Multimodal Breast Phantoms for Microwave, Ultrasound, Mammography, Magnetic Resonance and Computed Tomography Imaging
Abstract
:1. Introduction
2. Tissue-Mimicking Materials
2.1. Reference Values for Breast Tissues
2.2. Tissue-Mimicking Material Manufacturing Protocols
2.3. Characterization Protocols
2.4. Physical Properties Results
3. Anatomical Breast Phantom Mold Generation
4. Phantom Construction
5. Conventional Imaging Results
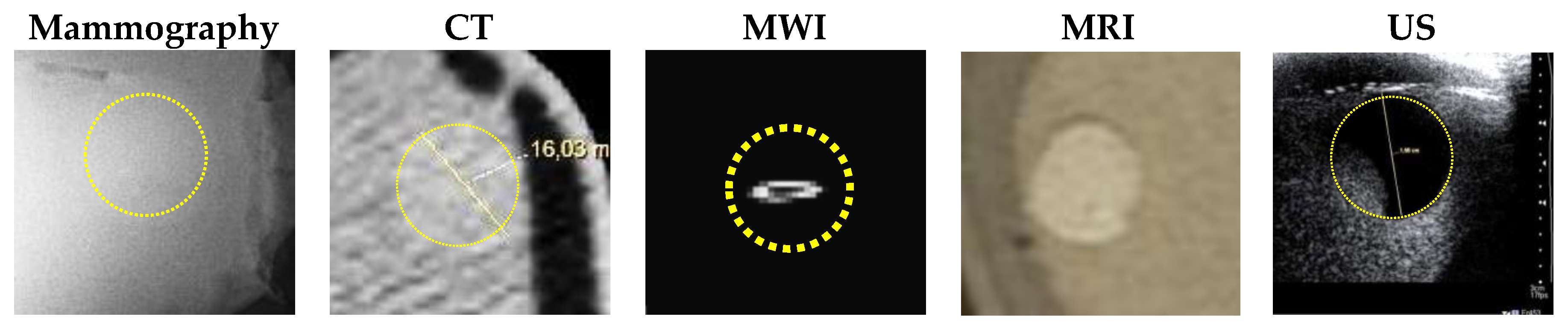
- MRI scanning was performed using a 3T system (Achieva, Philips Medical Systems, Netherlands). A high-resolution 3D T1-weighted image was acquired with a turbo gradient echo sequence, with an echo time TE of 2 ms, a repetition time TR of 4 ms and a flip angle of 10°. The spatial resolution was 0.4 × 0.4 × 0.8 mm3. A high-resolution 3D T2-weighted image was acquired using a 3D turbo spin echo sequence, with a TE of 378 ms and TR 2.5 ms. Resolution was 0.34 × 0.34 × 0.8 mm3. The high spatial and contrast resolutions of the resultant images facilitated their use as a reference for the other imaging modalities for localizing and characterizing the shape and size of the tumor. The MR images obtained for Phantom A and B are presented in Figure 4 and Figure 5. A low contrast both in terms of T1 and T2 can be observed between the tumor and the fibroglandular layer. However, the shape of the tumor can be distinguished from the fibroglandular background because of a chemical shift artefact at the interface between the two TMMs. This low contrast is consistent with that obtained clinically between malignant and fibroglandular tissues, which usually requires the injection of a contrast agent to enhance the visibility of the tumor.
- B-mode ultrasound scanning was performed using an Antares scanner (Siemens, Germany) with a VFX13-5 linear array transducer at a nominal frequency of 10 MHz. The output power was set to maximum and three focal zones were positioned at 10, 15 and 20 mm, which represented the entire length of the lesion. The maximum depth and FOV was set to 35 mm, which is representative of clinically relevant depth for breast ultrasound imaging. Representative ultrasound images of Phantom A and B are presented in Figure 6. All the layers can be distinguished, except for the pectoral muscle layer, which does not intersect with the scanning plane as this was beyond the typical clinically relevant field of view. Moreover, the indents in the tumor shape in Phantom A were accurately depicted in the ultrasound image. A mismatch in terms of speed of sound between the reference value (1479 ± 32 m/s) and the achieved one (1710 ± 17 m/s) for the breast fat, resulted in an underestimation of the thickness of the fat layer in the ultrasound image. This feature also occurs in clinical imaging where Coopers ligaments are present at the interface between fat and fibroglandular tissue; more importantly an accurate clinical contrast was achieved between the fibroglandular tissue and the tumor, with the tumor mimic being presented as a hypoechoic region within the image [45].
- Digital mammography scanning of Phantom A was performed with a Mammomat Novation DR system (Siemens Medical Solutions, Germany). The phantom was vertically taped to the device to reproduce the position of the breast during a mammographic exam (Figure 7). However, no compression was applied to the phantom as the fat-mimicking material was not elastically compressible. As a result, a poor contrast was observed between tumor and fibroglandular tissue. Phantom A was imaged at Mo/Rh 28 kVp and 140 mAs (Figure 8a) and at Mo/Rh 34 kVp and 160 mAs (Figure 8b), with a pixel spacing of 0.07 × 0.07 mm2, focal spot 0.3 mm and FOV 286 × 233 mm. The relative lack of contrast between the tumor and fibroglandular tissue was as a result of the lack of breast compression used during the acquisition, due to the lack of compressibility of the phantom materials. However, the relative contrast displayed between the fibroglandular and the tumor TMMs with the slightly higher kVp was slightly better. The bright external surface in each image is the skin TMM, while the darker irregular structure next to the skin is the subcutaneous fat TMM. The saturated white region is the fibroglandular tissue and the tumor mimic is within the red square; the contrast definition between both of these tissue types was challenging, which was representative of the clinical situation where dense breasts are imaged. Finally, as mentioned above, there was slightly better contrast between the fibroglandular tissue mimic and the tumor mimic with the slightly higher kVp. The same low contrast appears in the CT scanning results, performed with a Symbia TruePoint SPECT-CT scanner (Siemens Medical Solutions, Germany). The CT images were acquired at 80 kVp and 36 mAs, with a pixel spacing of 0.97 × 0.97 mm2 and slice thickness of 2 mm. In both phantoms, the tumor was localized using the MRI datasets as reference (Figure 5). Representative CT images of the phantoms are presented in Figure 9. As for the mammography images, a similarly low contrast was observed between the tumor and fibroglandular tissue. Nevertheless, the CT images demonstrated superior resolution of the internal structures, for example, showing the subcutaneous fat TMM fingers penetrating inside the fibroglandular layer.
6. Microwave Imaging Results
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madsen, E.L.; Zagzebski, J.A.; Frank, G.R.; Greenleaf, J.F.; Carson, P.L. Anthropomorphic breast phantoms for assessing ultrasonic imaging system performance and for training ultrasonographers: Part I. J. Clin. Ultrasound 1982, 10, 67–75. [Google Scholar] [CrossRef]
- Browne, J.E.; Cannon, L.M.; McDermott, R.; Ryan, M.; Fagan, A.J. Pilot Investigation into the Use of an Anthropomorphic Breast Sonography Phantom as a Training and Assessment Tool. Ultrasound Med. Biol. 2017, 43, 2733–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conceição, R.C.; Mohr, J.J.; O’Halloran, M. An Introduction to Microwave Imaging for Breast Cancer Detection; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Nikolova, N.K. Microwave imaging for breast cancer. IEEE Microw. Mag. 2011, 12, 78–94. [Google Scholar] [CrossRef]
- Grzegorczyk, T.M.; Meaney, P.M.; Kaufman, P.A.; Paulsen, K.D. Fast 3-D tomographic microwave imaging for breast cancer detection. IEEE Trans. Med. Imaging 2012, 31, 1584–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isernia, T.; Pascazio, V.; Pierri, R. On the local minima in a tomographic imaging technique. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1596–1607. [Google Scholar] [CrossRef]
- Li, X.; Bond, E.J.; Van Veen, B.D.; Hagness, S.C. An overview of ultra-wideband microwave imaging via space-time beamforming for early-stage breast-cancer detection. IEEE Antennas Propag. Mag. 2005, 47, 19–34. [Google Scholar]
- Fear, E.C.; Li, X.; Hagness, S.C.; Stuchly, M.A. Confocal microwave imaging for breast cancer detection: Localization of tumors in three dimensions. IEEE Trans. Biomed. Eng. 2002, 49, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Solimene, R.; Cuccaro, A.; Ruvio, G.; Tapia, D.F.; O’Halloran, M. Beamforming and holography image formation methods: An analytic study. Opt. Exp. 2016, 24, 9077–9093. [Google Scholar] [CrossRef]
- Ruvio, G.; Solimene, R.; Cuccaro, A.; Ammann, M.J. Comparison of noncoherent linear breast cancer detection algorithms applied to a 2-D numerical model. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 853–856. [Google Scholar] [CrossRef]
- Ruvio, G.; Solimene, R.; Cuccaro, A.; Gaetano, D.; Browne, J.E.; Ammann, M.J. Breast cancer detection using interferometric MUSIC: Experimental and numerical assessment. Med. Phys. 2014, 41, 103101. [Google Scholar] [CrossRef] [Green Version]
- Zastrow, E.; Davis, S.K.; Lazebnik, M.; Kelcz, F.; Van Veen, B.D.; Hagness, S.C. Development of anatomically realistic numerical breast phantoms with accurate dielectric properties for modeling microwave interactions with the human breast. IEEE Trans. Biomed. Eng. 2008, 55, 2792–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashal, A.; Gao, F.; Hagness, S.C. Heterogeneous anthropomorphic phantoms with realistic dielectric properties for microwave breast imaging experiments. Microw. Opt. Technol. Lett. 2011, 53, 1896–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burfeindt, M.J.; Colgan, T.J.; Mays, R.O.; Shea, J.D.; Behdad, N.; Van Veen, B.D.; Hagness, S.C. MRI-derived 3-D-printed breast phantom for microwave breast imaging validation. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 1610–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joachimowicz, N.; Duchêne, B.; Conessa, C.; Meyer, O. Anthropomorphic Breast and Head Phantoms for Microwave Imaging. Diagnostics 2018, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Conceição, R.C.; Rodrigues, D.B.; Oliveira, B.L.; Koutsoupidou, M.; Ruvio, G. Overview of microwave medical applications in Europe since the beginning of the COST action TD1301—MiMed. In Proceedings of the IEEE Proceedings of 11th European Conference on Antennas and Propagation (EuCAP), Paris, France, 19–24 March 2017. [Google Scholar]
- King, D.M.; Fagan, A.J.; Moran, C.M.; Browne, J.E. Comparative imaging study in ultrasound, MRI, CT, and DSA using a multimodality renal artery phantom. Med. Phys. 2011, 38, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Browne, J.E.; Gu, C.; Fazzio, R.T.; Fagan, A.J.; Tradup, D.J.; Hangiandreou, N.J. Use of Novel Anthropomorphic Breast Ultrasound Phantoms for Radiology Resident Education. J. Am. College Radiol. 2019, 16, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Yezitronix Group Inc. Phantom Technologies Medical Simulation & Training. Available online: https://www.yezitronix.com/ (accessed on 22 April 2020).
- Freed, M.; Zwart, J.A.; Loud, J.T.; El Khouli, R.H.; Myers, K.J.; Greene, M.H.; Duyn, J.H.; Badano, A. An anthropomorphic phantom for quantitative evaluation of breast MRI. Med. Phys. 2011, 38, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Cirs Multi-Modality Breast Biopsy and Sonographic Trainer. Available online: http://www.cirsinc.com/products/all/105/multi-modality-breast-biopsy-and-sonographic-trainer/ (accessed on 22 April 2020).
- Chmarra, M.K.; Hansen, R.; Mårvik, R.; Langø, T. Multimodal phantom of liver tissue. PLoS ONE 2013, 8, e64180. [Google Scholar] [CrossRef] [Green Version]
- Andreuccetti, D. An Internet Resource for the Calculation of the Dielectric Properties of Body Tissues in the Frequency Range 10 Hz–100 GHz. 2012. Available online: http://niremf.ifac.cnr.it/tissprop/ (accessed on 22 April 2020).
- Lazebnik, M.; McCartney, L.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; Okoniewski, M. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys. Med. Biol. 2007, 52, 2637–2656. [Google Scholar] [CrossRef] [Green Version]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093–6115. [Google Scholar] [CrossRef]
- Sugitani, T.; Kubota, S.-i.; Kuroki, S.-i.; Sogo, K.; Arihiro, K.; Okada, M.; Kadoya, T.; Hide, M.; Oda, M.; Kikkawa, T. Complex permittivities of breast tumor tissues obtained from cancer surgeries. Appl. Phys. Lett. 2014, 104, 253702. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, Y.; Tong, J.; Tang, Z. Ultrasonic Propagation Parameters in Human Tissues. In Proceedings of the IEEE 1986 Ultrasonics Symposium, Williamsburg, VA, USA, 17–19 November 1986; pp. 905–908. [Google Scholar]
- Scherzinger, A.L.; Belgam, R.A.; Carson, P.L.; Meyer, C.R.; Sutherland, J.V.; Bookstein, F.L.; Silver, T.M. Assessment of ultrasonic computed tomography in symptomatic breast patients by discriminant analysis. Ultrasound Med. Biol. 1989, 15, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Goss, S.; Johnston, R.; Dunn, F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J. Acoust. Soc. Am. 1978, 64, 423–457. [Google Scholar] [CrossRef]
- Dussik, K.T.; Fritch, D.J.; Kyriazidou, M.; Sear, R.S. Measurements of articular tissues with ultrasound. Am. J. Phys. Med. Rehab. 1958, 37, 160–165. [Google Scholar] [CrossRef]
- d’Astous, F.; Foster, F. Frequency dependence of ultrasound attenuation and backscatter in breast tissue. Ultrasound Med. Biol. 1958, 12, 795–808. [Google Scholar] [CrossRef]
- Rakow-Penner, R.; Daniel, B.; Yu, H.; Sawyer-Glover, A.; Glover, G.H. Relaxation times of breast tissue at 1.5 T and 3T measured using IDEAL. J. Magn. Reson. Imaging 2006, 23, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Database of Tissue Properties: Relaxation Time. Available online: https://www.itis.ethz.ch/virtual-population/tissue-properties/database/relaxation-times/ (accessed on 22 April 2020).
- Partridge, S.C.; DeMartini, W.B.; Kurland, B.F.; Eby, P.R.; White, S.W.; Lehman, C.D. Differential diagnosis of mammographically and clinically occult breast lesions on diffusion-weighted MRI. J. Magn. Reson. Imaging 2010, 31, 562–570. [Google Scholar] [CrossRef]
- Johns, P.C.; Yaffe, M.J. X-ray characterisation of normal and neoplastic breast tissues. Phys. Med. Biol. 1987, 32, 675. [Google Scholar] [CrossRef]
- Teirlinck, C.J.; Bezemer, R.A.; Kollmann, C.; Lubbers, J.; Hoskins, P.R.; Ramnarine, K.V.; Fish, P.; Fredfeldt, K.-E.; Schaarschmidt, U.G. Development of an example flow test object and comparison of five of these test objects, constructed in various laboratories. Ultrasonics 1998, 36, 653–660. [Google Scholar] [CrossRef]
- King, D.M.; Moran, C.M.; McNamara, J.D.; Fagan, A.J.; Browne, J.E. Development of a vessel-mimicking material for use in anatomically realistic Doppler flow phantoms. Ultrasound Med. Biol. 2011, 37, 813–826. [Google Scholar] [CrossRef] [Green Version]
- Cournane, S.; Cannon, L.; Browne, J.; Fagan, A. Assessment of the accuracy of an ultrasound elastography liver scanning system using a PVA-cryogel phantom with optimal acoustic and mechanical properties. Phys. Med. Biol. 2010, 55, 5965. [Google Scholar] [CrossRef] [PubMed]
- Cannon, L.M.; Fagan, A.J.; Browne, J.E. Novel tissue mimicking materials for high frequency breast ultrasound phantoms. Ultrasound Med. Biol. 2011, 37, 122–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AIUM. Methods for Specifying Acoustic Properties of Tissue Mimicking Phantoms and Objects, Stage I; American Institute of Ultrasound in Medicine: Laurel, MD, USA, 1995.
- Brewin, M.; Pike, L.; Rowland, D.; Birch, M. The acoustic properties, centered on 20 MHz, of an IEC agar-based tissue-mimicking material and its temperature, frequency and age dependence. Ultrasound Med. Biol. 2008, 34, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Farina, L.; Ruvio, G.; Pinto, R.; Vannucci, L.; Cavagnaro, M.; Lopresto, V. Development of a portable setup suitable for in vivo measurement of the dielectric properties of biological tissues. Proceedings of 10th European Conference on Antennas and Propagation (EuCAP), IEEE, Paris, France, 19–24 March 2017; pp. 2732–2736. [Google Scholar]
- Ruvio, G.; Vaselli, M.; Lopresto, V.; Pinto, R.; Farina, L.; Cavagnaro, M. Comparison of different methods for dielectric properties measurements in liquid sample media. Int. J. RF Microw. Comput.-Aided Eng. 2018, 28, e21215. [Google Scholar] [CrossRef]
- Carson, P.; Wang, B.; LeCarpentier, G.; Goodsitt, M.; Lashbrook, C.; Pinsky, R.; Narayanasamy, G.; Fowlkes, J.B.; Saitou, K. Local Compression in Automated Breast Ultrasound in the Mammographic Geometry. In Proceedings of the IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; pp. 1787–1790. [Google Scholar]
- Simonetti, F.; Huang, L.; Duric, N.; Littrup, P. Diffraction and coherence in breast ultrasound tomography: A study with a toroidal array. Med. Phys. 2009, 36, 2955–2965. [Google Scholar] [CrossRef]
- Ruvio, G.; Cuccaro, A.; Solimene, R.; Brancaccio, A.; Basile, B.; Ammann, M.J. Microwave bone imaging: A preliminary scanning system for proof-of-concept. IEEE Healthc. Technol. Lett. 2016, 3, 218. [Google Scholar] [CrossRef] [Green Version]
- Solimene, R.; Cuccaro, A. Front wall clutter rejection methods in TWI. IEEE Geosci. Remote Sens. Lett. 2014, 11, 1158–1162. [Google Scholar] [CrossRef]
- Tivive, F.H.C.; Amin, M.G.; Bouzerdoum, A. Wall clutter mitigation based on eigen-analysis in through-the-wall radar imaging. Proceedings of 17th IEEE International Conference on Digital Signal Processing (DSP), Corfu, Greece, 6–8 July 2011. [Google Scholar]
- Solimene, R.; D’Alterio, A. Entropy-based clutter rejection for intrawall diagnostics. Int. J. Geophys. 2012, 2012, 418084. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.F.; Rutt, B.K.; Holdsworth, D.W. Anthropomorphic carotid bifurcation phantom for MRI applications. J. Magn. Reson. Imaging 1999, 10, 533–544. [Google Scholar] [CrossRef]
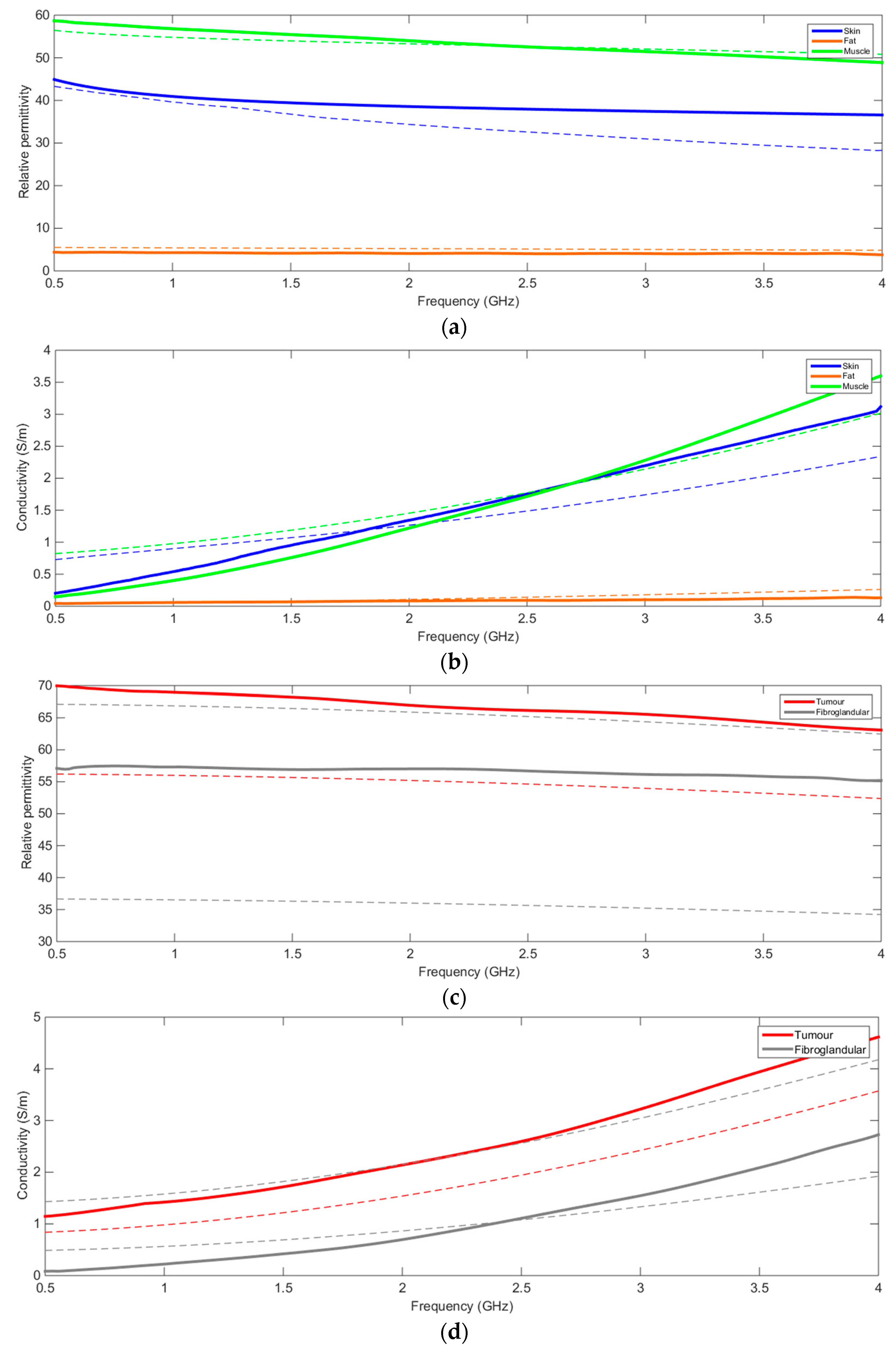










| Dielectric Properties | Acoustic Properties | Magnetic Resonance Relaxometry Properties | X-Ray Attenuation Properties | ||||
|---|---|---|---|---|---|---|---|
| Relative Permittivity at 2.5 GHz | Conductivity (S/m) at 2.5 GHz | Speed of Sound () | Attenuation Coefficient at 7 MHz () | Spin-Lattice Relaxation Time @ 3T | Spin-Spin Relaxation Time @ 3T | HU at 80 kVp | |
| Skin | 38.007 [23] | 1.464 [25] | 1537 [29] | 1.84 ± 0.44 [31] | (No reference) | (No reference) | (No reference) |
| Subcutaneous Fat | 5.1467 [25] | 0.137 [25] | 1479 ± 32 [30] | 0.6 ± 0.1 [31] | 367 ± 8 [32] | 53 ± 2 [32,33] | −102 −80 [35] |
| Fibroglandular tissue | 35.7–65.3 [26,27] | 1.52–2.37 [26,27] | 1553 ± 35 [30] | 2.0 ± 0.7 [31] | 1445 ± 93 [32] | 54 ± 9 [33] | 12 56 [35] |
| Carcinoma | 54.69 [28] | 1.89 [28] | 1550 ± 35 [30] | 1.0 ± 0.2 [31] | 876 ± 28 at 1.5T [33] | 75 ± 4 at 1.5T [33] | 23 78 [35] |
| Pectoral Muscle | 52.729 [25] | 1.7388 [25] | 1545 ± 5 [30] | (No reference) | 607 ± 85 [33] | 36 ± 7 [33] | (No reference) |
| Skin | Fibroglandular | Tumor | Pectoral Muscle | |
|---|---|---|---|---|
| Polyvinyl Alcohol Cryogel | 80 | - | - | - |
| Agar | - | 24 | 30 | 27 |
| SiC | - | 4.24 | - | - |
| Al2O3 (3 μm) | - | 7.6 | - | - |
| Al2O3 (0.3 μm) | - | 7.04 | - | - |
| Sugar | 480 | - | 200 | 360 |
| NaCl | - | - | 10 | - |
| Glycerol | - | 89.7 | - | - |
| Deionized water | 720 | 663.8 | 8.73 | 873 |
| Benzalkonium Chloride | 4 | 3.7 | 4.14 | 4.14 |
| 10% Synperonic A7 Surfactant | 40 | 40 | - | 20 |
| Olive Oil | 160 | 160 | - | 80 |
| Speed of Sound () @ 7.5 MHz | Attenuation Coefficient () @ 7.5 MHz | (ms) at 3T | (ms) at 3T | HU at 80 kVp | |
|---|---|---|---|---|---|
| Skin | 1657 ± 1 (+8%) | 1.77 ± 0.01 (+4%) | 292 ± 6 (No reference) | 33 ± 0.5 (No reference) | 100.75 ± 28.9 (No reference) |
| Fat | 1710 ± 17 (+15%) | 4.16 ± 0.05 (+593%) | 300–521 (Within range) | 40–84 (Within range) | −108.78 ± 17.2 (+7%) |
| Fibroglandular tissue | 1532 ± 4 (−1%) | 0.73 ± 0.01 (−22%) | 624 ± 10 (−57%) | 35.5 ± 0.6 (−36%) | 46.88 ± 23.9 (+280%) |
| Tumor | 1573 ± 1 (+1.5%) | 0.09 ± 0.01 (−91%) | 915 ± 14 (+4%) | 36 ± 0.6 (−50%) | 65.94 ± 31.5 (+183%) |
| Pectoral Muscle | 1577 ± 2.5 (+2%) | 0.29 ± 0.01 (No reference) | 786 ± 20 (Within range) | 39 ± 1 (+8%) | 52.25 ± 25.6 (No reference) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruvio, G.; Solimene, R.; Cuccaro, A.; Fiaschetti, G.; Fagan, A.J.; Cournane, S.; Cooke, J.; Ammann, M.J.; Tobon, J.; Browne, J.E. Multimodal Breast Phantoms for Microwave, Ultrasound, Mammography, Magnetic Resonance and Computed Tomography Imaging. Sensors 2020, 20, 2400. https://doi.org/10.3390/s20082400
Ruvio G, Solimene R, Cuccaro A, Fiaschetti G, Fagan AJ, Cournane S, Cooke J, Ammann MJ, Tobon J, Browne JE. Multimodal Breast Phantoms for Microwave, Ultrasound, Mammography, Magnetic Resonance and Computed Tomography Imaging. Sensors. 2020; 20(8):2400. https://doi.org/10.3390/s20082400
Chicago/Turabian StyleRuvio, Giuseppe, Raffaele Solimene, Antonio Cuccaro, Gaia Fiaschetti, Andrew J. Fagan, Sean Cournane, Jennie Cooke, Max J. Ammann, Jorge Tobon, and Jacinta E. Browne. 2020. "Multimodal Breast Phantoms for Microwave, Ultrasound, Mammography, Magnetic Resonance and Computed Tomography Imaging" Sensors 20, no. 8: 2400. https://doi.org/10.3390/s20082400
APA StyleRuvio, G., Solimene, R., Cuccaro, A., Fiaschetti, G., Fagan, A. J., Cournane, S., Cooke, J., Ammann, M. J., Tobon, J., & Browne, J. E. (2020). Multimodal Breast Phantoms for Microwave, Ultrasound, Mammography, Magnetic Resonance and Computed Tomography Imaging. Sensors, 20(8), 2400. https://doi.org/10.3390/s20082400









