A Fibre-Optic Platform for Sensing Nitrate Using Conducting Polymers
Abstract
1. Introduction
2. Materials and Methods
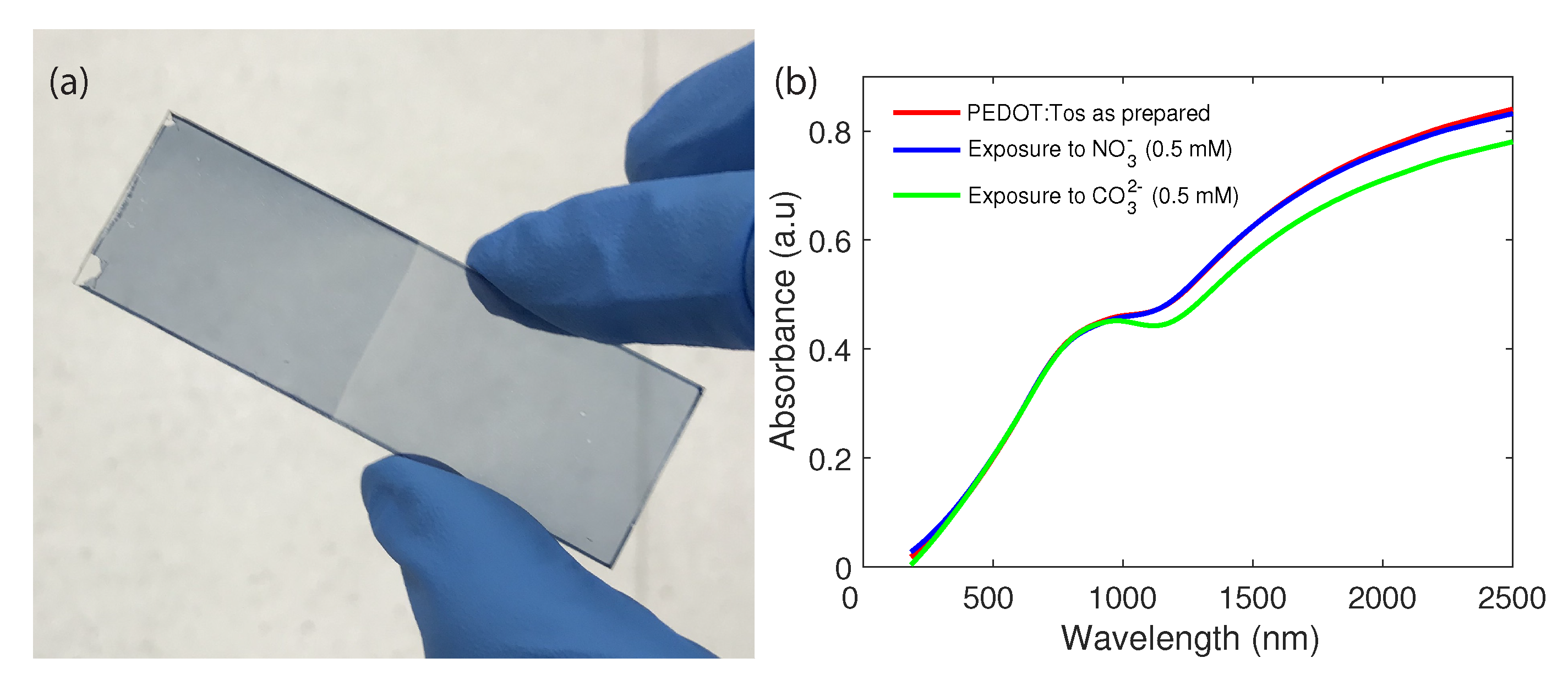
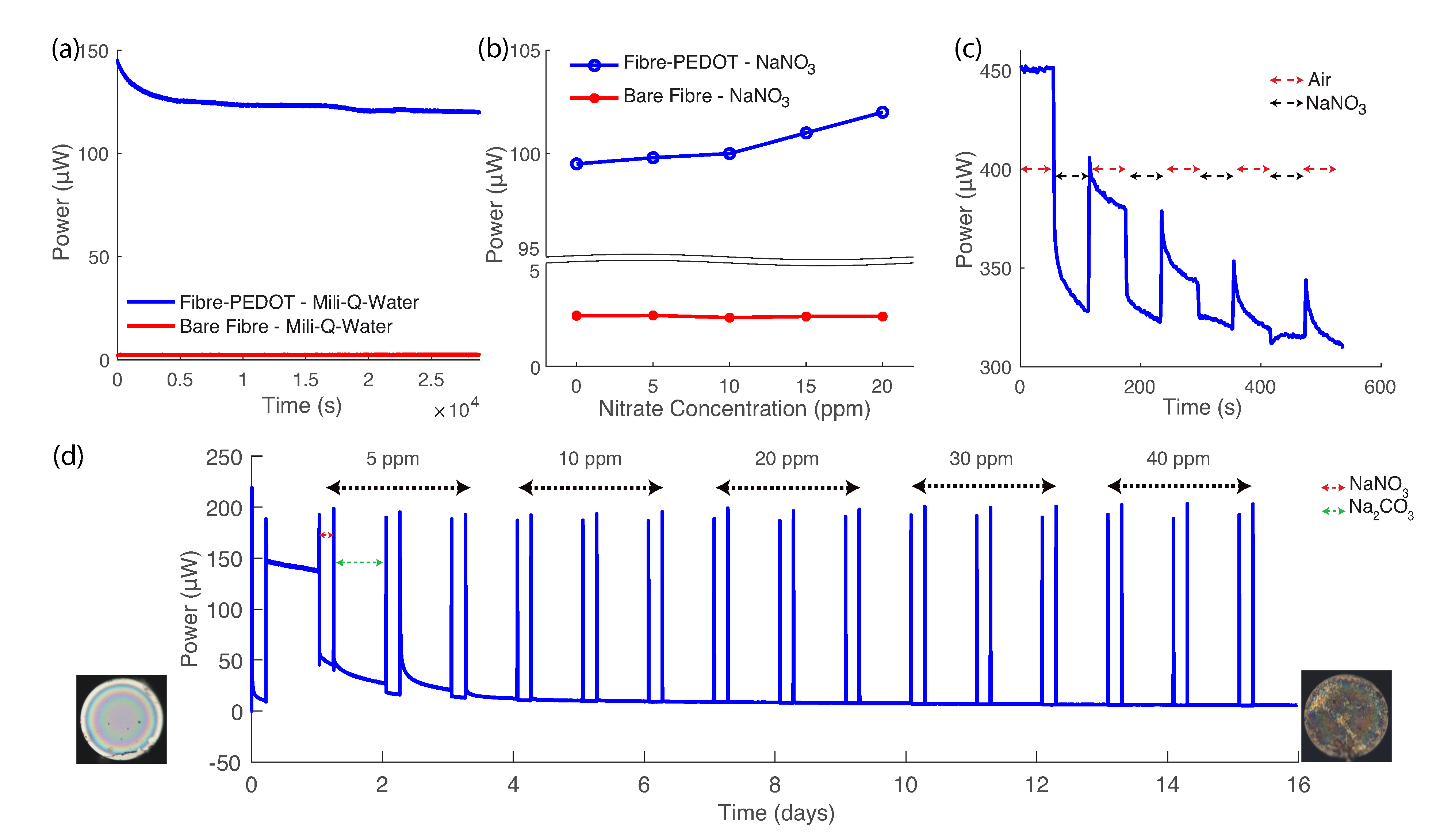
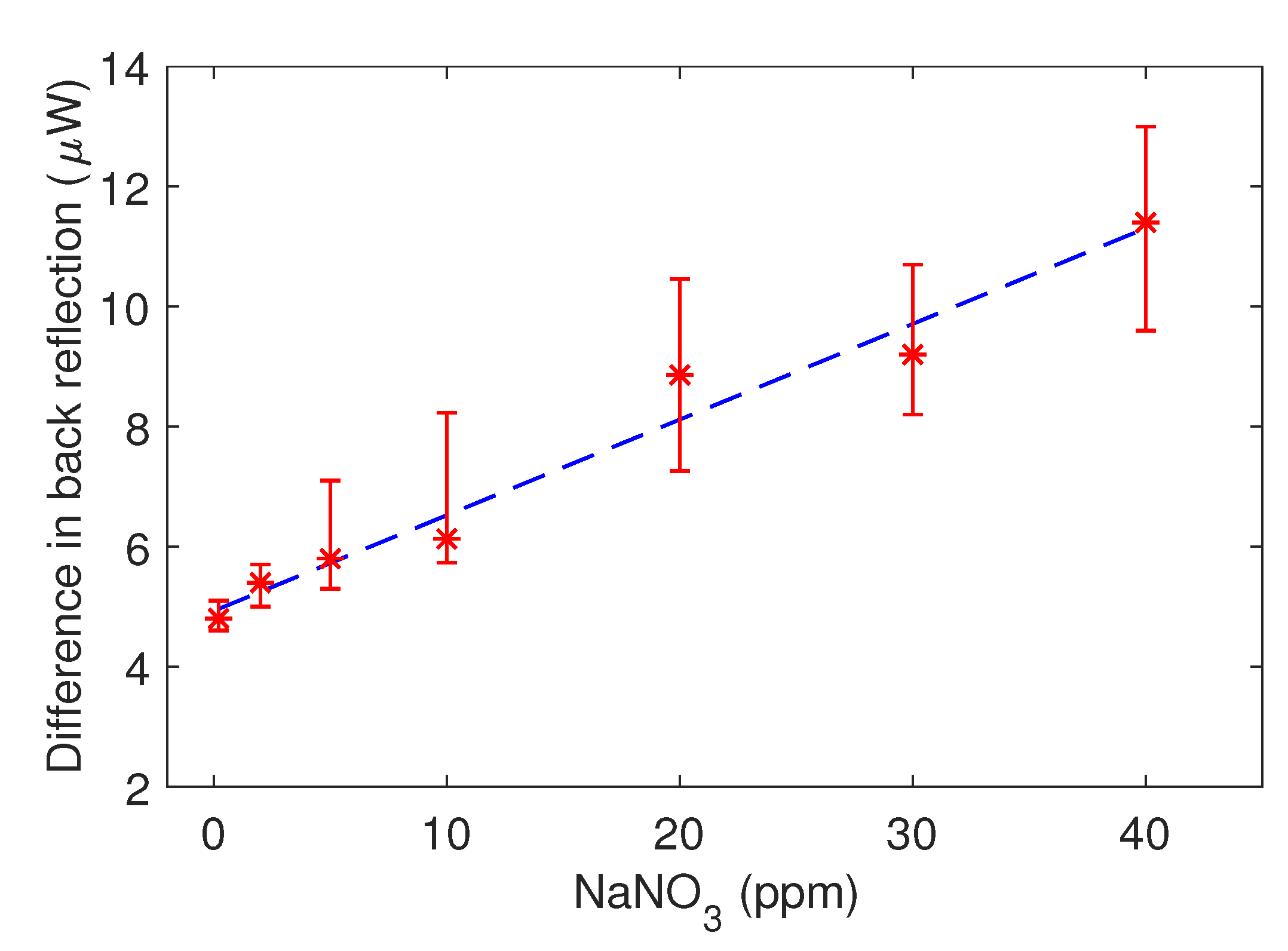
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sethumadhavan, V.; Rudd, S.; Switalska, E.; Zuber, K.; Teasdale, P.; Evans, D. Recent advances in ion sensing with conducting polymers. BMC Mater. 2019, 1, 4. [Google Scholar] [CrossRef]
- Badea, M.; Amine, A.; Palleschi, G.; Moscone, D.; Volpe, G.; Curulli, A. New electrochemical sensors for detection of nitrites and nitrates. J. Electroanal. Chem. 2001, 509, 66–72. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Xie, L.; Mukhopadhyay, S.; Burkitt, L. A temperature compensated smart nitrate-sensor for agricultural industry. IEEE Trans. Ind. Electron. 2017, 64, 7333–7341. [Google Scholar] [CrossRef]
- Kuddushi, M.; Mata, J.; Malek, N. Self-Sustainable, self-healable, Load Bearable and Moldable stimuli responsive ionogel for the Selective Removal of Anionic Dyes from aqueous medium. J. Mol. Liq. 2020, 298, 112048. [Google Scholar] [CrossRef]
- Mahmud, M.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbasi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere 2020. [Google Scholar] [CrossRef]
- Pięk, M.; Piech, R.; Paczosa-Bator, B. All-solid-state nitrate selective electrode with graphene/tetrathiafulvalene nanocomposite as high redox and double layer capacitance solid contact. Electrochim. Acta 2016, 210, 407–414. [Google Scholar] [CrossRef]
- Carlström, M.; Larsen, F.J.; Nyström, T.; Hezel, M.; Borniquel, S.; Weitzberg, E.; Lundberg, J.O. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17716–17720. [Google Scholar] [CrossRef]
- Hilmy, A.; El-Domiaty, N.; Wershana, K. Acute and chronic toxicity of nitrite to Clarias lazera. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1987, 86, 247. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C.; Burkitt, L. Imprinted polymer coated impedimetric nitrate sensor for real-time water quality monitoring. Sens. Actuators B Chem. 2018, 259, 753–761. [Google Scholar] [CrossRef]
- Bendikov, T.A.; Kim, J.; Harmon, T.C. Development and environmental application of a nitrate selective microsensor based on doped polypyrrole films. Sens. Actuators B Chem. 2005, 106, 512–517. [Google Scholar]
- Jahn, B.; Linker, R.; Upadhyaya, S.; Shaviv, A.; Slaughter, D.; Shmulevich, I. Mid-infrared spectroscopic determination of soil nitrate content. Biosyst. Eng. 2006, 94, 505–515. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.K.; Beg, Y.R.; Nishad, G.R. A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples. Talanta 2019, 191, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Cadman, J. Electrochemical and optical sensing of anions by transition metal based receptors. Coord. Chem. Rev. 2000, 205, 131–155. [Google Scholar] [CrossRef]
- Murray, E.; Roche, P.; Briet, M.; Moore, B.; Morrin, A.; Diamond, D.; Paull, B. Fully automated, low-cost ion chromatography system for in-situ analysis of nitrite and nitrate in natural waters. Talanta 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pampati, N.; McCormick, W.M.; Bhattacharyya, L. Protein nitrogen determination by kjeldahl digestion and ion chromatography. J. Pharm. Sci. 2016, 105, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.S.; de Araujo, W.R.; Paixão, T.R.; Angnes, L. Direct nitrate sensing in water using an array of copper-microelectrodes from flat flexible cables. Sens. Actuators B Chem. 2013, 188, 94–98. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue methode zur bestimmung des stickstoffs in organischen körpern. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Shinn, M.B. Colorimetric method for determination of nitrate. Ind. Eng. Chem. Anal. Ed. 1941, 13, 33–35. [Google Scholar] [CrossRef]
- Sancenón, F.; Martínez-Máñez, R.; Soto, J. A selective chromogenic reagent for nitrate. Angew. Chem. Int. Ed. 2002, 41, 1416–1419. [Google Scholar] [CrossRef]
- Khanfar, M.F.; Al-Faqheri, W.; Al-Halhouli, A. Low cost lab on chip for the colorimetric detection of nitrate in mineral water products. Sensors 2017, 17, 2345. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, F. Zur lehre von der wirkung der salze. Arch. Für Exp. Pathol. Und Pharmakol. 1888, 25, 1–30. [Google Scholar] [CrossRef]
- Andres, L.; Boateng, K.; Borja-Vega, C.; Thomas, E. A review of in-situ and remote sensing technologies to monitor water and sanitation interventions. Water 2018, 10, 756. [Google Scholar] [CrossRef]
- Rudd, S.; Desroches, P.; Switalska, E.; Gardner, E.; Dalton, M.; Buss, P.; Charrault, E.; Evans, D. Relationship between structure/properties of vapour deposited PEDOT and sensitivity to passive nitrate doping. Sens. Actuators B Chem. 2019, 281, 582–587. [Google Scholar] [CrossRef]
- Shahnia, S.; Rehmen, J.; Lancaster, D.G.; Monro, T.M.; Ebendorff-Heidepriem, H.; Evans, D.; Afshar, S. Towards new fiber optic sensors based on the vapor deposited conducting polymer PEDOT: Tos. Opt. Mater. Express 2019, 9, 4517–4531. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Kung, C.W.; Chou, L.Y.; Vittal, R.; Ho, K.C. Poly(3,4-ethylenedioxythiophene)(PEDOT) hollow microflowers and their application for nitrite sensing. Sens. Actuators B Chem. 2014, 192, 762–768. [Google Scholar] [CrossRef]
- Brooke, R.; Cottis, P.; Talemi, P.; Fabretto, M.; Murphy, P.; Evans, D. Recent advances in the synthesis of conducting polymers from the vapour phase. Prog. Mater. Sci. 2017, 86, 127–146. [Google Scholar] [CrossRef]
- Kateb, M.; Ahmadi, V.; Mohseni, M. Fast switching and high contrast electrochromic device based on PEDOT nanotube grown on ZnO nanowires. Sol. Energy Mater. Sol. Cells 2013, 112, 57–64. [Google Scholar] [CrossRef]
- Lock, J.P.; Lutkenhaus, J.L.; Zacharia, N.S.; Im, S.G.; Hammond, P.T.; Gleason, K.K. Electrochemical investigation of PEDOT films deposited via CVD for electrochromic applications. Synth. Met. 2007, 157, 894–898. [Google Scholar] [CrossRef]
- Sethumadhavan, V.; Zuber, K.; Evans, D. Hydrolysis of doped conducting polymers. Commun. Chem. 2020, 3, 153. [Google Scholar] [CrossRef]
- Khan, Z.U.; Bubnova, O.; Jafari, M.J.; Brooke, R.; Liu, X.; Gabrielsson, R.; Ederth, T.; Evans, D.R.; Andreasen, J.W.; Fahlman, M. Acido-basic control of the thermoelectric properties of poly (3, 4-ethylenedioxythiophene) tosylate (PEDOT-Tos) thin films. J. Mater. Chem. C 2015, 3, 10616–10623. [Google Scholar] [CrossRef] [PubMed]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly (3, 4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Zozoulenko, I.; Singh, A.; Singh, S.K.; Gueskine, V.; Crispin, X.; Berggren, M. Polarons, bipolarons, and absorption spectroscopy of PEDOT. ACS Appl. Polym. Mater. 2018, 1, 83–94. [Google Scholar] [CrossRef]
- Pozo-Gonzalo, C.; Mecerreyes, D.; Pomposo, J.A.; Salsamendi, M.; Marcilla, R.; Grande, H.; Vergaz, R.; Barrios, D.; Sánchez-Pena, J.M. All-plastic electrochromic devices based on PEDOT as switchable optical attenuator in the near IR. Sol. Energy Mater. Sol. Cells 2008, 92, 101–106. [Google Scholar] [CrossRef]
- Luo, S.C.; Mohamed Ali, E.; Tansil, N.C.; Yu, H.H.; Gao, S.; Kantchev, E.A.; Ying, J.Y. Poly(3,4-ethylenedioxythiophene)(PEDOT) nanobiointerfaces: Thin, ultrasmooth, and functionalized PEDOT films with in vitro and in vivo biocompatibility. Langmuir 2008, 24, 8071–8077. [Google Scholar] [CrossRef]
- Venkatraman, S.; Hendricks, J.; King, Z.A.; Sereno, A.J.; Richardson-Burns, S.; Martin, D.; Carmena, J.M. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 307–316. [Google Scholar] [CrossRef]
- Fani, N.; Hajinasrollah, M.; Asghari Vostikolaee, M.; Baghaban Eslaminejad, M.; Mashhadiabbas, F.; Tongas, N.; Rasoulianboroujeni, M.; Yadegari, A.; Ede, K.; Tahriri, M. Influence of conductive PEDOT: PSS in a hard tissue scaffold: In vitro and in vivo study. J. Bioact. Compat. Polym. 2019, 34, 436–441. [Google Scholar] [CrossRef]
- Carli, S.; Bianchi, M.; Zucchini, E.; Di Lauro, M.; Prato, M.; Murgia, M.; Fadiga, L.; Biscarini, F. Electrodeposited PEDOT: Nafion composite for neural recording and stimulation. Adv. Healthc. Mater. 2019, 8, 1900765. [Google Scholar] [CrossRef]
- Evans, D.; Fabretto, M.; Mueller, M.; Zuber, K.; Short, R.; Murphy, P. Structure-directed growth of high conductivity PEDOT from liquid-like oxidant layers during vacuum vapor phase polymerization. J. Mater. Chem. 2012, 22, 14889–14895. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Zhang, H.; Duan, X.; Xu, J.; Wen, Y. Electrochemical sensing application of poly (acrylic acid modified EDOT-co-EDOT): PSS and its inorganic nanocomposite with high soaking stability, adhesion ability and flexibility. RSC Adv. 2015, 5, 12237–12247. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J. Scientific Importance of Water-Processable PEDOT–PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1121–1150. [Google Scholar] [CrossRef]
- Esteban, O.; Cruz-Navarrete, M.; González-Cano, A.; Bernabeu, E. Measurement of the degree of salinity of water with a fiber-optic sensor. Appl. Opt. 1999, 38, 5267–5271. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.Y.; Wang, Q.; Wang, B.T. Refractive index sensing characteristics of carbon nanotube-deposited photonic crystal fiber SPR sensor. Opt. Fiber Technol. 2018, 43, 137–144. [Google Scholar] [CrossRef]
- Quan, X.; Fry, E.S. Empirical equation for the index of refraction of seawater. Appl. Opt. 1995, 34, 3477–3480. [Google Scholar] [CrossRef]
- Ramaswami, S.; Gulyas, H.; Behrendt, J.; Otterpohl, R. Measuring nitrate concentration in wastewaters with high chloride content. Int. J. Environ. Anal. Chem. 2017, 97, 56–70. [Google Scholar] [CrossRef]
- Parveen, S.; Pathak, A.; Gupta, B. Fiber optic SPR nanosensor based on synergistic effects of CNT/Cu-nanoparticles composite for ultratrace sensing of nitrate. Sens. Actuators B Chem. 2017, 246, 910–919. [Google Scholar] [CrossRef]
- Allsop, T.; Neal, R. A Review: Evolution and Diversity of Optical Fibre Plasmonic Sensors. Sensors 2019, 19, 4874. [Google Scholar] [CrossRef]
- Pham, T.B.; Bui, H.; Le, H.T.; Pham, V.H. Characteristics of the fiber laser sensor system based on etched-Bragg grating sensing probe for determination of the low nitrate concentration in water. Sensors 2017, 17, 7. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahnia, S.; Ebendorff-Heidepriem, H.; Evans, D.; Afshar, S. A Fibre-Optic Platform for Sensing Nitrate Using Conducting Polymers. Sensors 2021, 21, 138. https://doi.org/10.3390/s21010138
Shahnia S, Ebendorff-Heidepriem H, Evans D, Afshar S. A Fibre-Optic Platform for Sensing Nitrate Using Conducting Polymers. Sensors. 2021; 21(1):138. https://doi.org/10.3390/s21010138
Chicago/Turabian StyleShahnia, Soroush, Heike Ebendorff-Heidepriem, Drew Evans, and Shahraam Afshar. 2021. "A Fibre-Optic Platform for Sensing Nitrate Using Conducting Polymers" Sensors 21, no. 1: 138. https://doi.org/10.3390/s21010138
APA StyleShahnia, S., Ebendorff-Heidepriem, H., Evans, D., & Afshar, S. (2021). A Fibre-Optic Platform for Sensing Nitrate Using Conducting Polymers. Sensors, 21(1), 138. https://doi.org/10.3390/s21010138





