1. Introduction
Nowadays, an ageing society is one of the problems emerging on a global scale. The demographics of the global human population from the last decades have revealed that the fraction of individuals older than 60 years increased from 9.2% in 1990 to 11.7% in 2013 and it is expected to reach 21.1% (2 billion) by 2050 [
1]. In light of this trend, it can be assumed that conventional healthcare in terms of a hospital-centric concept will not be able to keep up with future demands. Therefore, the concept of preventive healthcare or home healthcare has become a promising solution, which allows us to regularly and systematically measure our health status in an environment outside of hospitals (i.e., at home, in the office, etc.). Monitoring devices should be small, low-power, portable, and in wearable forms [
2,
3,
4]. According to the World Health Organization, cardiovascular diseases are the leading cause of mortality worldwide [
5]. The elderly also have high risks of cardiovascular disease, and as a person’s age increases, it becomes more problematic. The symptoms of cardiovascular diseases are uncertain or intermittent. Therefore, it is preferable to monitor a person’s heart activity at any time or place when the symptoms occur. The electrocardiogram (ECG) is the most common diagnostic modality of cardiovascular diseases. The ECG is capable of detecting almost any kind of heart abnormality at an early stage and represents an essential tool for assessment of the cardiovascular system [
6]. The twelve-electrode ECGs (e.g., Holter) are considered the gold standard in clinical practice [
7]. However, three-electrode ECGs provide a sufficient sensitivity (≈98%) and specificity (≈74%) for distinguishing between native and pathological heart electrical activity [
8,
9]. In this configuration, two electrodes are used to measure a body surface’s potential difference, and a third electrode provides a low-impedance return path for noise reduction. It is desirable to have few electrodes, in order to reduce the costs of the ECG acquisition system and increase patient comfort. However, the removal of the third electrode is challenging due to the significantly higher electromagnetic interference (EMI) and lower signal-to-noise ratio (SNR) in two-electrode compared to three-electrode ECG acquisition systems [
10].
Biopotential recordings, such as the ECG, are frequently contaminated with 50 Hz (in Europe and most of Asia) or 60 Hz (in the USA and Canada) power-line interference. This interference results from the capacitive coupling of the patient’s body and electrode cables, on the one hand, and the power lines, on the other hand. The sources of power-line interference are summarized in
Figure 1.
The C
1 and C
2 capacitors act as coupling capacitors between the patient’s body and the power line, and the patient’s body and the ground, respectively. The C
S capacitor represents the capacitance between the power line and the ECG acquisition system, whilst C
CB capacitors symbolize capacitances between the power line and the electrode cables. Another source of noise is due to the different grounding of the ECG acquisition system and the AC power supply, which results in the coupling capacitance
CISO between the AC ground and the ground of the ECG acquisition system [
11,
12]. In
Figure 1, the one-lead ECG acquisition system utilizing three electrodes is depicted. It measures the biopotential between electrodes LA (left arm) and RA (right arm). The third RL (right leg) electrode, sometimes called a reference electrode, is used to minimize power-line interference by means of decreasing the common-mode voltage obtained from the patient’s body.
There are some general techniques for enhancing common-mode rejection (CMR). The C
S coupling capacitance can be eliminated by placing the ECG acquisition system (i.e., analogue front-end) into a shielded case and the
CCB cable coupling capacitances can be eliminated by using shielded electrode cables. The effect of the cable shielding was presented in [
13,
14]. The CMR system can be enhanced by improving the isolation between the device ground and the patient ground (C
ISO). Therefore, the battery-powered ECG acquisition systems display very high CMR [
11]. The removal of noise caused by the coupling capacitors C
1 and C
2 is the most crucial step, because the power-line noise is collected from the patient’s body and transferred as a common-mode voltage (
VCM) to the differential amplifier’s inputs. The common-mode signal is a signal that appears simultaneously and in-phase on both amplifier inputs. The powerline noise coming from the body represents the common-mode voltage. It should be effectively mitigated by using both the differential input amplifier with a high common-mode rejection ratio (CMRR) and a high input impedance. The CMRR represents the capability of an amplifier to reject common-mode signals, and it is defined as the ratio between the amplitude of the common-mode signal and the amplitude of an equivalent differential signal [
15,
16]. Unfortunately, in both inputs, the common-mode signals are not the same due to mismatches in electrode-skin impedances, cable impedances, input protection circuits (typically including resistors, capacitors, diodes, etc.), and amplifier input impedances [
11,
17]. This transformation of the common-mode voltage into differential-mode voltage (
VDM) interference must also be considered.
Differential-mode (DM) interference is caused by many effects, and it is also the reason why the shielded cables are needed. Unshielded cables suffer from power line interferences through the
CCB capacities shown in
Figure 2. The current flows from the mains power line through the
CCB capacities, the electrode-skin impedances
ZE1 and
ZE2, and the
C2 body-ground capacitance to the ground, while generating a
VDM voltage difference between the electrodes due to the different electrode-skin impedances (
ZE1 ≠
ZE2). This type of interference is known as DM interference [
18,
19].
The differential voltage
VDM can be calculated as follows [
18,
20]:
where
i1 and
i2 are displacement currents coupled to the electrode leads. The
VDM voltage is dependent on the distance between power lines and electrode cables and the length of the electrode cables. If the length of the electrode leads is the same, and the leads run close together, then the displacement currents will be equal (
i1 =
i2). If the
i1 and
i2 currents in
Figure 2 have a typical value of 10 nA
p-p and the imbalance in electrode-skin impedances (
Ze1-
Ze2) is only 20 kΩ, then the magnitude of the bipolar signal in the ECG system input at 50 Hz will be as large as 200 µV
p-p [
21]. According to the AAMI EC 11 standard, the maximum system noise allowed is 30 µV
p-p for an ECG. In practice, it is not possible to have
i1 =
i2 because leads cannot run close together side by side, so
i1 ≠
i2. If we balance electrode-skin impedances so that
ZE1 =
ZE2, e.g., by the method described in [
20], the
VDM differential voltage will not be zero in Equation (6), because leads have different capacitive couplings of
CCB and thus, different currents flow to the leads. The cable shielding ensures that power-line currents cannot flow to the electrode leads, and the interference is maximally transformed into a common-mode signal. A previous study [
14] showed that the cable shielding increased the attenuation of power-line noise by 19.3 dB, which is why the shielded cables were used in our experiments.
Another source of DM interference is the
ib displacement current flowing into the body from the power line through
C1 and C
2 capacitance to the ground. If we assume typical values of 3 and 300pF for
C1 and C
2, respectively, then an
ib current of less than 1 µA
p-p flows from the power line through the body to the ground [
21]. The model situation in
Figure 2 assumes that some fraction of the
ib current is flowing along the
Zb internal body impedance. This impedance is dependent on the patient orientation and position concerning the power line cables [
22]. Then, the DM interferencing voltage caused by
Zb impedance is
If using the maximal values
ib = 1 µA
p-p and
Zb = 500 Ω, then
VDM = 0.5 mV
p-p, which is at a voltage level of the measured ECG signal [
19]. We can reduce the DM interference by balancing the electrode and amplifier common-mode input, but there is still some portion of remaining interference because of
Zb.
A total input interfering voltage
Vi is defined as follows [
22,
23]:
where
VDM and
VCM are the differential-mode and common-mode voltages, respectively. Both voltages are the product of the
ib displacement current. The
CMRR1 in (3) describes the effect of the electrode impedance imbalance Δ
ZE =
ZE1 –
ZE2:
where
Zc is the amplifier CM input impedance at the power line frequency (see
Figure 2). A high differential-mode impedance of the amplifier is represented by
Zd. A typical value of
CMRR1 is 60 dB for shielded electrode leads [
24]. The
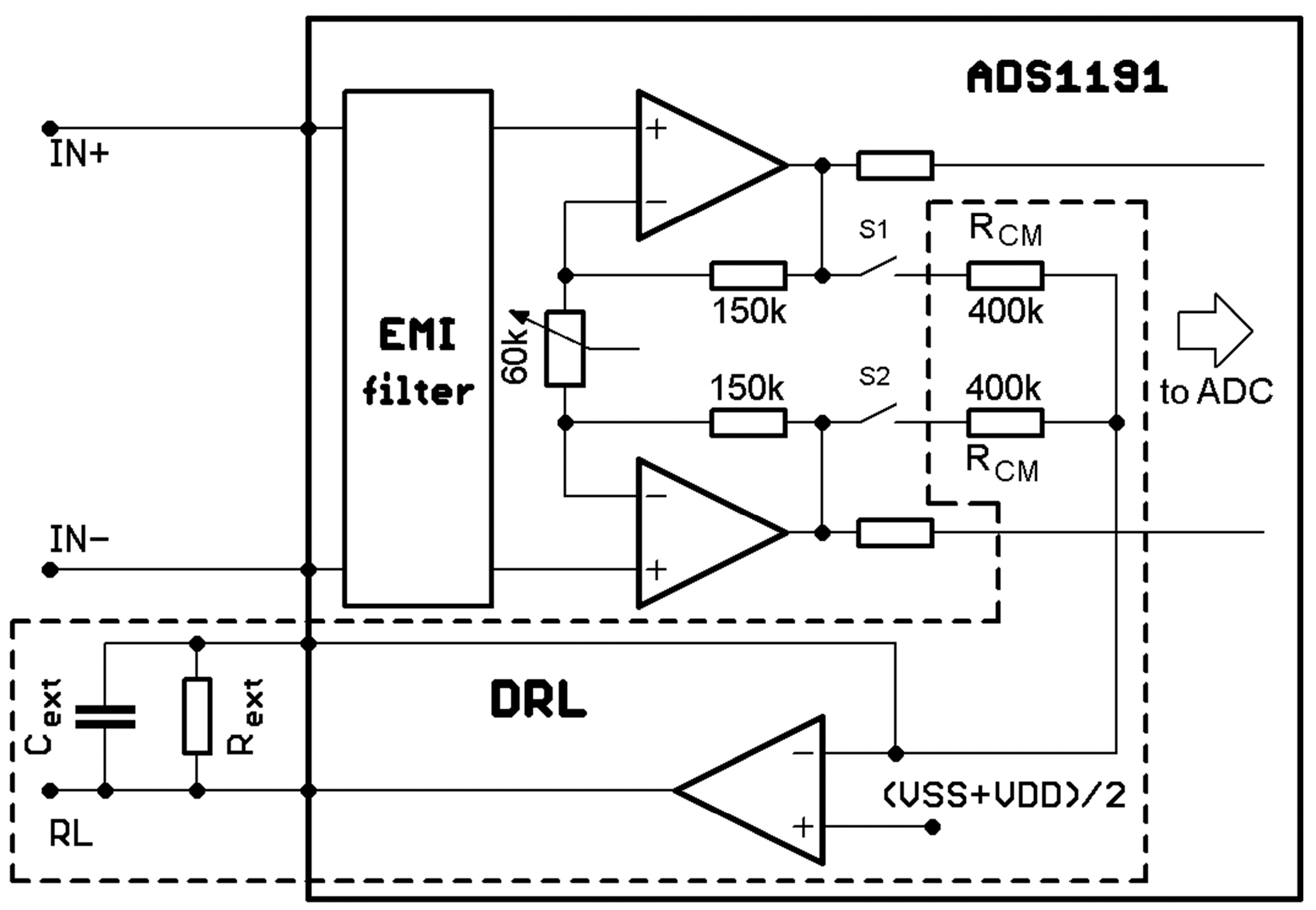
CMRR2 in (3) is a common-mode rejection ratio of a used amplifier (95 dB for ADS1191 used in this paper). The typical value of
VCM can range from millivolts to tens of millivolts, but it can reach a value of 200 mV
p-p [
21,
22,
24]. In general, if we use
VDM = 0.5 mV
p-p,
VCM = 10 mV
p-p,
CMRR1 = 60 dB, and
CMRR2 = 90 dB, then the total interference voltage
Vi according to (3) will be 510 μV
p-p. If we change the value of
VCM to 200 mV
p-p, then
Vi will be 704 μV
p-p.
Although a differential amplifier with a high CMRR is used, the differential amplifier does not completely suppress the noise. Moreover, a very high magnitude of the noise can cause saturation of the amplifier input and then it is impossible to extract an ECG from a noisy signal. An additional noise suppression method must then be used. A Driven-Right-Leg (DRL) circuit is often used to reduce the common-mode voltage. The DRL circuit senses the input common-mode voltage at the differential amplifier inputs [
25] or outputs [
11]. It enhances the CMRR by driving the right leg (RL) electrode through the inverting amplifier, acting as a low-pass filter. In acquisition systems using three electrodes, noise suppression using the DRL circuit is very efficient. In contrast, due to the missing third reference electrode, the power-line noise reduction in the two-electrode system is more complicated. Such a system is usually a portable, battery-powered acquisition system that records the ECG signal from two measuring points located on various body parts, e.g., palms [
26], wrists [
27,
28], and thumbs [
29,
30,
31]. Additionally, these systems usually use contactless gel-free (i.e., dry) electrodes, resulting in increased interference in ECG recordings.
The research community has already published many power-line noise suppression solutions for the two-electrode ECG system [
30,
31,
32]. The research papers present electrical schemes consisting of different operational amplifier combinations and discrete components. However, they could not significantly reduce the overall size of the ECG system, which is a crucial parameter for such a system. Therefore, we focused on using a commercial analogue front-end ECG chip with noise suppression solutions adopted from published research papers. In this paper, we present a comparison of three noise suppression methods for two-electrode ECG system design. The remainder of this paper is organized as follows.
Section 2 details our design in terms of the measurement system architecture, hardware, and software required.
Section 3 reports the performance of the noise suppression methods, experiments with a simulator and living subjects, measurement outcomes, and current consumption of the proposed solutions. Finally, conclusions are drawn in
Section 4.
3. Results
We tested the practical implementation of the proposed solutions for the two-electrode ECG system. The one-minute-long sine wave signal with a frequency of 5 Hz and amplitude of 1 mV was measured while being generated by the FLUKE ProSim 2 simulator (FLUKE Biomedical, Cleveland, USA). The PSD estimates of measured signals are shown in
Figure 9.
The PSD estimates are very similar for all three cases with very close values of the SNR. The calculated SNRs are shown in the title of
Figure 9. The frequency component of 50 Hz in the power spectrum is reduced when compared to the spectrum in
Figure 6. Furthermore, the power spectrum is very similar to the spectrum of the system, which uses three electrodes (
Figure 5). According to the results, we can suggest that all three alternatives should be suitable for suppressing power-line noise in two-electrode ECG systems.
Experiments with a living subject were also performed, where ECG clamp electrodes were placed on the proband wrists. The methods, according to
Figure 7, for active noise suppression, were used to avoid saturation of the inputs. The ECG system was placed in the shielded box and powered by a 5 V voltage adapter (a red scenario in
Figure 3). The ECG signal measured from three electrodes by the BIOPAC MP36 acquisition system (BIOPAC Systems, USA) is considered to be the golden standard. The DRL electrode was placed on the ankle of the right leg. All measurements were provided for the same subject in sequence, with the minimum idle time between them. The sampling rate was set to 500 SPS for all measurements. Measured signals are shown in
Figure 10. The magnitude of signals was normalized to the interval from 0 to 1 to compare the shape of the ECG signal and the amount of noise between specific hardware solutions. At first sight, minimal noise is visible in the solution with 10 MΩ resistors on the ADS inputs. This fact is also confirmed by the PSD estimation shown in
Figure 11. Although only five seconds of signals are shown in
Figure 10, the PSD in
Figure 11 was calculated for one-minute-long signals. The PSD shows the presence of the power-line noise in all cases, except for the golden standard, which was not high enough to cause the differential amplifier inputs to be saturated. The power of the signal at a frequency of 50 Hz was −28.0, −24.8, and −26.0 dB for the solution with 10 MΩ resistors, voltage followers, and the combination, respectively.
Time-domain or frequency-domain analysis, or their combination, may be used to evaluate the signal quality of the acquired ECG [
37]. In our case, we used frequency analysis of the signal quality obtained by the gold standard and three proposed solutions of the two-electrode ECG system. We started the work in accordance with [
38], dividing the frequency band similarly into three areas:
At the same time, the LF band includes isoline changes and artifacts, such as the breath waveform. HF noise contains power-line noise and its higher harmonic components or muscle EMG artifacts. For the analysis, we selected the signal segments with a length of 15 s. Firstly, the DC component was removed from the signals, and then, we normalized them using the maximum value of the R wave. For each signal, the fast Fourier transform (FFT) was calculated using a Hamming window. Subsequently, we summed the components for each frequency band. The result of the analysis is depicted in
Figure 12.
Looking at the ECG band, the best result was achieved by the BIOPAC system, which also serves as the gold standard. The worst result was the voltage follower solution. In terms of low-frequency noise contamination, the 10 MΩ resistor system, in combination with a virtual DRL circuit, shows the most considerable LF noise resistance. The high-frequency noise mostly occurs in the fourth solution and thus in the combination of voltage followers and 10 MΩ resistors. This correlates with the PSD shown in
Figure 11. As a result, the solution using virtual DRL and 10 MΩ resistors seems to be the best in terms of LF and HF noise, as well as the useful ECG signal. This noise can be additionally removed by several techniques, e.g., implementing a notch filter, wavelet filtering, or changing the DRL circuit’s gain, etc.
The raw signals were post-processed to enhance the signal quality and to demonstrate the effectivity of the proposed hardware solutions for high-quality ECG measurement. The signal denoted as the golden standard did not undergo postprocessing in the following figures because the power-line noise was minimal. At first, a notch filter was applied to raw signals. The filter was designed with attenuation of 60 dB at 50 Hz. The resulting signals are shown in
Figure 13.
After using a notch filter, the signals were further enhanced and smoothed by wavelet denoising. Signals were decomposed into five levels using the
Symlets 4 wavelet and filtered in the wavelet space using soft thresholding. Filtered signals were then composed by using the inverse discrete wavelet transform (
Figure 14).
Finally, an experiment was carried out without using any hardware techniques of noise suppression. The resulting signal and its PSD are shown in
Figure 15. In this figure, the ECG signal is lost in a high quantity of noise. In some intervals, the noise saturates the inputs of the differential amplifier (signal magnitudes overreached the input range of the differential 16-bit AD converter). Therefore, at the signal postprocessing stage, it is not feasible to fully extract an ECG from such a noisy signal.
4. Discussion
Comparing all three hardware solutions for noise suppression in ECG measurement, it is evident that the first solution actively suppresses the common noise component in the form of power-line noise. It also features active compensation for a possible common-mode noise reduction in the form of a DRL circuit when the measuring electrodes have distinct characteristics (i.e., electrical impedance at the interfaces: electrode, gel, and skin). This compensation is also included in the second and third noise suppression solutions, where the voltage followers ensure impedance separation of the measuring circuit and the electrode-gel-skin interfaces.
The cable shielding is necessary for designs where voltage buffers are used on the ECG device inputs (second and third method). Unshielded cables suffer from common-mode and differential-mode interferences through
CCB capacities (
Figure 1 and
Figure 2). A high magnitude of such interference can cause saturation of the amplifier inputs and it is then not possible to measure the ECG signal. The cable shielding is not needed in the first method because the DRL circuit provides active noise reduction by driving the inverted and amplified common-mode signal back to the electrode leads.
Depending on the current physiological state of the subject being measured, the impedance of the electrode-gel-skin interfaces may considerably vary. This fact is also supported by the final measurement (
Figure 15), wherein the measured signal was directly applied to the ADS1191 inputs, without any active suppression technique of the common-mode interference or impedance isolation by the voltage followers. The imbalance between both inputs significantly degrades the CMRR and makes it impossible to analyze the measured ECG (even after digital signal filtering) due to saturation of the ADS1191 inputs.
The last part, which must be discussed, is aimed at the current consumption of proposed hardware solutions which are intended to be used as battery-powered devices. The current consumption is measured in the configuration, according to the diagram shown in
Figure 3. To reduce the power consumption, the MCU ATmega328P runs at a frequency of 2 MHz, which is sufficient for the highest sampling rate of ADS1191 (500 SPS). The MCU peripherals, such as the AD converter and timers, are powered down. The MCU and ADS1191 are powered by a rechargeable lithium-polymer (LiPo) battery with a nominal voltage of 3.7 V with a capacity of 1200 mAh. The external dimensions of the battery are 10 × 30 × 40 mm, and the weight is only 18 g. These parameters make the battery ideal for wearable devices. The UART/USB converter is powered by an external power source because the converter is not essential in applications when the data are transmitted wirelessly to the receiver, such as a PC, tablet, smartphone, etc. The current consumption was measured by using the digital multimeter Agilent 34401A (Agilent Technologies, USA) placed between the battery and the LDO regulator. The battery had a voltage of 3.68 V during the measurement. Three power modes were investigated, and the current consumption values are shown in
Table 1.
The current consumption is slightly higher in the solution with voltage followers because op-amps (OPA2333) used as voltage followers consume a little extra power. The third solution, which is a combination of 10 MΩ resistors and voltage followers, is not mentioned in the table because the consumption does not differ from that of the voltage followers solution. Some current is drawn from the battery when the MCU and ADS1191 are powered down (third variant). This current results in internal consumption of the LDO regulator, external pull-up or pull-down resistors, and quiescent power-down currents of MCU and ADS. The second power mode variant allows us to discover the current consumption of a particular solution, excluding MCU consumption. The current consumption of the solution with 10 MΩ resistors and voltage followers is 187 and 205 µA, respectively. According to the ADS1191 datasheet [
33], the power consumption in normal mode with a DRL circuit disabled at 3 V is typically 420 µW, which means a current consumption of 140 µA. The DRL is enabled in the first solution, and additional voltage followers are used in the second solution, so the current consumption is higher and comparable to datasheet values. The current consumption in the first power mode variant is approximately 1.5 mA in both solutions. These values are measured while sending ECG data at 500 SPS over USART to a PC. In most cases, the data from the battery-powered device are transferred wirelessly. The Bluetooth Low Energy (BLE) is aimed at novel applications in healthcare and offers considerably reduced power consumption. As was stated by the authors in [
39], the RN4020 BLE module by Microchip drains only 7.6 mA at 3 V while sending data at 500 SPS. This BLE module, in combination with our two-electrode ECG solutions, ensures that the current consumption does not increase above 10 mA. If we consider a battery with a capacity of 1200 mAh, then the proposed ECG device is able to send the data continuously over Bluetooth for 120 h (5 days).
The best power-line noise suppression was achieved by using the first method with 10 MΩ resistors on the ADS1191 inputs (see
Figure 11). The battery-powered ECG system, according to the blue scenario of the measurement system in
Figure 3, implementing the first method (
Figure 7, case (a)), was tested in three different environments contaminated by power-line noise. The ECG was measured three times for three days and the representative raw signals are shown in
Figure 16. The first signal was measured in an office, inside of a university building; the second one was measured outside of the university in a park area; and the last measurement was performed in the exterior, under high-voltage transmission lines (see
Figure 17 for ECG waveforms). The PSD estimates of signals are depicted in
Figure 16. As can be seen in
Figure 17, the 50 Hz power-line frequency is significantly reduced inside and outside of the building, while under the high-voltage cables, it has a power of about 10 dB. The reason for such attenuation of the power-line frequency when compared to PSD in
Figure 11 rests in powering the ECG system with a battery, causing high isolation impedance
ZISO between the power-line ground and the acquisition system ground (see
Figure 8).