Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples
Abstract
:1. Introduction
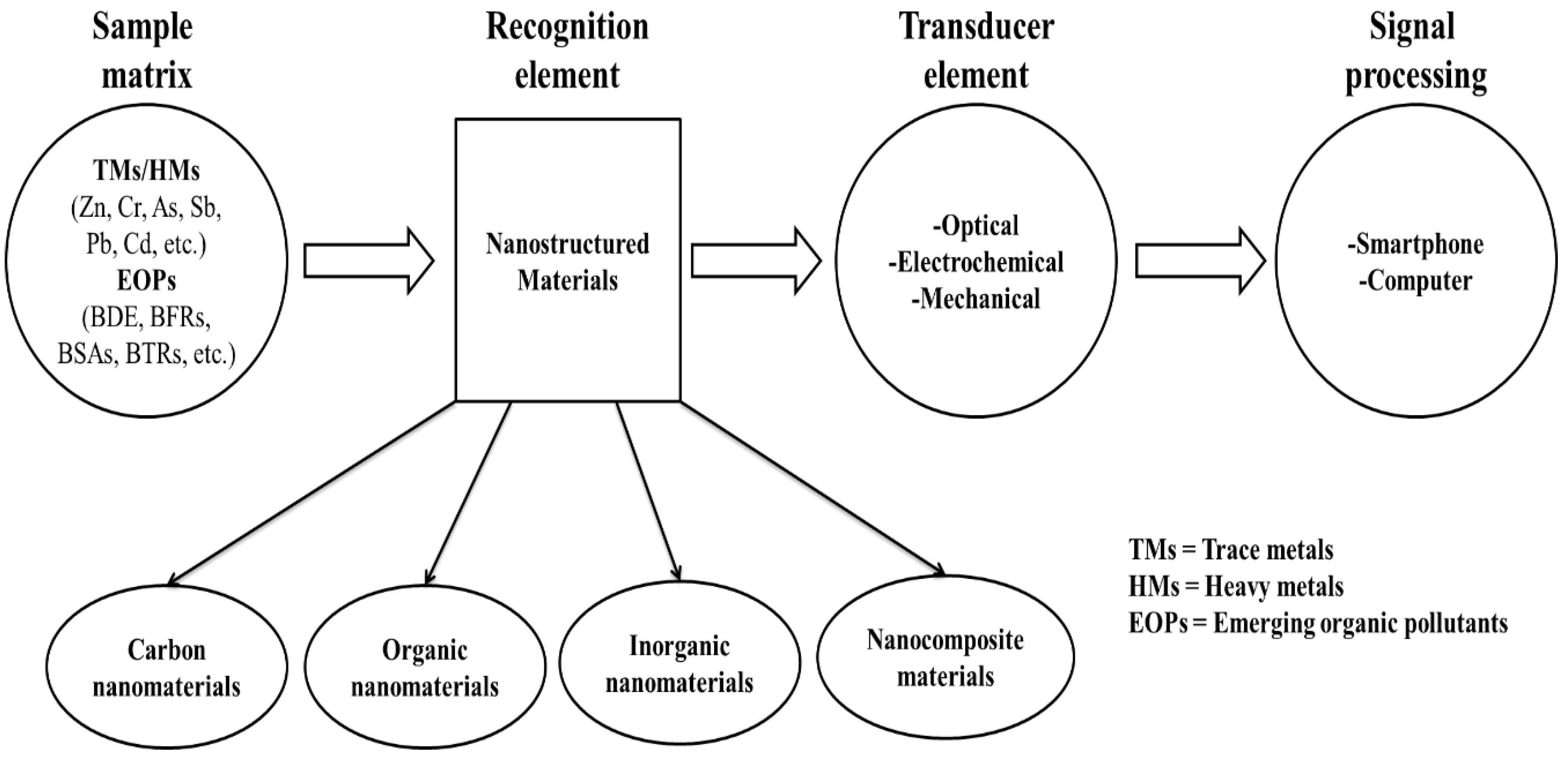
2. Nanosensors
3. Nanosensor Fabrication
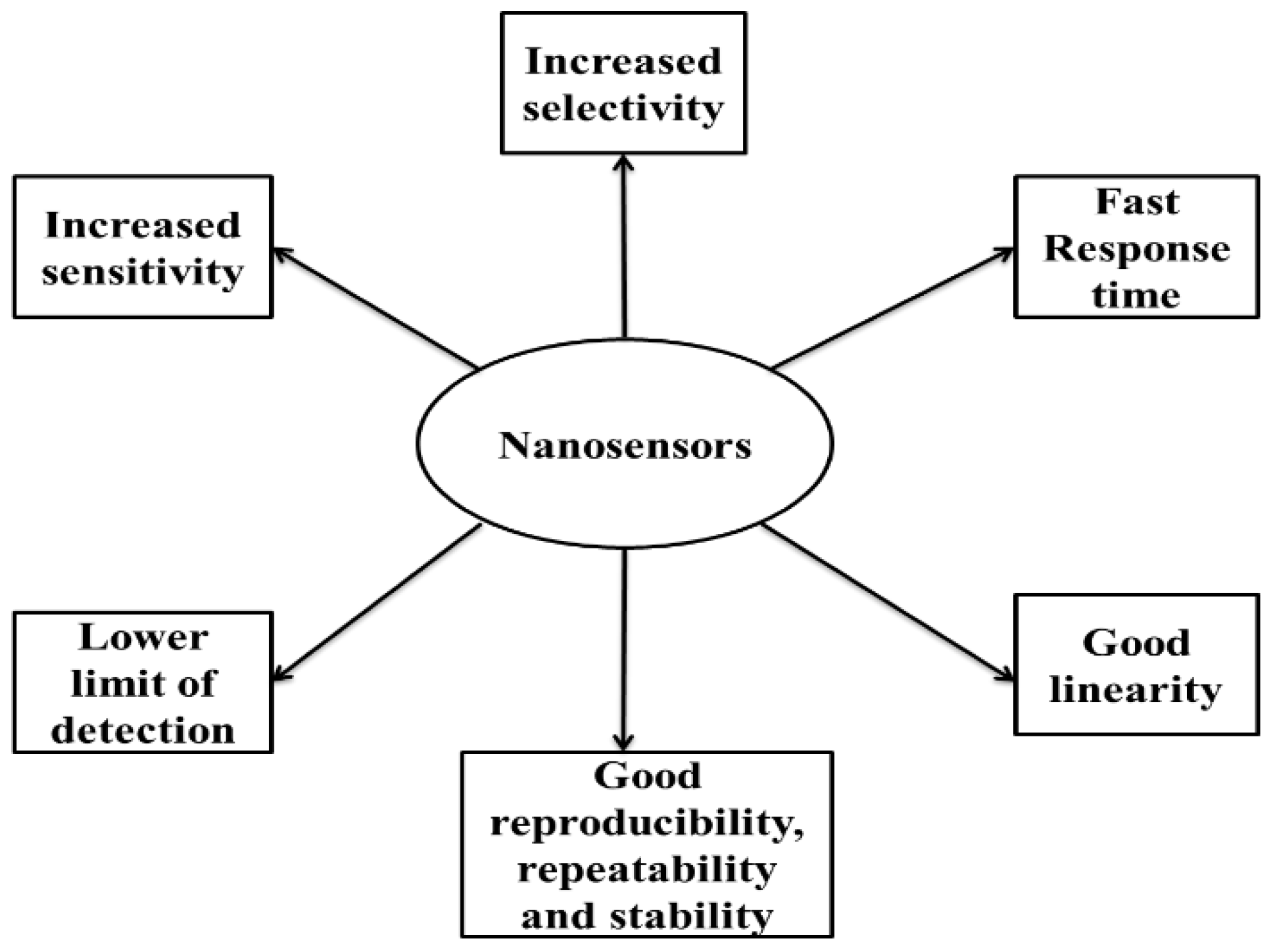
4. Performance Parameters of Nanosensors
4.1. Increased Sensitivity
4.2. Good Linearity
4.3. Lower Limit of Detection
4.4. Increased Selectivity
4.5. Fast Response Time
4.6. Good Reproducibility, Repeatability and Stability
5. Nanocomposites in Electrochemical Sensors for Trace Metal Detection
5.1. Carbon Based Nanomaterials
5.1.1. Graphene-Based Nanocomposites
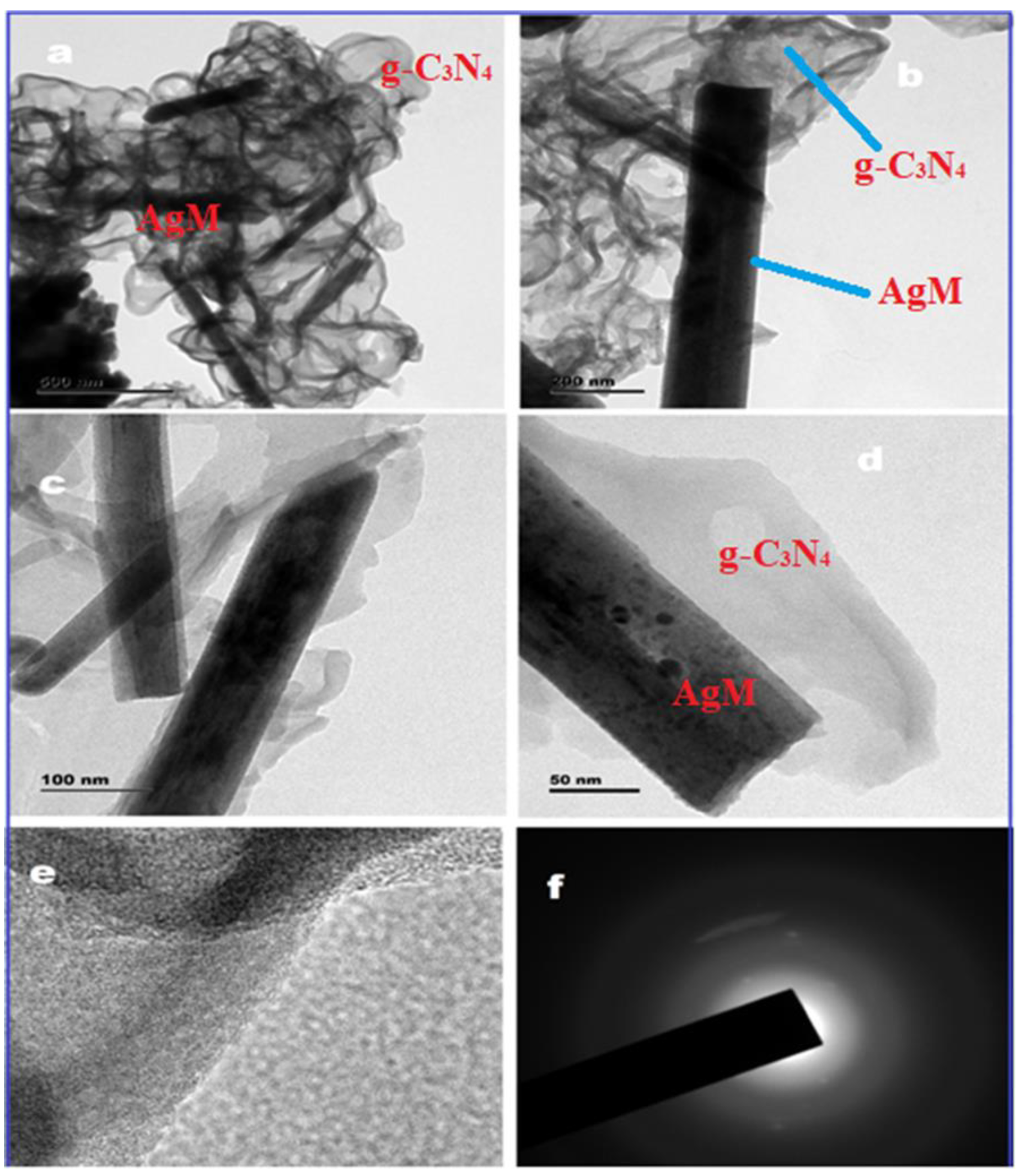
5.1.2. Graphitic Carbon Nitride-Based Nanocomposites
| Nanomaterial/Electrode | Synthesis Method | Target Analyte | Technique | LOD | Linear Range | RSD (%) | n | Sensitivity | Ref |
|---|---|---|---|---|---|---|---|---|---|
| g-C3N4/AgM/Nf/GCE | Sonochemical | Cr6+ | Amp | 0.002 (µM) | 0.1–0.7 (µM) | 2.83 | 30 | 65.8 (µA µM−1 cm−2) | [89] |
| Pt/g-C3N4/polythiophene/GCE | Chemical reduction | Hg2+ | DPV | 0.009 (nM) | 1–500 (nM) | 1.80 | 3 | 1.08 (µA nM−1 cm−2) | [90] |
| rGO/Ala/PANI/GCE | Chemical reduction/polymerization | Cd2+ Pb2+ Cu2+ | SWASV | 0.030 (nM) 0.045 (nM) 0.063 (nM) | 0.08–100 (nM) | 3.30 2.60 3.00 | 10 | 0.43 0.71 0.61 (μA nM−1 cm−2) | [91] |
| Nf/CLS/PGR/GCE | Hydrothermal/thermal reduction | Cd2+ Pb2+ | DPASV | 0.010 (μM) 0.003 (µM) | 0.05–5.00 (µM) | 4.54 3.63 | 8 | 9.77 32.7 (µA µM−1 cm−2) | [92] |
| Bi-NCNF/GCE | pyrolysis | Cd2+ Pb2+ | SWASV | 0.020 (μM) 0.030 (μM) | 1–120 (μM) | 7.10 4.30 | 10 | 0.207 0.273 (μA µM−1) | [93] |
5.2. Inorganic Metallic Nanoparticles
5.2.1. Noble Metal Based Nanomaterials
Gold Nanoparticle-Based Nanocomposites
Silver Nanoparticle-Based Nanocomposites
5.2.2. Non-Noble Metal-Based Nanomaterials
Bismuth Based Nanocomposites
5.3. Inorganic Non-Metallic Nanomaterials
5.3.1. Silica-Based Nanocomposites
5.3.2. Quantum Dot-Based Nanocomposites
| Nanomaterial/Electrode | Synthesis Method | Target Analyte | Electrochem Method | LOD | Linear Range | RSD (%) | n | Sensitivity | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Fe-Chitosan/CE | Electrodeposition | As3+ | SWASV | 1.12 ppb | 2–24 ppb | 2.90 | 8 | 3.66 µA/ppb | [125] |
| Modified nanoporous bismuth film/CE | co-electroplating | Cd2+ | SWASV | 1.30 (ppb) | 2–40 (ppb) | 3.10 | 40 | 0.035 (µA/ppb) | [96] |
| Pb2+ | 1.50 (ppb) | 4.30 | 0.125 (µA/ppb) | ||||||
| ZIF-67/EG | One-pot hydrothermal reaction | Cd2+ | SWASV | 1.13 (nM) | 500–3000 (ppt) | - | - | - | [126] |
| Pb2+ | 1.11 (nM) | ||||||||
| Cu2+ | 2.23 (nM) | ||||||||
| Hg2+ | 1.28 (nM) | ||||||||
| Gold nanostars (AuNSs)/CE | Chemical reduction | As3+ | SWASV | 0.80 (ppb) | 2.5–764.2 (ppb) | 2.50 | 3 | - | [94] |
| Hg2+ | 0.50 (ppb) | 1.5–538.9 (ppb) | 3.20 | ||||||
| Pb2+ | 4.30 (ppb) | 13.0–323.6 (ppb) | 4.60 | ||||||
| Cu based MOFs/GCE | Co-precipitation | Cd2+ | Ratiometric DPV | 33.0 (nM) | 10 nM to 10 μM | 2.21 | 5 | - | [127] |
| Pb2+ | 50.0 (nM) | 10 nM to 10 mM |
5.4. Nanostructured Metal Oxides/Hydroxides
5.4.1. Fe3O4-Based Nanocomposites
5.4.2. Layered Double Hydroxide-Based Nanocomposites
6. Smart Electrochemical Nanosensors
7. Summary and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akanji, S.P.; Ama, O.M.; Ray, S.S.; Osifo, P.O. Metal Oxide Nanomaterials for Electrochemical Detection of Heavy Metals in Water. In Nanostructured Metal-Oxide Electrode Materials for Water Purification; Springer: Berlin/Heidelberg, Germany, 2020; pp. 113–126. [Google Scholar]
- Sihlahla, M.; Mouri, H.; Nomngongo, P.N. Uptake of trace elements by vegetable plants grown on agricultural soils: Evaluation of trace metal accumulation and potential health risk. J. Afr. Earth Sci. 2019, 160, 103635. [Google Scholar] [CrossRef]
- Donner, M.W.; Arshad, M.; Ullah, A.; Siddique, T. Unravelled keratin-derived biopolymers as novel biosorbents for the simultaneous removal of multiple trace metals from industrial wastewater. Sci. Total Environ. 2019, 647, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Embaby, A.; Redwan, M. Sources and behavior of trace elements in groundwater in the South Eastern Desert, Egypt. Environ. Monit. Assess. 2019, 191, 686. [Google Scholar] [CrossRef]
- Strzelec, M.; Proemse, B.C.; Barmuta, L.A.; Gault-Ringold, M.; Desservettaz, M.; Boyd, P.W.; Perron, M.M.G.; Schofield, R.; Bowie, A.R. Atmospheric Trace Metal Deposition from Natural and Anthropogenic Sources in Western Australia. Atmosphere 2020, 11, 474. [Google Scholar] [CrossRef]
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preconcentration and speciation of chromium species using ICP-OES after ultrasound-assisted magnetic solid phase extraction with an amino-modified magnetic nanocomposite prepared from Fe3O4, MnO2 and Al2O3. Microchim. Acta 2017, 184, 1223–1232. [Google Scholar] [CrossRef]
- Jakavula, S.; Biata, N.R.; Dimpe, K.M.; Pakade, V.E.; Nomngongo, P.N. A Critical Review on the Synthesis and Application of Ion-Imprinted Polymers for Selective Preconcentration, Speciation, Removal and Determination of Trace and Essential Metals from Different Matrices. Crit. Rev. Anal. Chem. 2020, 1–13. [Google Scholar] [CrossRef]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Environ. Res. 2019, 177, 108599. [Google Scholar] [CrossRef] [PubMed]
- Nnorom, I.C.; Ewuzie, U.; Eze, S.O. Multivariate statistical approach and water quality assessment of natural springs and other drinking water sources in Southeastern Nigeria. Heliyon 2019, 5, e01123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotruvo, J.A. 2017 WHO Guidelines for Drinking Water Quality: First Addendum to the Fourth Edition. J. Am. Water Work. Assoc. 2017, 109, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Khound, N.J.; Phukon, P.; Bhattacharyya, K.G. Toxic Trace Metals in the Surface Water Sources of Jia–Bharali river basin, North Brahmaputra Plain, India—A Hydrochemical Elucidation. Water Resour. 2019, 46, 117–127. [Google Scholar] [CrossRef]
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preparation of magnetic Fe3O4 nanocomposites modified with MnO2, Al2O3, Au and their application for preconcentration of arsenic in river water samples. J. Environ. Chem. Eng. 2018, 6, 1673–1681. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Ionic Liquid Based Dispersive Liquid-Liquid Microextraction Combined with Magnetic-Based Dispersive Micro-Solid-Phase Extraction for Determination of Trace Cobalt in Water Samples by FAAS. Curr. Anal. Chem. 2017, 13, 456–463. [Google Scholar] [CrossRef]
- Han, Q.; Huo, Y.; Yang, L.; Yang, X.; He, Y.; Wu, J. Determination of Trace Nickel in Water Samples by Graphite Furnace Atomic Absorption Spectrometry after Mixed Micelle-Mediated Cloud Point Extraction. Molecules 2018, 23, 2597. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Yuan, Z.; Cheng, Q.; Zhang, Z.; Yang, J. Rapid in situ determination of heavy metal concentrations in polluted water via portable XRF: Using Cu and Pb as example. Environ. Pollut. 2018, 243, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, S.; Suo, X.-K.; Liao, H. The Processes for Fabricating Nanopowders. In Advanced Nanomaterials and Coatings by Thermal Spray; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–25. [Google Scholar]
- Kokab, T.; Shah, A.; Nisar, J.; Khan, A.M.; Khan, S.B.; Shah, A.H. Tripeptide Derivative-Modified Glassy Carbon Electrode: A Novel Electrochemical Sensor for Sensitive and Selective Detection of Cd2+ Ions. ACS Omega 2020, 5, 10123–10132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munir, A.; Shah, A.; Nisar, J.; Ashiq, M.N.; Akhter, M.S.; Shah, A.H. Selective and simultaneous detection of Zn2+, Cd2+, Pb2+, Cu2+, Hg2+ and Sr2+ using surfactant modified electrochemical sensors. Electrochim. Acta 2019, 323, 134592. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Li, M.; Li, W.; Zhao, Y.; Qi, J.; Cui, X. Graphene quantum dots decorated graphene as an enhanced sensing platform for sensitive and selective detection of copper(II). J. Electroanal. Chem. 2017, 797, 113–120. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef] [Green Version]
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019, 9, 6793–6803. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, Y.; Yu, H.; Tian, L.; Wang, Z. Portable and smart devices for monitoring heavy metal ions integrated with nanomaterials. TrAC Trends Anal. Chem. 2018, 98, 190–200. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.; Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 2016, 8, 1237–1259. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Huang, H.; Chen, L.; Wang, S.; Kang, P.; Chen, X.; Guo, Z.; Huang, X.-J. Electrochemical monitoring of persistent toxic substances using metal oxide and its composite nanomaterials: Design, preparation, and application. TrAC Trends Anal. Chem. 2019, 119, 115636. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, J.; Dai, W.; Lin, X.; Ye, J.; Ye, J. A screen-printed carbon electrode modified with a bismuth film and gold nanoparticles for simultaneous stripping voltammetric determination of Zn(II), Pb(II) and Cu(II). Microchim. Acta 2017, 184, 4731–4740. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. New approaches to antimony film screen-printed electrodes using carbon-based nanomaterials substrates. Anal. Chim. Acta 2016, 916, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Yu, Y. Graphene-derived nanomaterials as recognition elements for electrochemical determination of heavy metal ions: A review. Microchim. Acta 2019, 186, 1–17. [Google Scholar] [CrossRef]
- De Barros, A.; Constantino, C.J.L.; Da Cruz, N.C.; Bortoleto, J.R.R.; Ferreira, M. High performance of electrochemical sensors based on LbL films of gold nanoparticles, polyaniline and sodium montmorillonite clay mineral for simultaneous detection of metal ions. Electrochim. Acta 2017, 235, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Eranjaneya, H.; Adarakatti, P.S.; Siddaramanna, A.; Malingappa, P.; Chandrappa, G.T. Citric acid assisted synthesis of manganese tungstate nanoparticles for simultaneous electrochemical sensing of heavy metal ions. Mater. Sci. Semicond. Process. 2018, 86, 85–92. [Google Scholar] [CrossRef]
- Jin, W.; Fu, Y.; Hu, M.; Wang, S.; Liu, Z. Highly efficient SnS-decorated Bi2O3 nanosheets for simultaneous electrochemical detection and removal of Cd(II) and Pb(II). J. Electroanal. Chem. 2020, 856, 113744. [Google Scholar] [CrossRef]
- Mohamed, M.A.; El-Badawy, F.M.; El-Desoky, H.S.; Ghoneim, M.M. Magnetic cobalt ferrite nanoparticles CoFe2O4 platform as an efficient sensor for trace determination of Cu(ii) in water samples and different food products. New J. Chem. 2017, 41, 11138–11147. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H. Understanding signal amplification strategies of nanostructured electrochemical sensors for environmental pollutants. Curr. Opin. Electrochem. 2019, 17, 56–64. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Gao, J.; Jiang, J.; Jiang, X.; Wu, C. Three-dimensional porous Ni, N-codoped C networks for highly sensitive and selective non-enzymatic glucose sensing. Sens. Actuators B Chem. 2019, 299, 126945. [Google Scholar] [CrossRef]
- Chen, H.; Liang, X.; Liu, Y.; Ai, X.; Asefa, T.; Zou, X. Active Site Engineering in Porous Electrocatalysts. Adv. Mater. 2020, e2002435. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal–Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019, 6, 1802373. [Google Scholar] [CrossRef]
- Tuantranont, A. Applications of nanomaterials in sensors and diagnostics. In Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kranz, C. Carbon-Based Nanosensor Technology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 17, ISBN 3030118649. [Google Scholar]
- Wang, Z.L. Self-Powered Nanosensors and Nanosystems. Adv. Mater. 2012, 24, 280–285. [Google Scholar] [CrossRef]
- Vikesland, P.J. Nanosensors for water quality monitoring. Nat. Nanotechnol. 2018, 13, 651–660. [Google Scholar] [CrossRef]
- Fuertes, G.; Soto, I.; Carrasco, R.; Vargas, M.; Sabattin, J.; Lagos, C. Intelligent Packaging Systems: Sensors and Nanosensors to Monitor Food Quality and Safety. J. Sens. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.P.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-Dimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graboski, A.M.; Martinazzo, J.; Ballen, S.C.; Steffens, J.; Steffens, C. Nanosensors for water quality control. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–128. [Google Scholar]
- Mahbub, T.; Hoque, M.E. Introduction to nanomaterials and nanomanufacturing for nanosensors. In Nanofabrication for Smart Nanosensor Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20. [Google Scholar]
- Gregorczyk, K.; Knez, M. Hybrid nanomaterials through molecular and atomic layer deposition: Top down, bottom up, and in-between approaches to new materials. Prog. Mater. Sci. 2016, 75, 1–37. [Google Scholar] [CrossRef]
- Ganachari, S.V.; Banapurmath, N.R.; Salimath, B.; Yaradoddi, J.S.; Shettar, A.S.; Hunashyal, A.M.; Venkataraman, A.; Patil, P.; Shoba, H.; Hiremath, G.B. Synthesis techniques for preparation of nanomaterials. In Handbook of Ecomaterials; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Lim, T.-C. Nanosensors: Theory and Applications in Industry, Healthcare and Defense; CRC Press: Boca Raton, FL, USA, 2016; ISBN 143980737X. [Google Scholar]
- Jiang, H. Chemical Preparation of Graphene-Based Nanomaterials and Their Applications in Chemical and Biological Sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Rocha-Santos, T.A.P.; Cardoso, S.; Duarte, A.C. Strategies for enhancing the analytical performance of nanomaterial-based sensors. TrAC Trends Anal. Chem. 2013, 47, 27–36. [Google Scholar] [CrossRef]
- Fan, Y.Z.; Tang, Q.; Liu, S.G.; Yang, Y.Z.; Ju, Y.J.; Xiao, N.; Luo, H.Q.; Li, N.B. A smartphone-integrated dual-mode nanosensor based on novel green-fluorescent carbon quantum dots for rapid and highly selective detection of 2,4,6-trinitrophenol and pH. Appl. Surf. Sci. 2019, 492, 550–557. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, R.; Wang, J.; Zhang, Y.; Wen, C.; Tan, Y.; Yang, M. An ultrasensitive and selective fluorescent nanosensor based on porphyrinic metal–organic framework nanoparticles for Cu2+ detection. Analyst 2020, 145, 797–804. [Google Scholar] [CrossRef]
- Liu, J.; Pan, L.; Shang, C.; Lu, B.; Wu, R.; Feng, Y.; Chen, W.; Zhang, R.; Bu, J.; Xiong, Z. A highly sensitive and selective nanosensor for near-infrared potassium imaging. Sci. Adv. 2020, 6, eaax9757. [Google Scholar] [CrossRef] [Green Version]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: A review on recent advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Cui, L. Biomedical Sensor, Device and Measurement Systems. Adv. Bioeng. 2015, 177. [Google Scholar] [CrossRef] [Green Version]
- Leal-Junior, A.G.; Frizera, A.; Pontes, M.J. Sensitive zone parameters and curvature radius evaluation for polymer optical fiber curvature sensors. Opt. Laser Technol. 2018, 100, 272–281. [Google Scholar] [CrossRef]
- O’Riordan, A.; Barry, S. Electrochemical nanosensors: Advances and applications. Rep. Electrochem. 2016, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Li, J. Plasmon-Based Colorimetric Nanosensors for Ultrasensitive Molecular Diagnostics. ACS Sens. 2017, 2, 857–875. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Ghassabian, S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In Calibration and Validation of Analytical Methods—A Sampling of Current Approaches; IntechOpen Ltd.: London, UK, 2018; pp. 109–127. [Google Scholar]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, E.; Guo, X. Limit of detection and limit of quantification determination in gas chromatography. Adv. Gas Chromatogr. 2014, 3, 57–63. [Google Scholar]
- Hare, D.J.; New, E.J.; De Jonge, M.D.; McColl, G. Imaging metals in biology: Balancing sensitivity, selectivity and spatial resolution. Chem. Soc. Rev. 2015, 44, 5941–5958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef]
- De, A.; Chen, S.; Carlen, E.T. Probe-free semiconducting silicon nanowire platforms for biosensing. In Semiconducting Silicon Nanowires for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 229–265. [Google Scholar]
- Rowland, C.E.; Brown III, C.W.; Delehanty, J.B.; Medintz, I.L. Nanomaterial-based sensors for the detection of biological threat agents. Mater. Today 2016, 19, 464–477. [Google Scholar] [CrossRef]
- Mnyipika, S.H.; Nomngongo, P.N. Square Wave Anodic Stripping Voltammetry for Simultaneous Determination of Trace Hg(II) and Tl(I) in Surface Water Samples Using SnO2@MWCNTs Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci 2017, 12, 4811–4827. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Shi, S.Q.; Zhang, H. High-Temperature Gas Sensors for Harsh Environment Applications: A Review. CLEAN Soil Air Water 2019, 47, 1800491. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterial-based sensors. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–359. [Google Scholar]
- Yan, Q.-L.; Gozin, M.; Zhao, F.-Q.; Cohen, A.; Pang, S.-P. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 2016, 8, 4799–4851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syama, S.; Mohanan, P.V. Comprehensive application of graphene: Emphasis on biomedical concerns. Nano-Micro Lett. 2019, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.T.; Kabir, M.F.; Gurung, A.; Reza, K.M.; Pathak, R.; Ghimire, N.; Baride, A.; Wang, Z.; Kumar, M.; Qiao, Q. Graphene Oxide–Silver Nanowire Nanocomposites for Enhanced Sensing of Hg2+. ACS Appl. Nano Mater. 2019, 2, 4842–4851. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Dai, H.; Wang, N.; Wang, D.; Ma, H.; Lin, M. An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd(II) and Pb(II). Chem. Eng. J. 2016, 299, 150–155. [Google Scholar] [CrossRef]
- Tan, S.M.; Ambrosi, A.; Chua, C.K.; Pumera, M. Electron transfer properties of chemically reduced graphene materials with different oxygen contents. J. Mater. Chem. A 2014, 2, 10668–10675. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Nanomaterials in Biosensors: Fundamentals and Applications. Nanomater. Biosens. 2018, 1. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and its electrochemistry—An update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xu, Z.; Liu, M.; Deng, P.; Tang, S.; Jiang, J.; Feng, H.; Qian, D.; He, L. Ag/N-doped reduced graphene oxide incorporated with molecularly imprinted polymer: An advanced electrochemical sensing platform for salbutamol determination. Biosens. Bioelectron. 2017, 90, 210–216. [Google Scholar] [CrossRef]
- Hassanpoor, S.; Rouhi, N. Electrochemical sensor for determination of trace amounts of cadmium (II) in environmental water samples based on MnO2/RGO nanocomposite. Int. J. Environ. Anal. Chem. 2019, 1–20. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Ong, W.-J. 2D/2D Graphitic Carbon Nitride (g-C3N4) Heterojunction Nanocomposites for Photocatalysis: Why Does Face-to-Face Interface Matter? Front. Mater. 2017, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Lv, H.; Wang, C.; Ao, Z.; Wang, G. Fabrication of the protonated graphitic carbon nitride nanosheets as enhanced electrochemical sensing platforms for hydrogen peroxide and paracetamol detection. Electrochim. Acta 2016, 206, 259–269. [Google Scholar] [CrossRef]
- Ding, S.; Ali, A.; Jamal, R.; Xiang, L.; Zhong, Z.; Abdiryim, T. An Electrochemical Sensor of Poly (EDOT-pyridine-EDOT)/Graphitic Carbon Nitride Composite for Simultaneous Detection of Cd2+ and Pb2+. Materials 2018, 11, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Photocatalysis of graphene and carbon nitride-based functional carbon quantum dots. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 759–781. [Google Scholar]
- Zhang, J.; Zhu, Z.; Di, J.; Long, Y.; Li, W.; Tu, Y. A Sensitive Sensor for trace Hg2+ Determination Based on Ultrathin g-C3N4 Modified Glassy Carbon Electrode. Electrochimica Acta 2015, 186, 192–200. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Wang, M.; Shi, X.Z.; Wang, C.M. Palladium Nanoparticles/Graphitic Carbon Nitride Nanosheets-Carbon Nanotubes as a Catalytic Amplification Platform for the Selective Determination of 17α-ethinylestradiol in Feedstuffs. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Karthika, A.; Nikhil, S.; Suganthi, A.; Rajarajan, M. A facile sonochemical approach based on graphene carbon nitride doped silver molybdate immobilized nafion for selective and sensitive electrochemical detection of chromium (VI) in real sample. Adv. Powder Technol. 2020, 31, 1879–1890. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Basirun, W.J.; Alias, Y.; MengWoi, P. Investigating the effectiveness of g-C3N4 on Pt/g-C3N4/polythiophene nanocomposites performance as an electrochemical sensor for Hg2+ detection. J. Environ. Chem. Eng. 2020, 8, 104204. [Google Scholar] [CrossRef]
- Akhtar, M.; Tahir, A.; Zulfiqar, S.; Hanif, F.; Warsi, M.F.; Agboola, P.O.; Shakir, I. Ternary hybrid of polyaniline-alanine-reduced graphene oxide for electrochemical sensing of heavy metal ions. Synth. Met. 2020, 265, 116410. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Q.; Yang, B.; Xu, Q.; Xu, Q.; Hu, X. Electrochemical sensor construction based on Nafion/calcium lignosulphonate functionalized porous graphene nanocomposite and its application for simultaneous detection of trace Pb2+ and Cd2+. Sens. Actuators B Chem. 2018, 259, 540–551. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, W.; Lin, X.; Liu, B.; Zhang, J.; Ye, J.; Ye, J. Facile one-step fabrication of a novel 3D honeycomb-like bismuth nanoparticles decorated N-doped carbon nanosheet frameworks: Ultrasensitive electrochemical sensing of heavy metal ions. Electrochim. Acta 2018, 266, 94–102. [Google Scholar] [CrossRef]
- Dutta, S.; Strack, G.; Kurup, P. Gold nanostar electrodes for heavy metal detection. Sens. Actuators B Chem. 2019, 281, 383–391. [Google Scholar] [CrossRef]
- Eksin, E.; Erdem, A.; Fafal, T.; Kıvçak, B. Eco-friendly Sensors Developed by Herbal Based Silver Nanoparticles for Electrochemical Detection of Mercury (II) Ion. Electroanalysis 2019, 31, 1075–1082. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Wang, X.; Zhao, D.; Rex, M.M.; Cho, H.J.; Lee, W.H. A novel nanoporous bismuth electrode sensor for in situ heavy metal detection. Electrochim. Acta 2019, 298, 440–448. [Google Scholar] [CrossRef]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T.; Ghaedi, H.; Rezaeivala, M. Fabrication and application of a new modified electrochemical sensor using nano-silica and a newly synthesized Schiff base for simultaneous determination of Cd2+, Cu2+ and Hg2+ ions in water and some foodstuff samples. Anal. Chim. Acta 2013, 771, 21–30. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Umar, A.; Kumar, S. SnO2 quantum dots as novel platform for electrochemical sensing of cadmium. Electrochim. Acta 2015, 169, 97–102. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Ma, L.; Zhou, L.; Jiang, Y.; Gao, J. Synthesis of bismuth nanoparticle-loaded cobalt ferrite for electrochemical detection of heavy metal ions. RSC Adv. 2020, 10, 27697–27705. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Rong, X.; Wang, Y.; Ding, S. Facile fabrication of a novel 3D graphene framework/Bi nanoparticle film for ultrasensitive electrochemical assays of heavy metal ions. Anal. Chim. Acta 2017, 968, 21–29. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Wang, D.; Lu, L.; Wang, X.; Guo, G. A carbon-supported BiSn nanoparticles based novel sensor for sensitive electrochemical determination of Cd (II) ions. Talanta 2019, 202, 27–33. [Google Scholar] [CrossRef]
- Ting, S.L.; Ee, S.J.; Ananthanarayanan, A.; Leong, K.C.; Chen, P. Graphene quantum dots functionalized gold nanoparticles for sensitive electrochemical detection of heavy metal ions. Electrochim. Acta 2015, 172, 7–11. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Voltammetric Determination of Lead (II) and Cadmium (II) Using a Bismuth Film Electrode Modified with Mesoporous Silica Nanoparticles. Electrochim. Acta 2014, 132, 223–229. [Google Scholar] [CrossRef]
- Zhou, M.; Han, L.; Deng, D.; Zhang, Z.; He, H.; Zhang, L.; Luo, L. 4-mercaptobenzoic acid modified silver nanoparticles-enhanced electrochemical sensor for highly sensitive detection of Cu2+. Sens. Actuators B Chem. 2019, 291, 164–169. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, D.; Hu, X.; Han, H.; Lin, M.; Wang, C. An electrochemical sensor based on reduced graphene oxide/gold nanoparticles modified electrode for determination of iron in coastal waters. Sens. Actuators B Chem. 2017, 243, 1–7. [Google Scholar] [CrossRef]
- Cheng, Y.; Fa, H.; Yin, W.; Hou, C.; Huo, D.; Liu, F.; Zhang, Y.; Chen, C. A sensitive electrochemical sensor for lead based on gold nanoparticles/nitrogen-doped graphene composites functionalized with l-cysteine-modified electrode. J. Solid State Electrochem. 2016, 20, 327–335. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef]
- Çiftçi, H.; Tamer, U.; Metin, A.Ü.; Alver, E.; Kizir, N. Electrochemical copper (II) sensor based on chitosan covered gold nanoparticles. J. Appl. Electrochem. 2014, 44, 563–571. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Renedo, O.D.; Martínez, M.J.A. A novel method for the anodic stripping voltammetry determination of Sb (III) using silver nanoparticle-modified screen-printed electrodes. Electrochem. Commun. 2007, 9, 820–826. [Google Scholar] [CrossRef]
- Han, T.; Jin, J.; Wang, C.; Sun, Y.; Zhang, Y.; Liu, Y. Ag Nanoparticles-Modified 3D Graphene Foam for Binder-Free Electrodes of Electrochemical Sensors. Nanomaterials 2017, 7, 40. [Google Scholar] [CrossRef]
- Wu, T.; Xu, T.; Ma, Z. Sensitive electrochemical detection of copper ions based on the copper(ii) ion assisted etching of Au@Ag nanoparticles. Analyst 2015, 140, 8041–8047. [Google Scholar] [CrossRef]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wu, J.; Li, J.; Ge, Y.; Ju, H. Electrochemical detection of Cu2+ through Ag nanoparticle assembly regulated by copper-catalyzed oxidation of cysteamine. Biosens. Bioelectron. 2014, 55, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, M.R.; Basirun, W.J.; Woi, P.M.; Yousefi, R.; Alias, Y. l-Glutamine-assisted synthesis of ZnO oatmeal-like/silver composites as an electrochemical sensor for Pb2+ detection. Anal. Bioanal. Chem. 2019, 411, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Z.; Dang, H. A simple way to prepare bismuth nanoparticles. Mater. Lett. 2004, 58, 790–793. [Google Scholar] [CrossRef]
- He, Y.; Ma, L.; Zhou, L.; Liu, G.; Jiang, Y.; Gao, J. Preparation and Application of Bismuth/MXene Nano-Composite as Electrochemical Sensor for Heavy Metal Ions Detection. Nanomaterials 2020, 10, 866. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Bapat, G.; Labade, C.; Chaudhari, A.; Zinjarde, S. Silica nanoparticle based techniques for extraction, detection, and degradation of pesticides. Adv. Colloid Interface Sci. 2016, 237, 1–14. [Google Scholar] [CrossRef]
- Fang, Y.; Cui, B.; Huang, J.; Wang, L. Ultrasensitive electrochemical sensor for simultaneous determination of cadmium and lead ions based on one-step co-electropolymerization strategy. Sens. Actuators B Chem. 2019, 284, 414–420. [Google Scholar] [CrossRef]
- Ul Hassan Alvi, N.; Gómez, V.J.; Soto Rodriguez, P.E.D.; Kumar, P.; Zaman, S.; Willander, M.; Nötzel, R. An InN/InGaN quantum dot electrochemical biosensor for clinical diagnosis. Sensors 2013, 13, 13917–13927. [Google Scholar] [CrossRef]
- Bhanjana, G.; Dilbaghi, N.; Kumar, R.; Kumar, S. Zinc Oxide Quantum Dots as Efficient Electron Mediator for Ultrasensitive and Selective Electrochemical Sensing of Mercury. Electrochim. Acta 2015, 178, 361–367. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Shi, A.; You, T. Simultaneous stripping determination of cadmium and lead ions based on the N-doped carbon quantum dots-graphene oxide hybrid. Sens. Actuators B Chem. 2018, 255, 1762–1770. [Google Scholar] [CrossRef]
- Bakhsh, E.M.; Khan, S.B.; Marwani, H.M.; Danish, E.Y.; Asiri, A.M. Efficient electrochemical detection and extraction of copper ions using ZnSe–CdSe/SiO2 core–shell nanomaterial. J. Ind. Eng. Chem. 2019, 73, 118–127. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Pathak, P.; Wang, X.; Rodriguez, K.L.; Park, J.; Cho, H.J.; Lee, W.H. A novel Fe-Chitosan-coated carbon electrode sensor for in situ As (III) detection in mining wastewater and soil leachate. Sens. Actuators B Chem. 2019, 294, 89–97. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, X.; Ikram, M.; Ullah, M.; Wu, H.; Shi, K. Controllable synthesis of an intercalated ZIF-67/EG structure for the detection of ultratrace Cd2+, Cu2+, Hg2+ and Pb2+ ions. Chem. Eng. J. 2020, 395, 125216. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, X.; Chi, K.-N.; Yang, T.; Yang, Y. Bifunctional MOFs-Based Ratiometric Electrochemical Sensor for Multiplex Heavy Metal Ions. ACS Appl. Mater. Interfaces 2020, 12, 30770–30778. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef]
- Anu Prathap, M.U.; Kaur, B.; Srivastava, R. Electrochemical Sensor Platforms Based on Nanostructured Metal Oxides, and Zeolite-Based Materials. Chem. Rec. 2019, 19, 883–907. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Ali, A.; Hira Zafar, M.Z.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Blaney, L. Magnetite (Fe3O4): Properties, Synthesis, and Applications. 2007. Available online: http://preserve.lehigh.edu/cas-lehighreview-vol-15/5 (accessed on 2 December 2020).
- Wu, W.; Jia, M.; Zhang, Z.; Chen, X.; Zhang, Q.; Zhang, W.; Li, P.; Chen, L. Sensitive, selective and simultaneous electrochemical detection of multiple heavy metals in environment and food using a lowcost Fe3O4 nanoparticles/fluorinated multi-walled carbon nanotubes sensor. Ecotoxicol. Environ. Saf. 2019, 175, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Zhou, W.-Y.; Jiang, M.; Guo, Z.; Liu, J.-H.; Zhang, L.; Huang, X.-J. Surface Fe(II)/Fe(III) cycle promoted ultra-highly sensitive electrochemical sensing of arsenic (III) with dumbbell-like Au/Fe3O4 nanoparticles. Anal. Chem. 2018, 90, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Benício, L.P.F.; Silva, R.A.; Lopes, J.A.; Eulálio, D.; Dos Santos, R.M.M.; De Aquino, L.A.; Vergütz, L.; Novais, R.F.; Da Costa, L.M.; Pinto, F.G.; et al. Layered double hydroxides: Nanomaterials for applications in agriculture. Rev. Bras. Cienc. Solo 2015, 39, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nalawade, P.; Aware, B.; Kadam, V.J.; Hirlekar, R.S. Layered double hydroxides: A review. J. Sci. Ind. Res. 2009, 68, 267–272. [Google Scholar]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Forticaux, A.; Dang, L.; Liang, H.; Jin, S. Controlled Synthesis of Layered Double Hydroxide Nanoplates Driven by Screw Dislocations. Nano Lett. 2015, 15, 3403–3409. [Google Scholar] [CrossRef]
- Munonde, T.S.; Zheng, H.; Nomngongo, P.N. Ultrasonic exfoliation of NiFe LDH/CB nanosheets for enhanced oxygen evolution catalysis. Ultrason. Sonochemistry 2019, 59, 104716. [Google Scholar] [CrossRef]
- Munonde, T.S.; Zheng, H.; Matseke, M.S.; Nomngongo, P.N.; Wang, Y.; Tsiakaras, P. A green approach for enhancing the electrocatalytic activity and stability of NiFe2O4/CB nanospheres towards hydrogen production. Renew. Energy 2020, 154, 704–714. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Amini, R. A novel voltammetric sensor for mercury(II) based on mercaptocarboxylic acid intercalated layered double hydroxide nanoparticles modified electrode. Sens. Actuators B Chem. 2017, 246, 961–968. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A sensitive electrochemical sensor for simultaneous voltammetric sensing of cadmium and lead based on Fe3O4/multiwalled carbon nanotube/laser scribed graphene composites functionalized with chitosan modified electrode. Mater. Chem. Phys. 2019, 238, 121877. [Google Scholar] [CrossRef]
- Singh, S.; Pankaj, A.; Mishra, S.; Tewari, K.; Singh, S.P. Cerium oxide-catalyzed chemical vapor deposition grown carbon nanofibers for electrochemical detection of Pb(II) and Cu(II). J. Environ. Chem. Eng. 2019, 7, 103250. [Google Scholar] [CrossRef]
- Salimi, A.; Pourbahram, B.; Mansouri-Majd, S.; Hallaj, R. Manganese oxide nanoflakes/multi-walled carbon nanotubes/chitosan nanocomposite modified glassy carbon electrode as a novel electrochemical sensor for chromium (III) detection. Electrochim. Acta 2015, 156, 207–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.-Y.; He, L.; Zhang, X.; Liu, C.-S. Fe (III)-based metal–organic framework-derived core–shell nanostructure: Sensitive electrochemical platform for high trace determination of heavy metal ions. Biosens. Bioelectron. 2017, 94, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.H.B.; Rechotnek, F.; da Silva, E.P.; de Sousa Marques, V.; Rubira, A.F.; Silva, R.; Lourenço, S.A.; Muniz, E.C. A sensitive electrochemical sensor for Pb2+ ions based on ZnO nanofibers functionalized by L-cysteine. J. Mol. Liq. 2020, 309, 113041. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, P.; Dai, H.; Chen, L.; Ma, H.; Lin, M.; Shen, D. An electrochemical sensor based on Co3O4 nanosheets for lead ions determination. RSC Adv. 2017, 7, 39611–39616. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, A.C.; Silva, A.F. Smart devices: Micro-and nanosensors. In Bioinspired Materials for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 297–329. [Google Scholar]
- Hunter, G.W.; Stetter, J.R.; Hesketh, P.; Liu, C.-C. Smart Sensor Systems. Electrochem. Soc. Interface 2010, 19, 29. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R. Smart Micro/Nano Sensors and their Applications in Intelligent Sensory Network System. Int. J. Sens. Netw. Data Commun. 2018, 7, 1. [Google Scholar] [CrossRef]
- Dong, B.; Shi, Q.; Yang, Y.; Wen, F.; Zhang, Z.; Lee, C. Technology evolution from self-powered sensors to AIoT enabled smart homes. Nano Energy 2020, 79, 105414. [Google Scholar] [CrossRef]
- Saini, R.K.; Bagri, L.P.; Bajpai, A.K. Smart nanosensors for pesticide detection. In New Pesticides and Soil Sensors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 519–559. [Google Scholar]



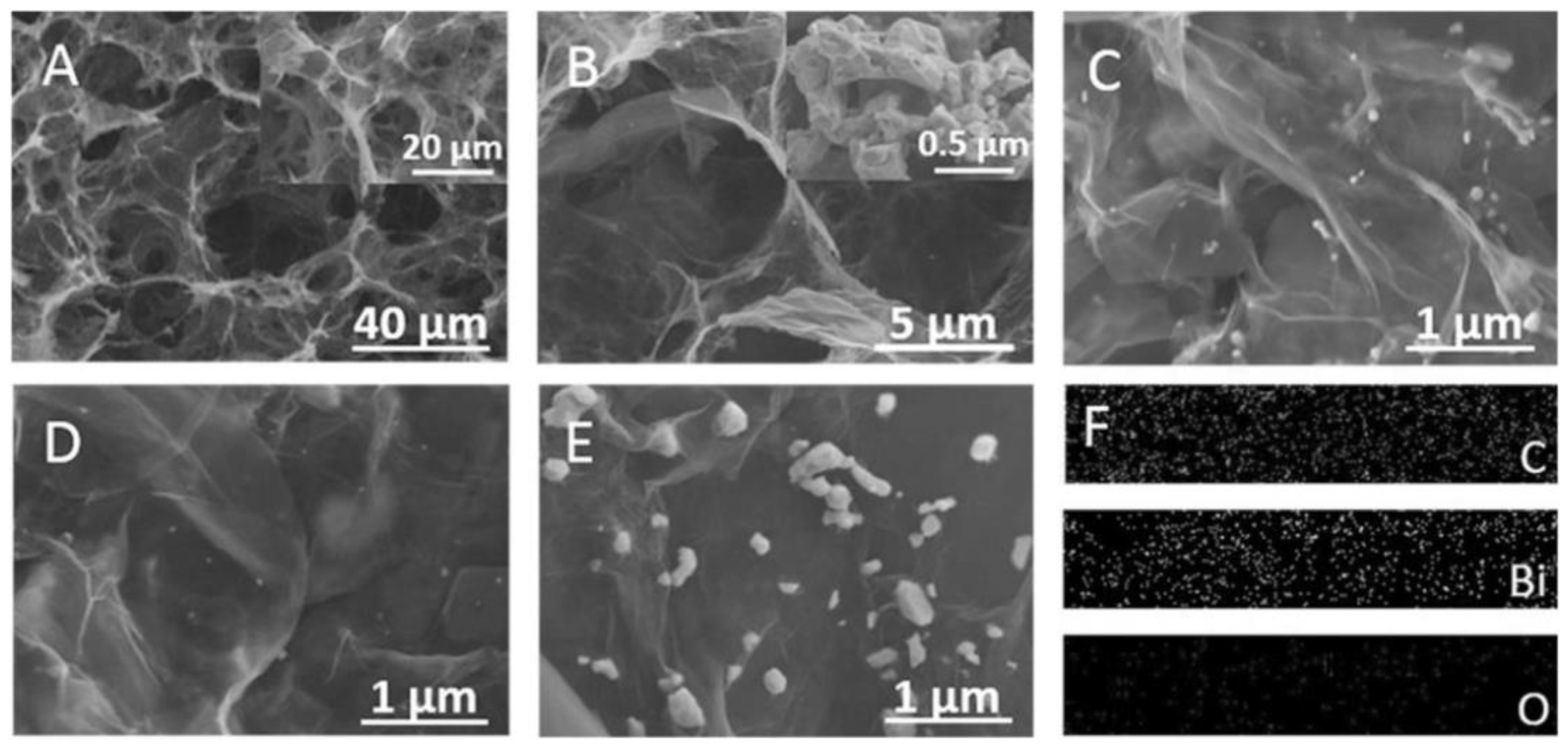

| Nanomaterial/Electrode | Synthesis Method | Target Analyte | Electrochem Method | LOD | Linear Range | RSD (%) | n | Sensitivity | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Fe3O4/MWCNTs/LSG/CS/GCE | Hydrothermal/laser scribing | Cd2+ | SWASV | 0.10 ppb | 1 to 200 ppb | 3.77 | 10 | - | [143] |
| Pb2+ | 0.07 ppb | 0.97 | |||||||
| CeO2-CNF | chemical vapor deposition | Pb2+ | DPV | 0.60 ppb | 0.9–2.1 ppb | - | - | - | [144] |
| Cu2+ | 0.30 ppb | 0.6–1.8 ppb | |||||||
| MnO2/MWCNT/CS | Sonochemical/Electrodeposition | Cr3+ | HC | 0.30 ppb | 3 to 200 ppb | 3.00 | 5 | 18.7 nA µM−1 cm−2 | [145] |
| Fe-MOF@mFe3O4@mC | Hydrothermal/temperature treatment | Pb2+ | EIS | 2.27 pM | 0.01 to 10.0 nM | 5.61 | 5 | - | [146] |
| As3+ | 6.73 pM | 4.66 | |||||||
| ZnO nanofibers/L-cysteine nanocomposite/GCE | Electrospinning/annealing | Pb2+ | SWASV | 0.40 ppb | 10–140 ppb | 4.50 | 30 | - | [147] |
| BiNPs@CoFe2O4/GCE | Hydrothermal reaction/colloidal dispersion | Cd2+ | SWASV | 7.30 nM | 0.06 to 0.6 mM | 3.20 | 10 | - | [99] |
| Pb2+ | 8.20 nM | 0.08 to 0.8 mM | 2.50 | ||||||
| Co3O4 nanosheets/ITO | Electrodeposition | Pb2+ | DPASV | 0.52 ppb | 1–100 ppb | 5.20 | 7 | - | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munonde, T.S.; Nomngongo, P.N. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors 2021, 21, 131. https://doi.org/10.3390/s21010131
Munonde TS, Nomngongo PN. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors. 2021; 21(1):131. https://doi.org/10.3390/s21010131
Chicago/Turabian StyleMunonde, Tshimangadzo S., and Philiswa N. Nomngongo. 2021. "Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples" Sensors 21, no. 1: 131. https://doi.org/10.3390/s21010131
APA StyleMunonde, T. S., & Nomngongo, P. N. (2021). Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors, 21(1), 131. https://doi.org/10.3390/s21010131






