Assessment of Electronic Sensing Techniques for the Rapid Identification of Alveolar Echinococcosis through Exhaled Breath Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples Collection
2.2. Gas Sensors System
- S1: TGS8100 sensor (commercial sensor acquired from Figaro USA, Inc), optimized for target gases such as ethanol and hydrogen.
- S2 and S3: Two MiCS-6814 sensors (commercial sensors acquired from SGX Sensortech Limited, UK), containing different gas-sensitive layers optimized for NH3 and CO detection, respectively. S2 also detects other gases such as ethanol, hydrogen, propane and iso-butane, and S3 gases such as H2S, ethanol, hydrogen, ammonia, methane, propane and iso-butane.
- S4: Custom sensor based on a ~159 nm thick WO3 nanowires layer doped with gold nanoparticles, fabricated by catalyst free, relatively low temperature single-step Aerosol Assisted Chemical Vapor Deposition (AACVD). Detailed information on the fabrication and characterization of this sensor can be found elsewhere [16].
2.3. Sensing Measurements
- Opening the test chamber and cleaning it with synthetic air flow (100 mL/min flow-rate) for at least 25 min.
- Closing the test chamber and waiting approximately 30 min for the stabilization of sensors baseline.
- Measuring DC and AC components by recording the voltage in independent channels for each sensor.
- Producing vacuum, up to 420 mmHg, for approximately 20 sec in the test chamber by means of an external pump (model SAILFO 1A50361), just before injecting the breath VOCs sample.
- Injecting 20 mL of breath VOCs sample in the test chamber by means of a commonly used disposable medical gas syringe (Becton-Dickinson Discordit II model). To this end, the sorbent Tenax TA material storing the breath sample was previously introduced in a sealed glass vial (20 mL volume) provided with a septum, which was heated at 200 ºC for 15 min in an oven. This produced the thermal desorption of the absorbed breath VOCs, which were captured by the gas syringe by introducing its needle through the septum, and then injected into the sensors test chamber.
- Waiting ~30 min for the stabilization of sensors responses.
- Measuring the DC and AC components by separately recording the voltages for the different sensors.
2.4. Data Analysis
- DC measurements:
- F1: ∆R/R0. Relative resistance change after sample exposure. ∆R = Rmin – R0 is the resistance drop after sample exposure, where Rmin is the minimum resistance of the sensor after sample exposure and R0 is the sensor’s resistance in synthetic air immediately before the short vacuum step that preceded sample exposure;
- F2: dR/dt. Dynamic slope of the resistance calculated at 30 s after sample injection;
- F3: AUC. Area under the curve during 13 min of sample exposure
- 2.
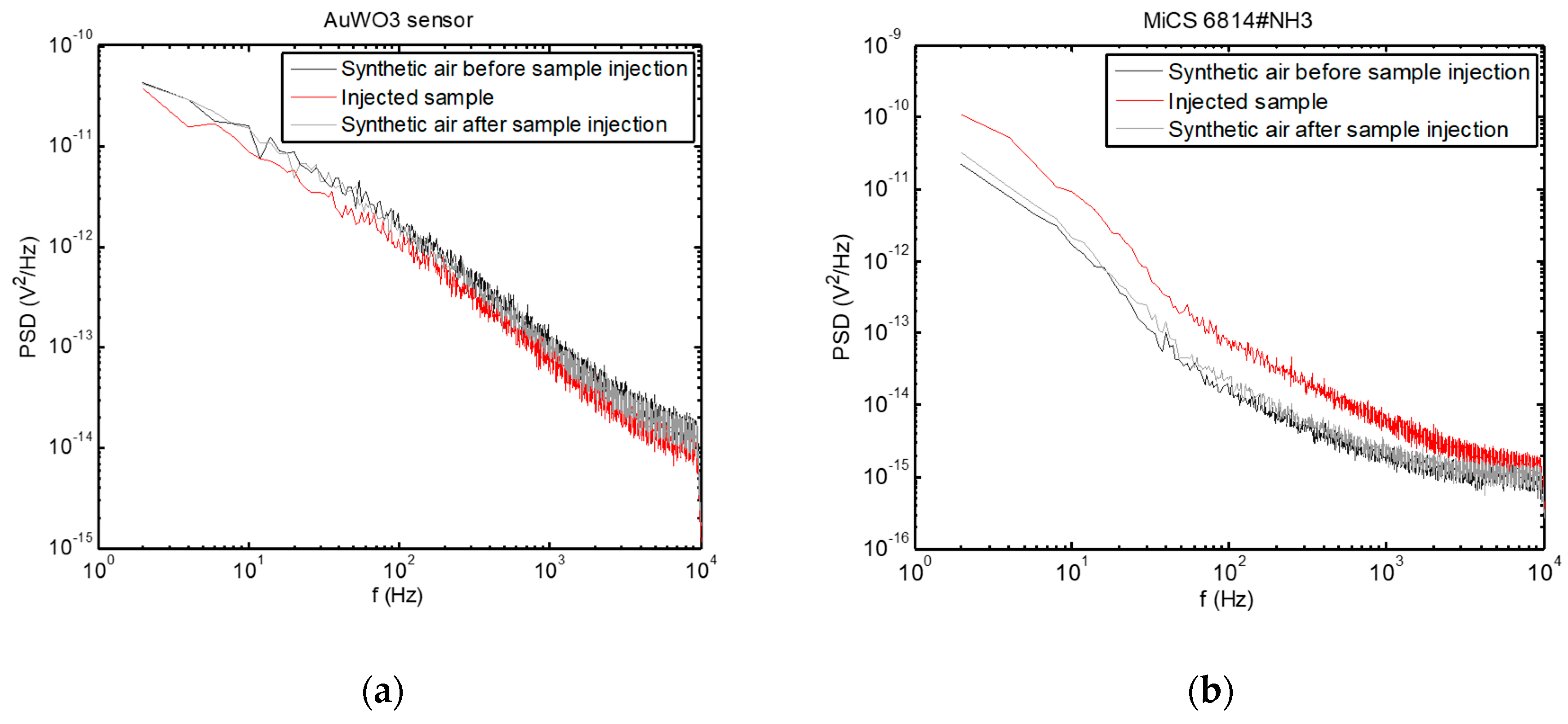
- AC measurements:
- F4: PSD value at 180 Hz, averaged over 11 points around this frequency
- F5: PSD value at 400 Hz, averaged over 11 points around this frequency
- F6: PSD value at 1000 Hz, averaged over 11 points around this frequency
- F7: PSD value at 5000 Hz, averaged over 11 points around this frequency
3. Results
3.1. Sensors Responses
3.2. Classification Results
4. Discussion
4.1. AC versus DC Measurements
4.2. Sensors Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethical Statement
References
- World Health Organisation. Echinococcosis. Available online: https://www.who.int/news-room/fact-sheets/detail/echinococcosis (accessed on 31 March 2020).
- Deplazes, P.; Rinaldi, L.; Rojas, C.A.; Torgerson, P.; Harandi, M.; Romig, T.; Antolova, D.; Schurer, J.; Lahmar, S.; Cringoli, G. Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar]
- Gottstein, B.; Stojkovic, M.; Vuitton, D.A.; Millon, L.; Marcinkute, A.; Deplazes, P. Threat of alveolar echinococcosis to public health—A challenge for Europe. Trends Parasitol. 2015, 31, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebezov, B.; Mamashev, N.; Umetaliev, T.; Ziadinov, I.; Craig, P.S.; Joekel, D.E.; Deplazes, P.; Grimm, F.; Torgerson, P.R. Intense focus of alveolar echinococcosis, South Kyrgyzstan. Emerg. Infect. Dis. 2018, 24, 1119. [Google Scholar] [CrossRef] [PubMed]
- Mihmanli, M.; Idiz, U.O.; Kaya, C.; Demir, U.; Bostanci, O.; Omeroglu, S.; Bozkurt, E. Current status of diagnosis and treatment of hepatic echinococcosis. World J. Hepatol. 2016, 8, 1169. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis-focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef]
- Ionescu, R.; Broza, Y.; Shaltieli, H.; Sadeh, D.; Zilberman, Y.; Feng, X.; Glass-Marmor, L.; Lejbkowicz, I.; Müllen, K.; Miller, A.; et al. Detection of multiple sclerosis from exhaled breath using bilayers of polycyclic aromatic hydrocarbons and single-wall carbon nanotubes. ACS Chem. Neurosci. 2011, 2, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Thaler, E.R.; Kennedy, D.W.; Hanson, C.W. Medical applications of electronic nose technology: Review of current status. Am. J. Rhinol. 2001, 15, 291–295. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Zhang, D.; Li, N.; Zhang, L.; Yang, J. A novel breath analysis system based on electronic olfaction. IEEE Trans. Biomed. Eng. 2010, 57, 2753–2763. [Google Scholar]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Konvalina, G.; Haick, H. Sensors for breath testing: From nanomaterials to comprehensive disease detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Lentka, Ł.; Kotarski, M.; Smulko, J.; Cindemir, U.; Topalian, Z.; Granqvist, C.G.; Calavia, R.; Ionescu, R. Fluctuation-enhanced sensing with organically functionalized gold nanoparticle gas sensors targeting biomedical applications. Talanta 2016, 160, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welearegay, T.G.; Diouani, M.F.; Österlund, L.; Borys, S.; Khaled, S.; Smadhi, H.; Ionescu, F.; Bouchekoua, M.; Aloui, D.; Laouini, D. Diagnosis of human echinococcosis via exhaled breath analysis: A promise for rapid diagnosis of infectious diseases caused by helminths. J. Infect. Dis. 2018, 219, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Saidi, T.; Palmowski, D.; Babicz-Kiewlicz, S.; Welearegay, T.G.; El Bari, N.; Ionescu, R.; Smulko, J.; Bouchikhi, B. Exhaled breath gas sensing using pristine and functionalized WO3 nanowire sensors enhanced by UV-light irradiation. Sens. Actuators B Chem. 2018, 273, 1719–1729. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar]
- Sinues, P.M.-L.; Kohler, M.; Zenobi, R. Human breath analysis may support the existence of individual metabolic phenotypes. PLoS ONE 2013, 8, e59909. [Google Scholar]
- Kish, L.; Vajtai, R.; Granqvist, C. Extracting information from noise spectra of chemical sensors: Single sensor electronic noses and tongues. Sens. Actuators B Chem. 2000, 71, 55–59. [Google Scholar] [CrossRef]
- Ionescu, R.; Llobet, E.; Vilanova, X.; Brezmes, J.; Sueiras, J.E.; Calderer, J.; Correig, X. Quantitative analysis of NO2 in the presence of CO using a single tungsten oxide semiconductor sensor and dynamic signal processing. Analyst 2002, 127, 1237–1246. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kolek, A.; Licznerski, B. Noise and nonlinearity of gas sensors—Preliminary results. In Proceedings of the 22nd International Spring Seminar on Electronics Technology, Dresden-Freital, Germany, 18–20 May 1999; pp. 99–104. [Google Scholar]
- Rumyantsev, S.; Liu, G.; Shur, M.S.; Potyrailo, R.A.; Balandin, A.A. Selective gas sensing with a single pristine graphene transistor. Nano Lett. 2012, 12, 2294–2298. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A. Low-frequency 1/f noise in graphene devices. Nat. Nanotechnol. 2013, 8, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Gomri, S.; Seguin, J.-L.; Guerin, J.; Aguir, K. Adsorption–desorption noise in gas sensors: Modelling using Langmuir and Wolkenstein models for adsorption. Sens. Actuators B Chem. 2006, 114, 451–459. [Google Scholar] [CrossRef]
- Kotarski, M.M.; Smulko, J.M. Hazardous gases detection by fluctuation-enhanced gas sensing. Fluct. Noise Lett. 2010, 9, 359–371. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.-B.; Loukas, A.; Lin, R.-Y.; Ito, A.; Zhang, L.-H.; Jones, M.; McManus, D.P. Functional expression and characterization of Echinococcus granulosus thioredoxin peroxidase suggests a role in protection against oxidative damage. Gene 2004, 326, 157–165. [Google Scholar] [CrossRef]
- Liebau, E.; Müller, V.; Lucius, R.; Walter, R.D.; Henkle-Dührsen, K. Molecular cloning, expression and characterization of a recombinant glutathione S-transferase from Echinococcus multilocularis. Mol. Biochem. Parasitol. 1996, 77, 49–56. [Google Scholar] [CrossRef]
- Lee, S.C.; San Tang, M.; Lim, Y.A.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl. Trop. Dis. 2014, 8, e2880. [Google Scholar] [CrossRef]
- Hibbard, T.; Killard, A.J. Breath ammonia analysis: Clinical application and measurement. Crit. Rev. Anal. Chem. 2011, 41, 21–35. [Google Scholar] [CrossRef]
- Lee, I.; Choi, S.-J.; Park, K.-M.; Lee, S.S.; Choi, S.; Kim, I.-D.; Park, C. The stability, sensitivity and response transients of ZnO, SnO2 and WO3 sensors under acetone, toluene and H2S environments. Sens. Actuators B Chem. 2014, 197, 300–307. [Google Scholar] [CrossRef]
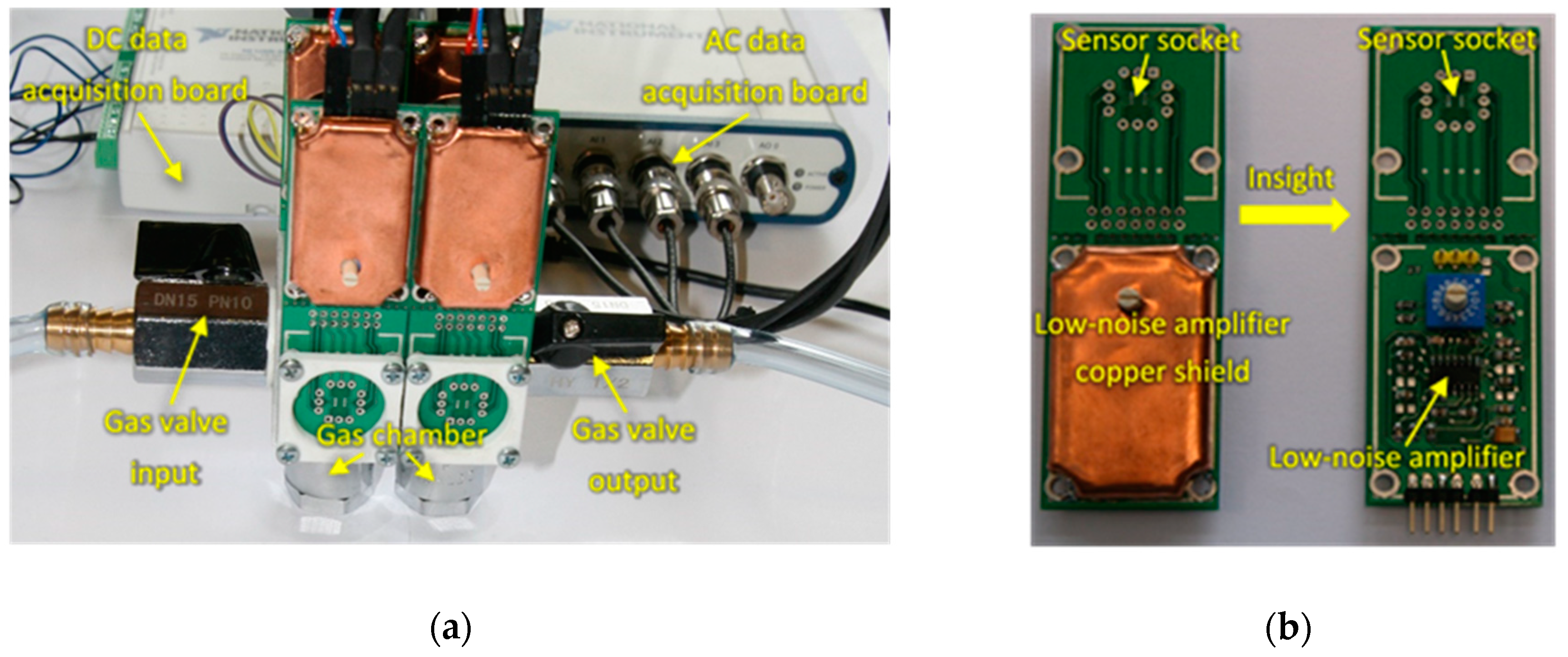

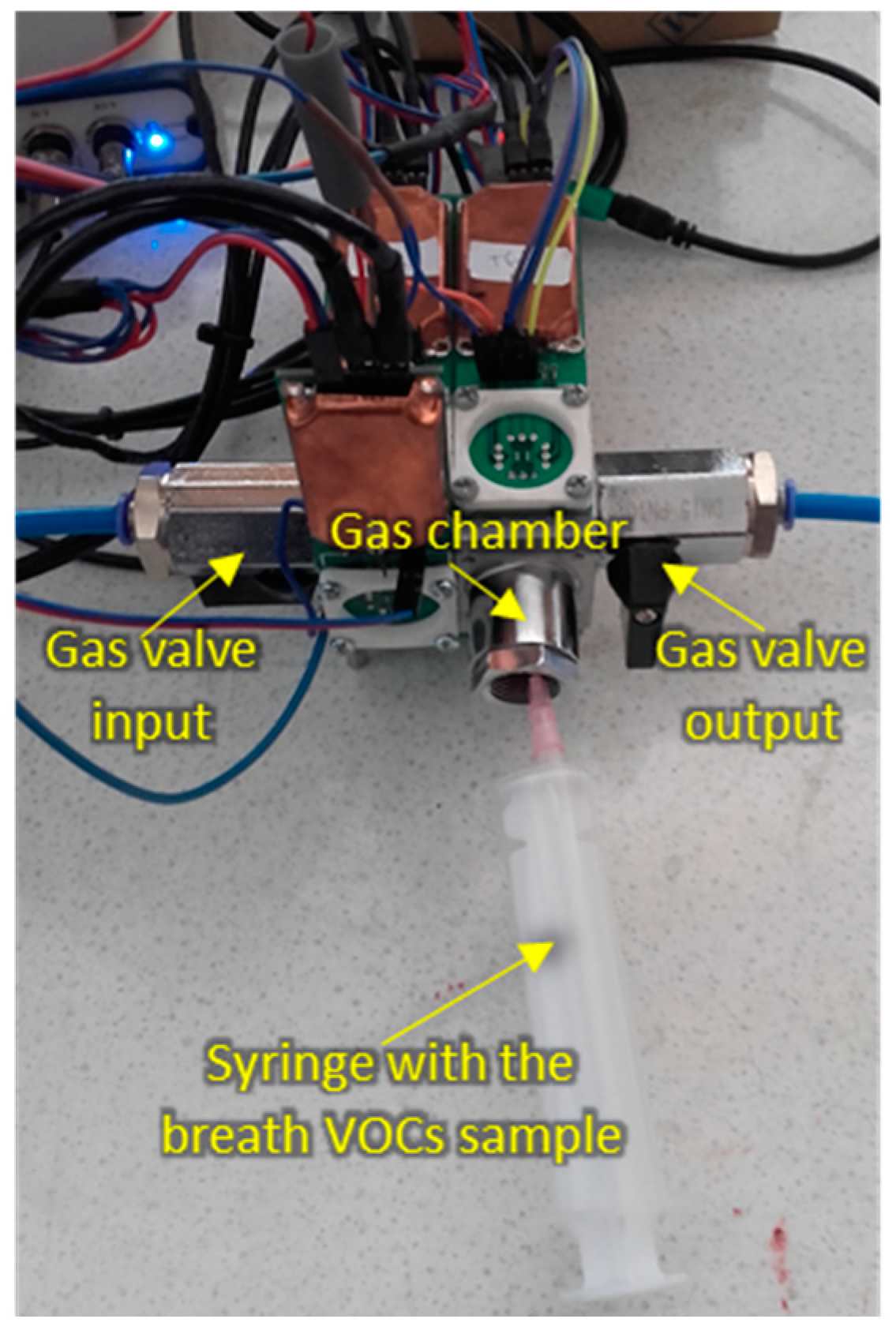




| Patient no. | Disease 1 | Age | Gender 2 | Smoking Habit | Medication |
|---|---|---|---|---|---|
| 1 5 | AE | 69 | F | No | Sulfasalazine, Acidum, Folicum |
| 2 | Not AE | 36 | M | No | - |
| 3 5 | AE | 58 | M | No | Ramipril, Nebivolol, Propafenone |
| 4 4 | AE | 49 | M | Yes | Albendazole, Amlodipine, Bisoprolol |
| 5 4 | AE | 74 | F | No | Enalapril, Amlodipine |
| 6 5 | AE | 73 | F | No | Albendazole, Indapamide, Ramipril, Bisoprolol |
| 7 | Not AE | 30 | F | No | - |
| 8 4 | AE | 61 | F | Yes | Albendazole, Furosemide, Spironolactone, Propranolol |
| 9 | Not AE | 50 | F | No | - |
| 10 5 | AE | 54 | F | No | Albendazole, Bisoprolol, Perindopril |
| 11 4 | AE | 60 | F | No | Albendazole, Furosemide, Spironolactone |
| 12 5 | AE | 75 | F | No | Albendazole, Valsartan, Metformin |
| 13 | Not AE | 46 | F | No | Ramipril |
| 14 | Not AE | 44 | F | No | - |
| 15 5 | AE | 35 | F | No | Albendazole |
| 16 3,5 | AE after liver transplantation | 59 | F | No | Albendazole, Tacrolimus, Calcium |
| 17 | None | 37 | M | No | - |
| Sensors | Features | Classification Success Rate | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Individual sensors | S1 | F1,F5,F6 | 49.1 ± 20.2% | 58.7 ± 29.5% | 31.2 ± 36.8% |
| S2 | F4,F6 | 49.7 ± 20.3% | 57.8 ± 30.7% | 34.5 ± 37.3% | |
| S3 | F5,F6 | 63.4 ± 20.0% | 66.3 ± 27.6% | 54.6 ± 41.9% | |
| S4 | F3,F6 | 45.6 ± 21.8% | 49.2 ± 30.9% | 37.4 ± 37.4% | |
| Sensors array | DC measure-ments | S1: F1,F3 S2: F1,F3 S4: F2 | 73.4 ± 19.3% | 75.9 ± 24.5% | |
| 64.2 ± 39.6% | |||||
| AC measure-ments | S1: F1,F4 S2: F1 S3: F1 S4: F1,F2 | 90.2 ± 16.4% | 93.6 ± 16.8% | 79.4 ± 35.8% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, A.; Chludziński, T.; Saidi, T.; Welearegay, T.G.; Jaimes-Mogollón, A.L.; El Bari, N.; Borys, S.; Bouchikhi, B.; Smulko, J.; Ionescu, R. Assessment of Electronic Sensing Techniques for the Rapid Identification of Alveolar Echinococcosis through Exhaled Breath Analysis. Sensors 2020, 20, 2666. https://doi.org/10.3390/s20092666
Kwiatkowski A, Chludziński T, Saidi T, Welearegay TG, Jaimes-Mogollón AL, El Bari N, Borys S, Bouchikhi B, Smulko J, Ionescu R. Assessment of Electronic Sensing Techniques for the Rapid Identification of Alveolar Echinococcosis through Exhaled Breath Analysis. Sensors. 2020; 20(9):2666. https://doi.org/10.3390/s20092666
Chicago/Turabian StyleKwiatkowski, Andrzej, Tomasz Chludziński, Tarik Saidi, Tesfalem Geremariam Welearegay, Aylen Lisset Jaimes-Mogollón, Nezha El Bari, Sebastian Borys, Benachir Bouchikhi, Janusz Smulko, and Radu Ionescu. 2020. "Assessment of Electronic Sensing Techniques for the Rapid Identification of Alveolar Echinococcosis through Exhaled Breath Analysis" Sensors 20, no. 9: 2666. https://doi.org/10.3390/s20092666
APA StyleKwiatkowski, A., Chludziński, T., Saidi, T., Welearegay, T. G., Jaimes-Mogollón, A. L., El Bari, N., Borys, S., Bouchikhi, B., Smulko, J., & Ionescu, R. (2020). Assessment of Electronic Sensing Techniques for the Rapid Identification of Alveolar Echinococcosis through Exhaled Breath Analysis. Sensors, 20(9), 2666. https://doi.org/10.3390/s20092666








