neural Network. Int. J. Inf. Electron. Eng. 2015, 5, 5. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Soleimani, M. Capacitively Coupled Electrical Impedance Tomography for Brain Imaging. IEEE Trans. Med. Imaging 2019, 38, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ren, S.; Zhao, S.; Dong, F. A Lagrange-Newton Method for EIT/UT Dual-Modality Image Reconstruction. Sensors 2019, 19, 1966. [Google Scholar] [CrossRef]
- Santos, S.A.; Robens, A.; Boehm, A.; Leonhardt, S.; Teichmann, D. System Description and First Application of an FPGA-Based Simultaneous Multi-Frequency Electrical Impedance Tomography. Sensors 2016, 16, 1158. [Google Scholar] [CrossRef] [PubMed]
- Lionheart, W.R. EIT reconstruction algorithms: Pitfalls, challenges and recent developments, review article. Physiol. Meas. 2004, 25, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Nagaraju, J. Electrical impedance spectroscopic study of broiler chicken tissues suitable for the development of practical phantoms in multifrequency EIT. J. Electr. Bioimpedance 2011, 2, 48–63. [Google Scholar] [CrossRef]
- Mari, L.-P.; Olli, K.; Aku, S.; Marek, R.; Raul, L.; Paul, A.; Mart, M.; Edite, F.; Jari, H. EIT in hybrid imaging setup for tissue engineering applications. In Proceedings of the 20th International Conference on Biomedical Applications of Electrical Impedance Tomography (EIT2019), London, UK, 1–3 July 2019. [Google Scholar]
- Singh, G.; Anand, S.; Lall, B.; Srivastava, A.; Singh, V. Development of a Microcontroller based Electrical Impedance Tomography System. In Proceedings of the 2015 Long Island Systems, Applications and Technology, Farmingdale, NY, USA, 1 May 2015. [Google Scholar]
- Khalighi, M.; Vahdat, B.V.; Mortazavi, M.; Soleimani, M. Practical Design of Low-cost Instrumentation for Industrial Electrical Impedance Tomography (EIT). Ijst Trans. Electr. Eng. 2014, 38, 1–20. [Google Scholar]
- Seward, R.; Ethan, M.; Courtney, M.; Fu, Z.; Hilda, G.; Badria, M.; Ryan, H. Using EIT to assess Pulmonary Function in ALS Patients. In Proceedings of the 20th International Conference on Biomedical Applications of Electrical Impedance Tomography (EIT2019), London, UK, 1–3 July 2019. [Google Scholar]
- Qiao, G.; Wang, W.; Wang, L.; He, Y.; Bramer, B.; Al-Akaidi, M. Investigation of biological Phantom for 2D and 3 D Breast EIT images. IMBE Proc. 2007, 17, 328–331. [Google Scholar]
- Gabriel, C. Compilation of the Dielectric Properties of Body tissue at RF and Microwave Frequencie; Report N. AL/OE -TR-1996-0037; Occupational and Environmental Health Directorate, Radiofrequency Radiation Division Broooks Air Force Base: San Antonio, TX, USA, June 1996. [Google Scholar]
- Mylott, E.; Kutschera, E.; Widenhor, R. Bioelectrical impedance analysis as a laboratory activity: At the interface of physics and the body. Am. J. Phys. 2014, 82, 521–528. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Biolelectrical Impedance Analysis-Part 1: Review of Principles And Metods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Pandya, A.S.; Arimoto, A.; Agarwal, A.; Kinouchi, Y. Anovel Approach for measuring Electrical Impedance Tomography for Local Tissue with Artificial Intelligent Algorithm. Int. J. Biom. Bioinform. 2010, 3, 66. [Google Scholar]
- Rizzoni, G. Principles and Applications of Electrical Engineering, 5th ed.; Mc Graw-Hill: New York, NY, USA; Singapore, 2007; ISBN 007-125444-7. [Google Scholar]
- Li, Z.; Xu, Z.; Ren, C.; Wang, W.; Zhao, D.; Zhang, H. Study of Voltage Control Current Source in Electrical Impedance Tomography System. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010. [Google Scholar] [CrossRef]
- Sapuan, I.; Ain, K.; Suryanto, A. Dual frequency electrical Impedance Tomografy to Obtain Functional Image. J. Phys. Conf. Ser. 2017, 853, 012002. [Google Scholar] [CrossRef]
- Gong, B.; Krueger-Ziolek, S.; Moeller, K.; Schullcke, B.; Zhao, Z. Electrical impedance tomography: Functional lung imaging on its way to clinical practice? J. Expert Rev. Respir. Med. 2015, 9, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, H.; Huang, Z.; Wang, B.; Li, H. Study on image reconstruction of capacitively coupled electrical impedance tomography (CCEIT). Meas. Sci. Technol. 2019, 30, 094002. [Google Scholar] [CrossRef]






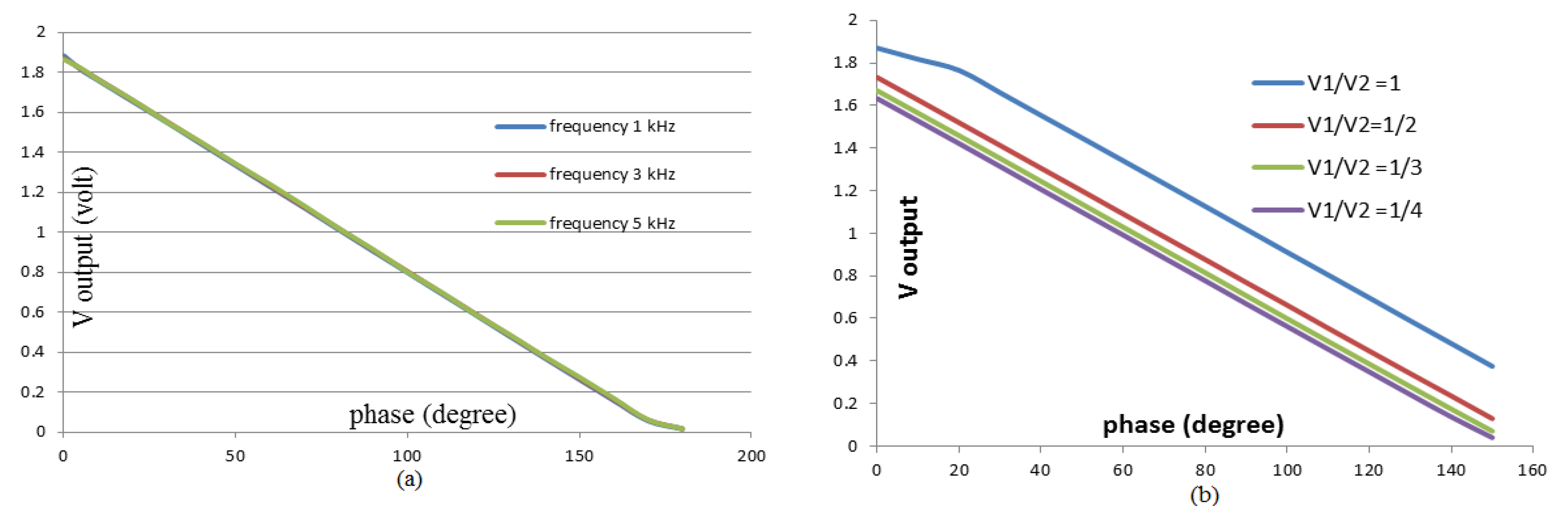


| No | Ratio V1/V2 | Constant | Slope |
|---|---|---|---|
| 1 | 1 | 1.9455 | 0.0104 |
| 2 | ½ | 1.7338 | 0.0107 |
| 3 | 1/3 | 1.6747 | 0.0107 |
| 4 | 1/4 | 1.6334 | 0.0107 |
| Frequency | ||||||
|---|---|---|---|---|---|---|
| 10 kHz | 100 kHz | |||||
| Heart | Deflated Lung | Distillated Water | Heart | Deflated Lung | Distillated Water | |
| Z (Ω) | 1.001 | 1.078 | 2000 | 2.676 | 2.988 | 2000 |
| Re (Ω) | 0.245 | 0.084 | 2000 | 0.66 | 0.313 | 2000 |
| Im (Ω) | 0.971 | 1.074 | 100 | 2.593 | 2.972 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapuan, I.; Yasin, M.; Ain, K.; Apsari, R. Anomaly Detection Using Electric Impedance Tomography Based on Real and Imaginary Images. Sensors 2020, 20, 1907. https://doi.org/10.3390/s20071907
Sapuan I, Yasin M, Ain K, Apsari R. Anomaly Detection Using Electric Impedance Tomography Based on Real and Imaginary Images. Sensors. 2020; 20(7):1907. https://doi.org/10.3390/s20071907
Chicago/Turabian StyleSapuan, Imam, Moh Yasin, Khusnul Ain, and Retna Apsari. 2020. "Anomaly Detection Using Electric Impedance Tomography Based on Real and Imaginary Images" Sensors 20, no. 7: 1907. https://doi.org/10.3390/s20071907
APA StyleSapuan, I., Yasin, M., Ain, K., & Apsari, R. (2020). Anomaly Detection Using Electric Impedance Tomography Based on Real and Imaginary Images. Sensors, 20(7), 1907. https://doi.org/10.3390/s20071907





