Facile Fabrication of an Ammonia-Gas Sensor Using Electrochemically Synthesised Polyaniline on Commercial Screen-Printed Three-Electrode Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polyaniline Synthesis
2.2. Material Characterisation
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2.3. Profilometer
2.3. Sensing Characterisation
3. Results and Discussion
3.1. Electrodeposition of Polyaniline (PANI)
3.2. Characterisation and Analysis of the Electrodeposited PANI
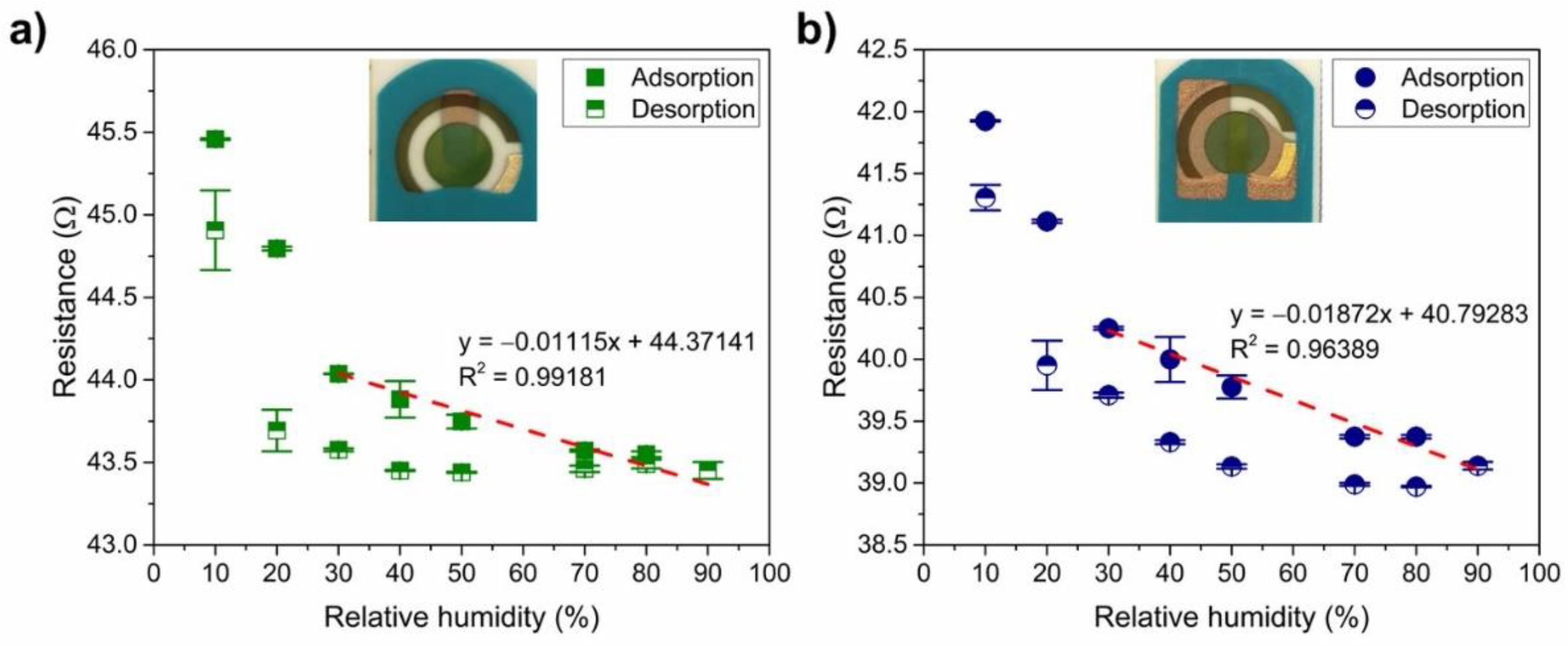
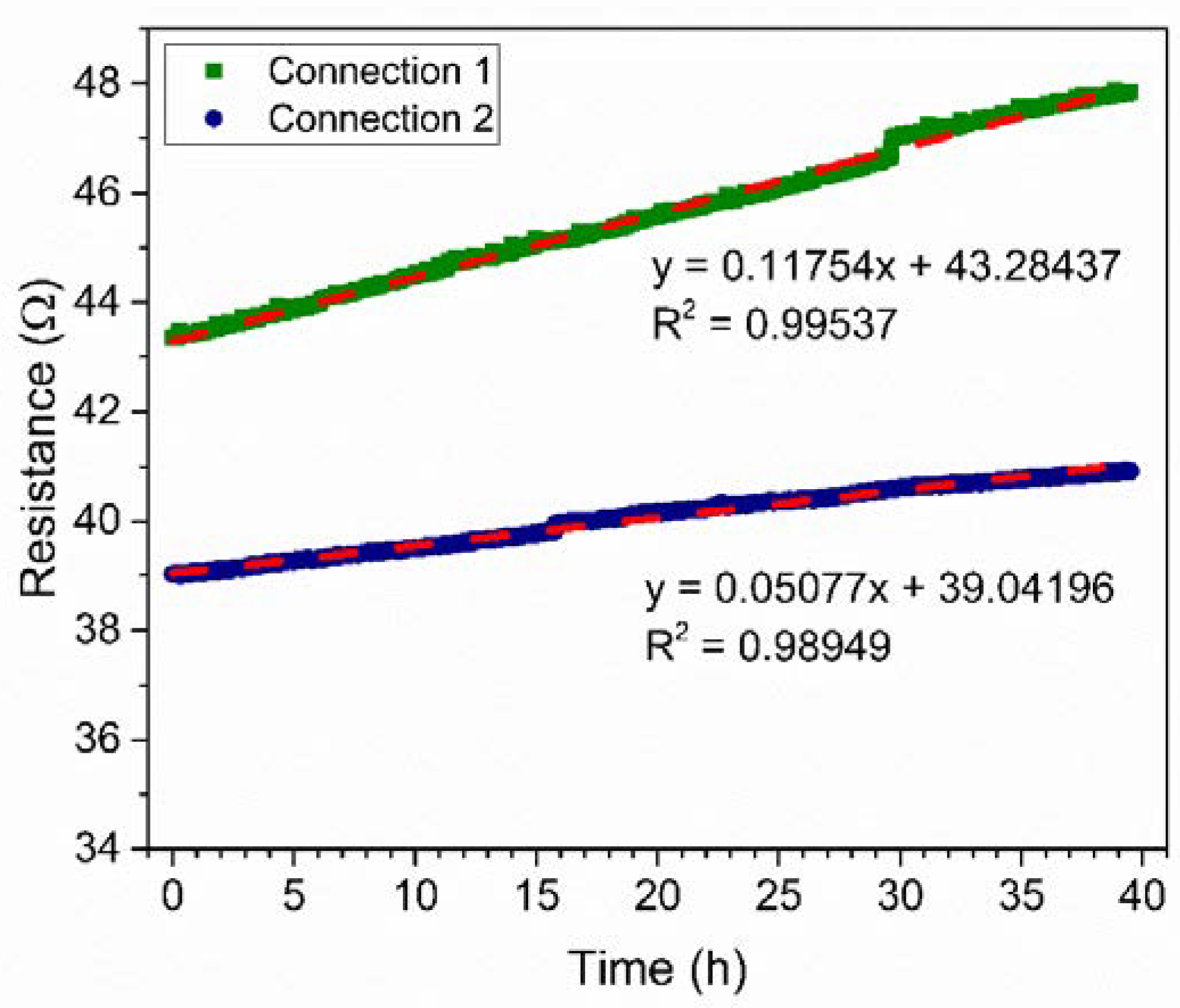
3.3. Impact of Humidity on the Resistance of the PANI-Au-SPE
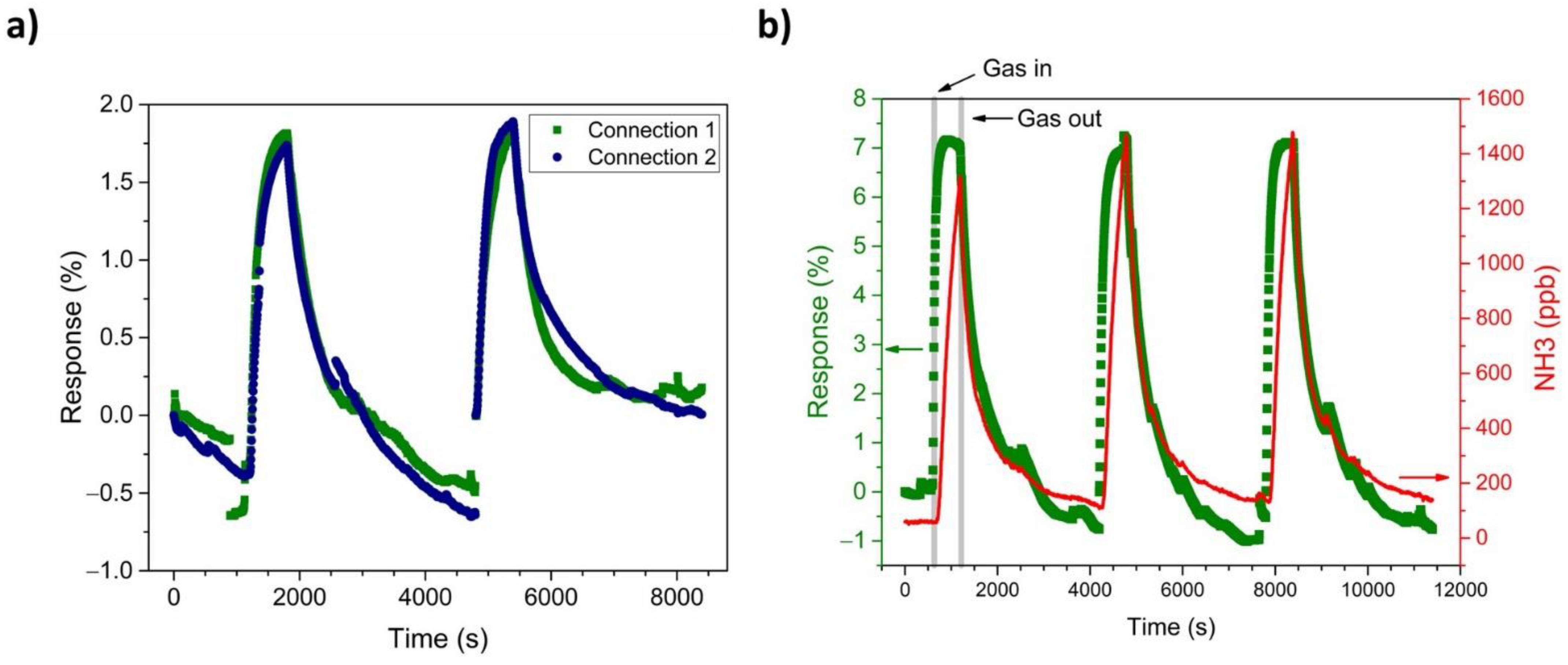
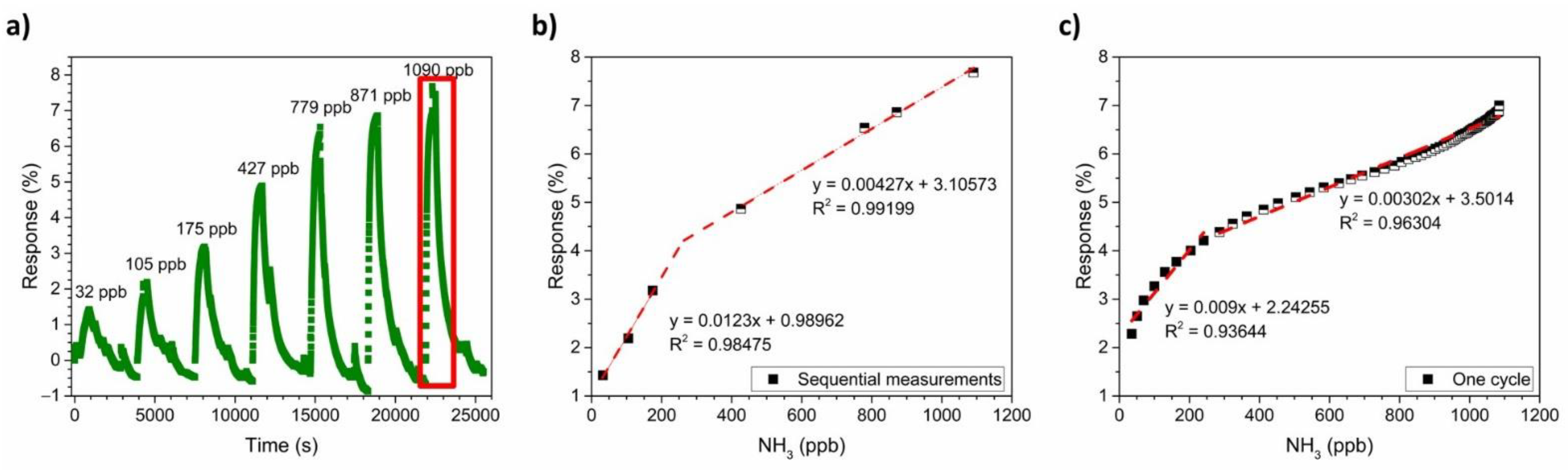
3.4. NH3-Sensing Performance of the PANI-Au-SPE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement.
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hangarter, C.M.; Chartuprayoon, N.; Hernández, S.C.; Choa, Y.; Myung, N.V. Hybridized conducting polymer chemiresistive nano-sensors. Nano Today 2013, 8, 39–55. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-electrical gas sensors based on conducting polymer hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting polymers as chemiresistive gas sensing materials: A review. J. Electrochem. Soc. 2020, 167, 037503. [Google Scholar] [CrossRef]
- Pandey, S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453. [Google Scholar] [CrossRef] [Green Version]
- Tanguy, N.R.; Thompson, M.; Yan, N. A review on advances in application of polyaniline for ammonia detection. Sens. Actuators B Chem. 2018, 257, 1044–1064. [Google Scholar] [CrossRef]
- Alfano, B.; Massera, E.; de Maria, A.; de Girolamo, A.; di Francia, G.; Delli Veneri, P.; Napolitano, T.; Borriello, A. Polyaniline Proton Doping for Sensor Application. In Proceedings of the 2015 18th AISEM Annual Conference, AISEM 2015, Trento, Italy, 26 March 2015. [Google Scholar]
- Wojkiewicz, J.L.; Bliznyuk, V.N.; Carquigny, S.; Elkamchi, N.; Redon, N.; Lasri, T.; Pud, A.A.; Reynaud, S. Nanostructured polyaniline-based composites for ppb range ammonia sensing. Sens. Actuators B Chem. 2011, 160, 1394–1403. [Google Scholar] [CrossRef]
- Mikhaylov, S.; Ogurtsov, N.; Noskov, Y.; Redon, N.; Coddeville, P.; Wojkiewicz, J.L.; Pud, A. Ammonia/amine electronic gas sensors based on hybrid polyaniline-TiO2 nanocomposites. The effects of titania and the surface active doping acid. RSC Adv. 2015, 5, 20218–20226. [Google Scholar] [CrossRef]
- Patni, N.; Jain, N.; Pillai, S.G. Polyaniline-based sensors for monitoring and detection of ammonia and carbon monoxide gases. In Trends and Applications in Advanced Polymeric Materials; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017; pp. 145–162. ISBN 9781119364795. [Google Scholar]
- Virji, S.; Kaner, R.B.; Weiller, B.H. Hydrogen sensors based on conductivity changes in polyaniline nanofibers. J. Phys. Chem. B 2006, 110, 22266–22270. [Google Scholar] [CrossRef]
- Nicolas-Debarnot, D.; Poncin-Epaillard, F. Polyaniline as a new sensitive layer for gas sensors. Anal. Chim. Acta 2003, 475, 1–15. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Administration Sampling and Analytical Methods; Ammonia in Workplace Atmospheres—Solid Sorbent. Available online: https://www.osha.gov/dts/sltc/methods/inorganic/id188/id188.html (accessed on 16 March 2020).
- The Fertilizer Institute. Health Effects of Ammonia; The Fertilizer Institute: Arlington, VA, USA, 2010. [Google Scholar]
- National Research Council (US); Committee on Acute Exposure Guideline Levels Ammonia. Acute exposure guideline levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, USA, 2008; Volume 6, pp. 58–114. ISBN 978-0-309-11213-0. [Google Scholar]
- Grabowska-Polanowska, B.; Faber, J.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Śliwka, I.; Amann, A. Detection of potential chronic kidney disease markers in breath using gas chromatography with mass-spectral detection coupled with thermal desorption method. J. Chromatogr. A 2013, 1301, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef]
- Le Maout, P.; Wojkiewicz, J.L.; Redon, N.; Lahuec, C.; Seguin, F.; Dupont, L.; Mikhaylov, S.; Noskov, Y.; Ogurtsov, N.; Pud, A. Polyaniline nanocomposites based sensor array for breath ammonia analysis. Portable e-nose approach to non-invasive diagnosis of chronic kidney disease. Sens. Actuators B Chem. 2018, 274, 616–626. [Google Scholar] [CrossRef]
- Carquigny, S.; Redon, N.; Plaisance, H.; Reynaud, S. Development of a polyaniline/fluoral-P chemical sensor for gaseous formaldehyde detection. IEEE Sens. J. 2012, 12, 1300–1306. [Google Scholar] [CrossRef]
- Antwi-Boampong, S.; Peng, J.S.; Carlan, J.; Belbruno, J.J. A molecularly imprinted fluoral-p/polyaniline double layer sensor system for selective sensing of formaldehyde. IEEE Sens. J. 2014, 14, 1490–1498. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on smart gas sensing technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [Green Version]
- Kukla, A.L.; Shirshov, Y.M.; Piletsky, S.A. Ammonia sensors based on sensitive polyaniline films. Sens. Actuators B Chem. 1996, 37, 135–140. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Wallace, G.G.; Teasdale, P.R.; Spinks, G.M. Conductive Electroactive Polymers: Intelligent Polymer Systems; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420067095. [Google Scholar]
- Ramohlola, K.E.; Monana, G.R.; Hato, M.J.; Modibane, K.D.; Molapo, K.M.; Masikini, M.; Mduli, S.B.; Iwuoha, E.I. Polyaniline-metal organic framework nanocomposite as an efficient electrocatalyst for hydrogen evolution reaction. Compos. Part B Eng. 2018, 137, 129–139. [Google Scholar] [CrossRef]
- Hirata, M.; Sun, L. Characteristics of an organic semiconductor polyaniline film as a sensor for NH3 gas. Sens. Actuators A. Phys. 1994, 40, 159–163. [Google Scholar] [CrossRef]
- Mérian, T.; Redon, N.; Zujovic, Z.; Stanisavljev, D.; Wojkiewicz, J.L.; Gizdavic-Nikolaidis, M. Ultra sensitive ammonia sensors based on microwave synthesized nanofibrillar polyanilines. Sens. Actuators B Chem. 2014, 203, 626–634. [Google Scholar] [CrossRef]
- Di Zhang, H.; Tang, C.C.; Long, Y.Z.; Zhang, J.C.; Huang, R.; Li, J.J.; Gu, C.Z. High-sensitivity gas sensors based on arranged polyaniline/PMMA composite fibers. Sens. Actuators A Phys. 2014, 219, 123–127. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Li, P.; Zong, X.; Dong, G.; Zhang, Y. Facile fabrication of polyaniline/multi-walled carbon nanotubes/molybdenum disulfide ternary nanocomposite and its high-performance ammonia-sensing at room temperature. Sens. Actuators B Chem. 2018, 258, 895–905. [Google Scholar] [CrossRef]
- Liu, C.; Tai, H.; Zhang, P.; Yuan, Z.; Du, X.; Xie, G.; Jiang, Y. A high-performance flexible gas sensor based on self-assembled PANI-CeO2 nanocomposite thin film for trace-level NH3 detection at room temperature. Sens Actuators B Chem. 2018, 261, 587–597. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, S.; Wu, Z.; Zhang, M.; Cao, Y.; Guo, J.; Zhong, F.; Duan, H.; Jia, D. High-performance gas sensor of polyaniline/carbon nanotube composites promoted by interface engineering. Sensors 2020, 20, 149. [Google Scholar] [CrossRef] [Green Version]
- Krutovertsev, S.A.; Sorokin, S.I.; Zorin, A.V.; Letuchy, Y.A.; Antonova, O.Y. Polymer film-based sensors for ammonia detection. Sens. Actuators B. Chem. 1992, 7, 492–494. [Google Scholar] [CrossRef]
- Zeng, F.-W.; Liu, X.-X.; Diamond, D.; Lau, K.T. Humidity sensors based on polyaniline nanofibres. Sens. Actuators B Chem. 2010, 143, 530–534. [Google Scholar] [CrossRef]
- Manjunatha, S.; Machappa, T.; Ravikiran, Y.T.; Chethan, B.; Sunilkumar, A. Polyaniline based stable humidity sensor operable at room temperature. Phys. B Condens. Matter 2019, 561, 170–178. [Google Scholar] [CrossRef]
- Chani, M.T.S.; Karimov, K.S.; Khalid, F.A.; Moiz, S.A. Polyaniline based impedance humidity sensors. Solid State Sci. 2013, 18, 78–82. [Google Scholar] [CrossRef]
- Jain, S.; Chakane, S.; Samui, A.B.; Krishnamurthy, V.N.; Bhoraskar, S.V. Humidity sensing with weak acid-doped polyaniline and its composites. Sens. Actuators B Chem. 2003, 96, 124–129. [Google Scholar] [CrossRef]
- Nohria, R.; Khillan, R.K.; Su, Y.; Dikshit, R.; Lvov, Y.; Varahramyan, K. Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sens. Actuators B Chem. 2006, 114, 218–222. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Gvozdenovic, M.; Jugovic, B.; Stevanovic, J.; Grgur, B. Electrochemical synthesis of electroconducting polymers. Hem. Ind. 2014, 68, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Rana, U.; Paul, N.D.; Mondal, S.; Chakraborty, C.; Malik, S. Water soluble polyaniline coated electrode: A simple and nimble electrochemical approach for ascorbic acid detection. Synth. Met. 2014, 192, 43–49. [Google Scholar] [CrossRef]
- Paul, E.W.; Ricco, A.J.; Wrighton, M.S. Resistance of polyaniline films as a function of electrochemical potential and the fabrication of polyaniline-based microelectronic devices. J. Phys. Chem. 1985, 89, 1441–1447. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.W. A selective hydrogen peroxide sensor based on chemiresistive polyaniline nanowires modified with silver catalytic nanoparticles. J. Micromech. Microeng. 2014, 24, 065004. [Google Scholar] [CrossRef]
- Wang, J.; Chan, S.; Carlson, R.R.; Luo, Y.; Ge, G.; Ries, R.S.; Heath, J.R.; Tseng, H.R. Electrochemically fabricated polyaniline nanoframework electrode junctions that function as resistive sensors. Nano Lett. 2004, 4, 1693–1697. [Google Scholar] [CrossRef]
- Mehto, A.; Mehto, V.R.; Chauhan, J.; IB, S.; RK, P. Preparation and characterization of polyaniline/ZnO composite sensor. J. Nanomed. Res. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhang, J.; Du, Y.; Yang, X.; Wang, E. Microfabricated on-chip integrated Au-Ag-Au three-electrode system for in situ mercury ion determination. Analyst 2010, 135, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Jiang, Y.; Duan, Z.; Liu, B.; Zhao, Q.; Wang, S.; Yuan, Z.; Tai, H. Ultrasensitive flexible NH3 gas sensor based on polyaniline/SrGe4O9 nanocomposite with ppt-level detection ability at room temperature. Sens. Actuators B Chem. 2020, 319. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Lima, J.; Quinaz, M.B. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef]
- Kellenberger, A.; Ambros, D.; Plesu, N. Scan rate dependent morphology of polyaniline films electrochemically deposited on nickel. Int. J. Electrochem. Sci. 2014, 9, 6821–6833. [Google Scholar]
- Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K. A correlative study of polyaniline electropolymerization and its electrochromic behavior. J. Electrochem. Soc. 2020, 167, 106504. [Google Scholar] [CrossRef]
- Plesu, N.; Kellenberger, A.; Mihali, M.; Vaszilcsin, N. Effect of temperature on the electrochemical synthesis and properties of polyaniline films. J. Non Cryst. Solids 2010, 356, 1081–1088. [Google Scholar] [CrossRef]
- DropSens. DropSens Screen-Printed Electrodes. Available online: http://www.dropsens.com/en/pdfs_productos/new_brochures/250at-250bt.pdf (accessed on 8 October 2019).
- Yano, J.; Yoshikawa, K.; Kitani, A. Kinetic study of the electropolymerization of aniline using chronoamperometric techniques. Anal. Sci. 1997, 13, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Molano Franco, D.; Arevalo-Rodriguez, I.; Roqué i Figuls, M.; Zamora, J. Interleukin-6 for diagnosis of sepsis in critically ill adult patients. Cochrane Database Syst. Rev. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Fitriyana, F.; Kurniawan, F. Polyaniline-invertase-gold nanoparticles modified gold electrode for sucrose detection. Indones. J. Chem. 2015, 15, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Wen, T.C.; Gopalan, A. Negative capacitance for polyaniline: An analysis via electrochemical impedance spectroscopy. Synth. Met. 2002, 128, 179–189. [Google Scholar] [CrossRef]
- Huang, W.S.; MacDiarmid, A.G. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Yin, M.; Liu, S. Preparation of ZnO hollow spheres with different surface roughness and their enhanced gas sensing property. Sens. Actuators B Chem. 2014, 197, 58–65. [Google Scholar] [CrossRef]
- Brožová, L.; Holler, P.; Kovářová, J.; Stejskal, J.; Trchová, M. The stability of polyaniline in strongly alkaline or acidic aqueous media. Polym. Degrad. Stab. 2008, 93, 592–600. [Google Scholar] [CrossRef]
- Rangel-Vazquez, N.-A.; Sánchez-López, C.; Felix, F.R. Spectroscopy analyses of polyurethane/polyaniline IPN using computational simulation (Amber, MM+ and PM3 method). Polímeros 2014, 24, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Electrochemical synthesis of polyaniline and its comparison with chemically synthesized polyaniline. J. Appl. Polym. Sci. 2007, 104, 1900–1904. [Google Scholar] [CrossRef]
- Dhivya, C.; Vandarkuzhali, S.A.A.; Radha, N. Antimicrobial activities of nanostructured polyanilines doped with aromatic nitro compounds. Arab. J. Chem. 2019, 12, 3785–3798. [Google Scholar] [CrossRef] [Green Version]
- Kinyanjui, J.M.; Hanks, J.; Hatchett, D.W.; Smith, A.; Josowicz, M. Chemical and electrochemical synthesis of polyaniline/gold composites. J. Electrochem. Soc. 2004, 151, D113–D120. [Google Scholar] [CrossRef]
- Travers, J.P.; Nechtschein, M. Water effects in polyaniline: A new conduction process. Synth. Met. 1987, 21, 135–141. [Google Scholar] [CrossRef]
- Cavallo, P.; Acevedo, D.F.; Fuertes, M.C.; Soler-Illia, G.J.A.A.; Barbero, C.A. Understanding the sensing mechanism of polyaniline resistive sensors. Effect of humidity on sensing of organic volatiles. Sens. Actuators B Chem. 2015, 210, 574–580. [Google Scholar] [CrossRef]
- He, L.; Jia, Y.; Meng, F.; Li, M.; Liu, J. Gas sensors for ammonia detection based on polyaniline-coated multi-wall carbon nanotubes. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2009, 163, 76–81. [Google Scholar] [CrossRef]
- Fan, G.; Chen, D.; Li, T.; Yi, S.; Ji, H.; Wang, Y.; Zhang, Z.; Shao, G.; Fan, B.; Wang, H.; et al. Enhanced room-temperature ammonia-sensing properties of polyaniline-modified WO3 nanoplates derived via ultrasonic spray process. Sens. Actuators B Chem. 2020, 127892. [Google Scholar] [CrossRef]
- Hibbard, T.; Crowley, K.; Killard, A.J. Direct measurement of ammonia in simulated human breath using an inkjet-printed polyaniline nanoparticle sensor. Anal. Chim. Acta 2013, 779, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Yan, X.; Xue, Q. NH3 and HCl sensing characteristics of polyaniline nanofibers deposited on commercial ceramic substrates using interfacial polymerization. Synth. Met. 2010, 160, 2452–2458. [Google Scholar] [CrossRef]
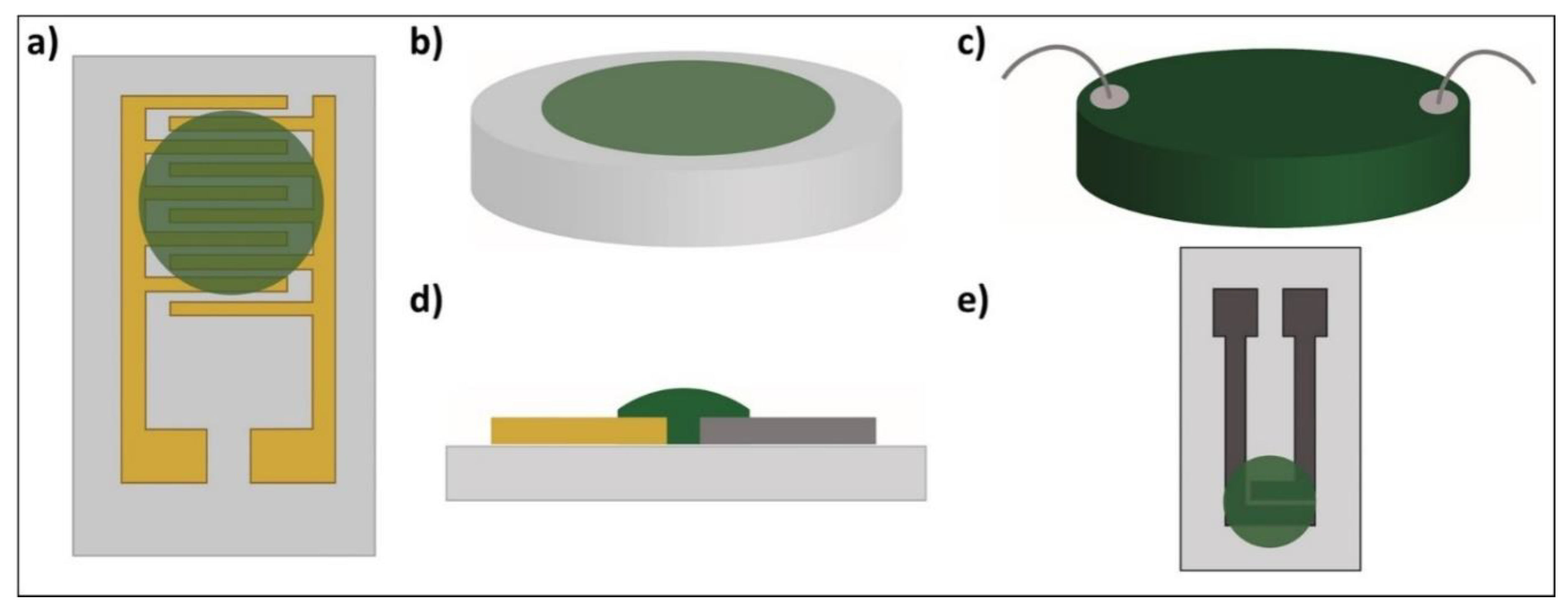

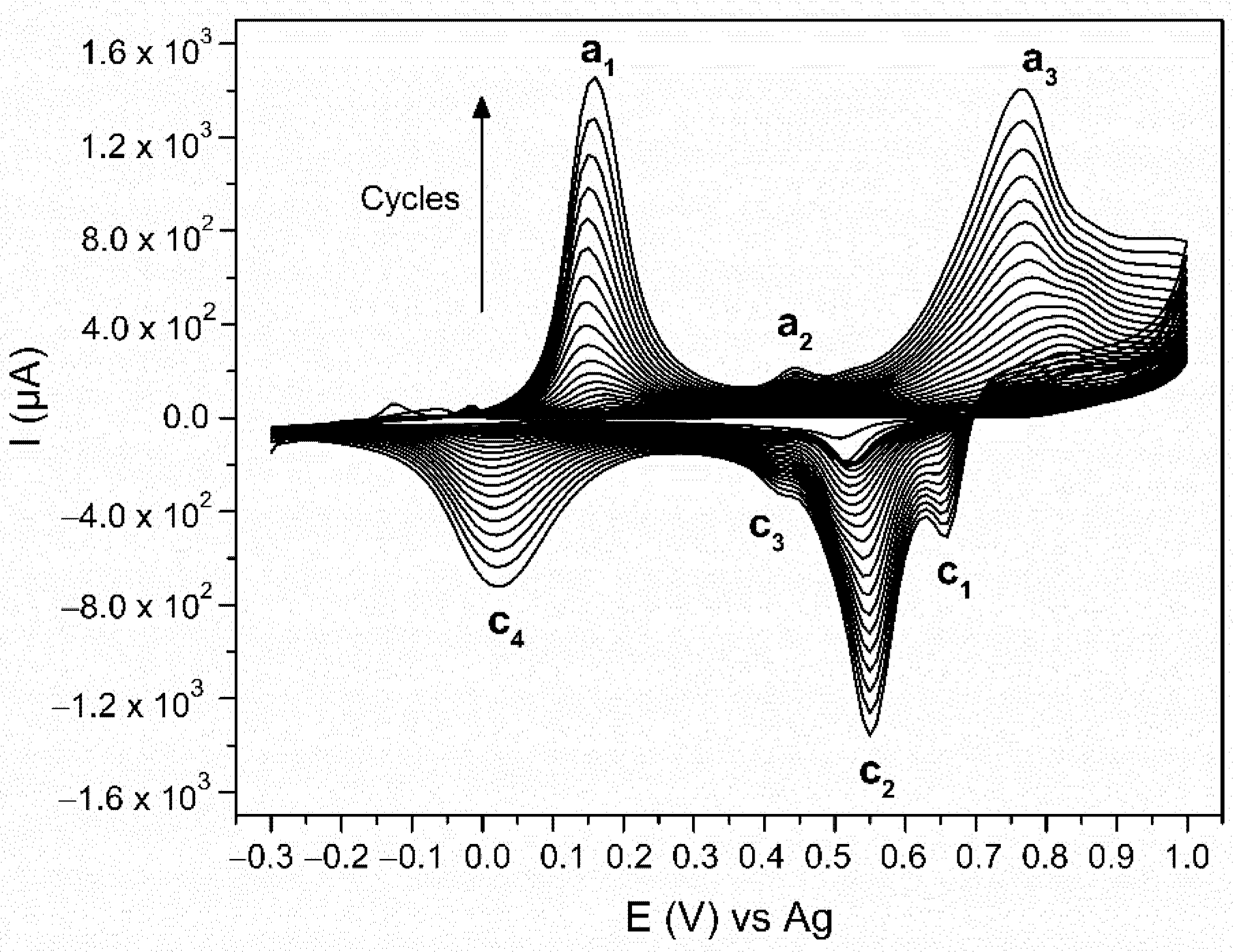
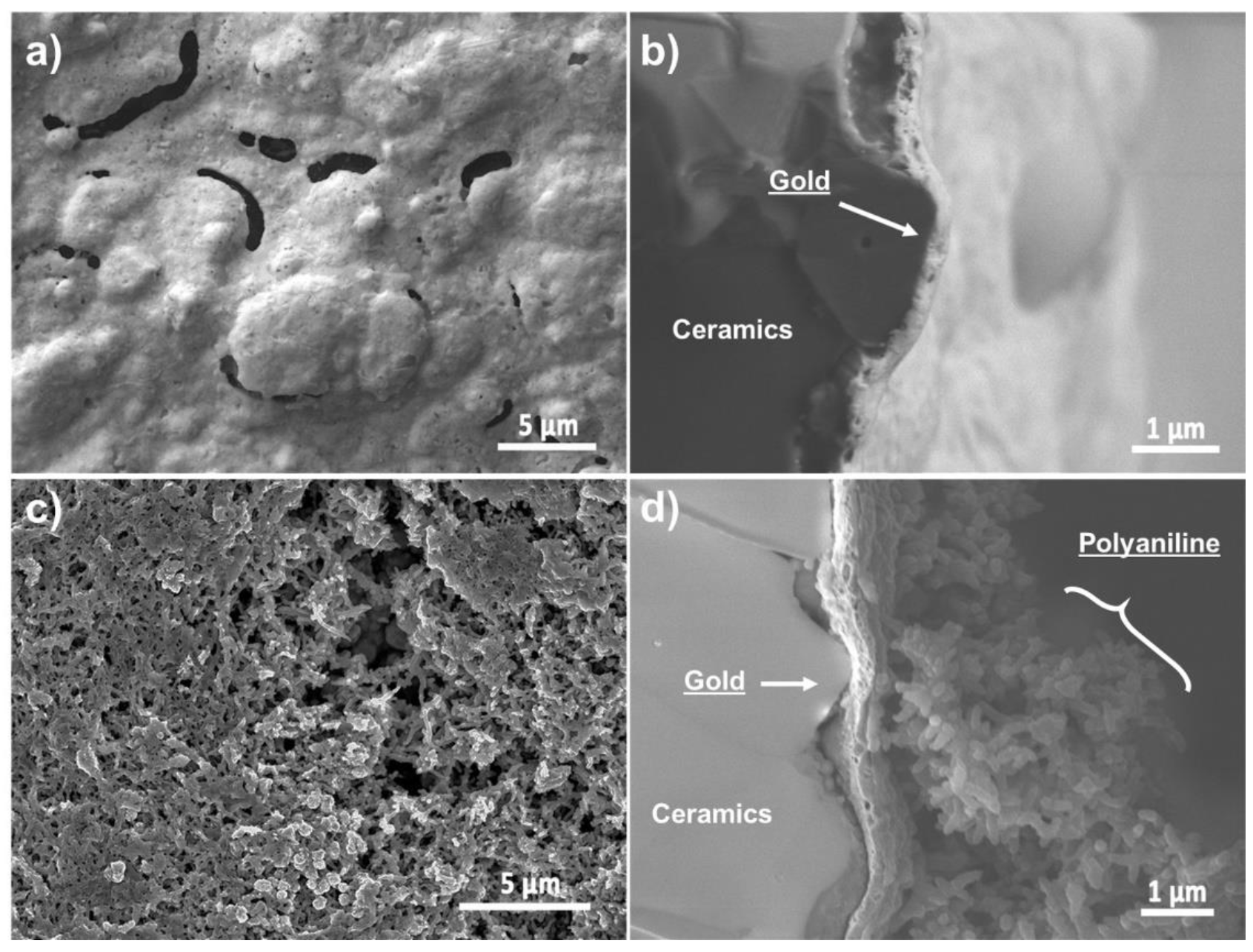

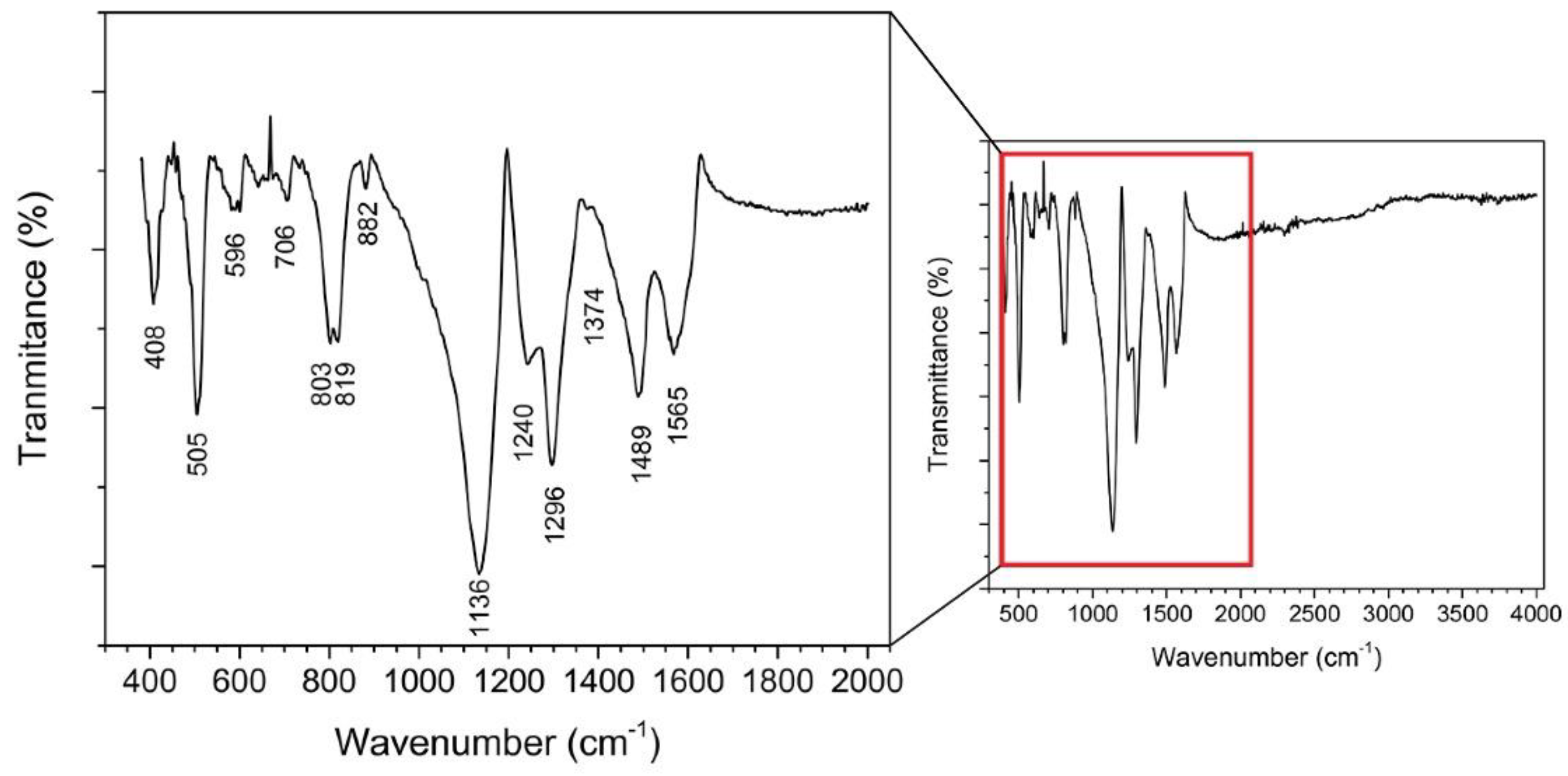


| Material | PANI Synthesis | Detection Limit 1 [ppb] | Quantification Limit 2 [ppb] | Sensitivity [% ppm−1] | Response Time [min] |
|---|---|---|---|---|---|
| PU-PANI (CSA) [8] | Chemical | / | 20 | 0.8 | 5 at 1 ppm |
| PBuA-PANI (HCl) [8] | Chemical | / | 250 | 10 | 2.5 at 1 ppm |
| PVDF-PANI (DBSA) [8] | Chemical | / | 100 | 17 | 2.5 at 1 ppm |
| WO3@PANI [71] | Chemical | 3 | 500 | / | 4.2 for >0.25 ppm |
| PANI (H2SO4) [30] | Chemical | 7 | / | 15 | 3 at 50 ppm |
| PANI-MWCNT (HCl) [70] | Chemical | / | 200 | / | 1.3 at 12 ppm |
| PANI (DBSA) 3 [72] | Chemical | / | 40 | 7900 | / |
| PANI (HCl) [73] | Chemical | / | 1000 | / | / |
| PANI (HCl) [this work] | Electrochemical | 23 | 32 | 12.3 [32–200 ppb] & 4.27 [200–1000 ppb] | 5.2 at 1 ppm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K.; Redon, N.; Wojkiewicz, J.-L.; Duc, C. Facile Fabrication of an Ammonia-Gas Sensor Using Electrochemically Synthesised Polyaniline on Commercial Screen-Printed Three-Electrode Systems. Sensors 2021, 21, 169. https://doi.org/10.3390/s21010169
Korent A, Žagar Soderžnik K, Šturm S, Žužek Rožman K, Redon N, Wojkiewicz J-L, Duc C. Facile Fabrication of an Ammonia-Gas Sensor Using Electrochemically Synthesised Polyaniline on Commercial Screen-Printed Three-Electrode Systems. Sensors. 2021; 21(1):169. https://doi.org/10.3390/s21010169
Chicago/Turabian StyleKorent, Anja, Kristina Žagar Soderžnik, Sašo Šturm, Kristina Žužek Rožman, Nathalie Redon, Jean-Luc Wojkiewicz, and Caroline Duc. 2021. "Facile Fabrication of an Ammonia-Gas Sensor Using Electrochemically Synthesised Polyaniline on Commercial Screen-Printed Three-Electrode Systems" Sensors 21, no. 1: 169. https://doi.org/10.3390/s21010169






