Recognition of Bimolecular Logic Operation Pattern Based on a Solid-State Nanopore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Instrument
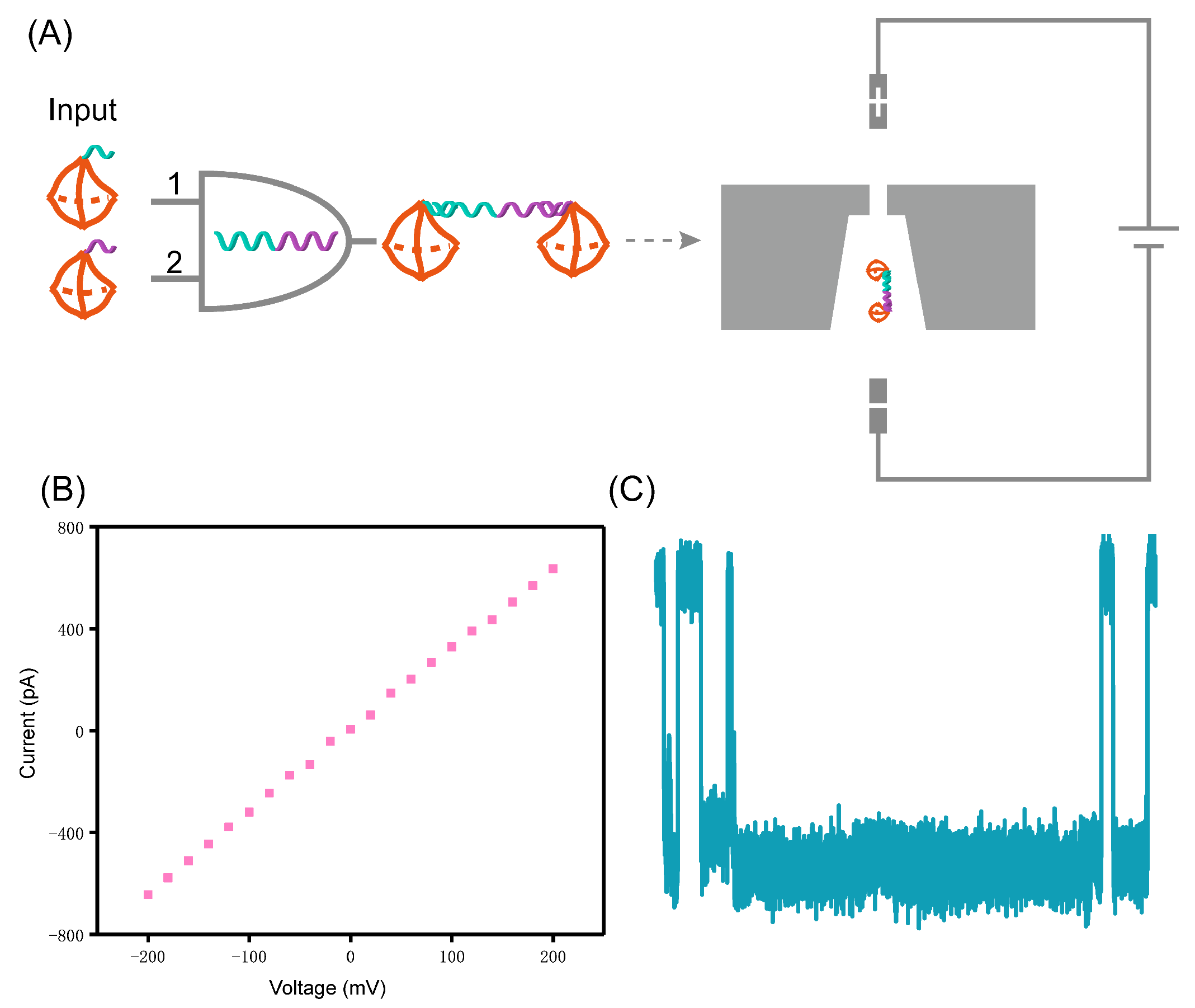
2.2. Nanopore Fabrication and Measurement
2.3. Synthesis of Tetrahedron Probes
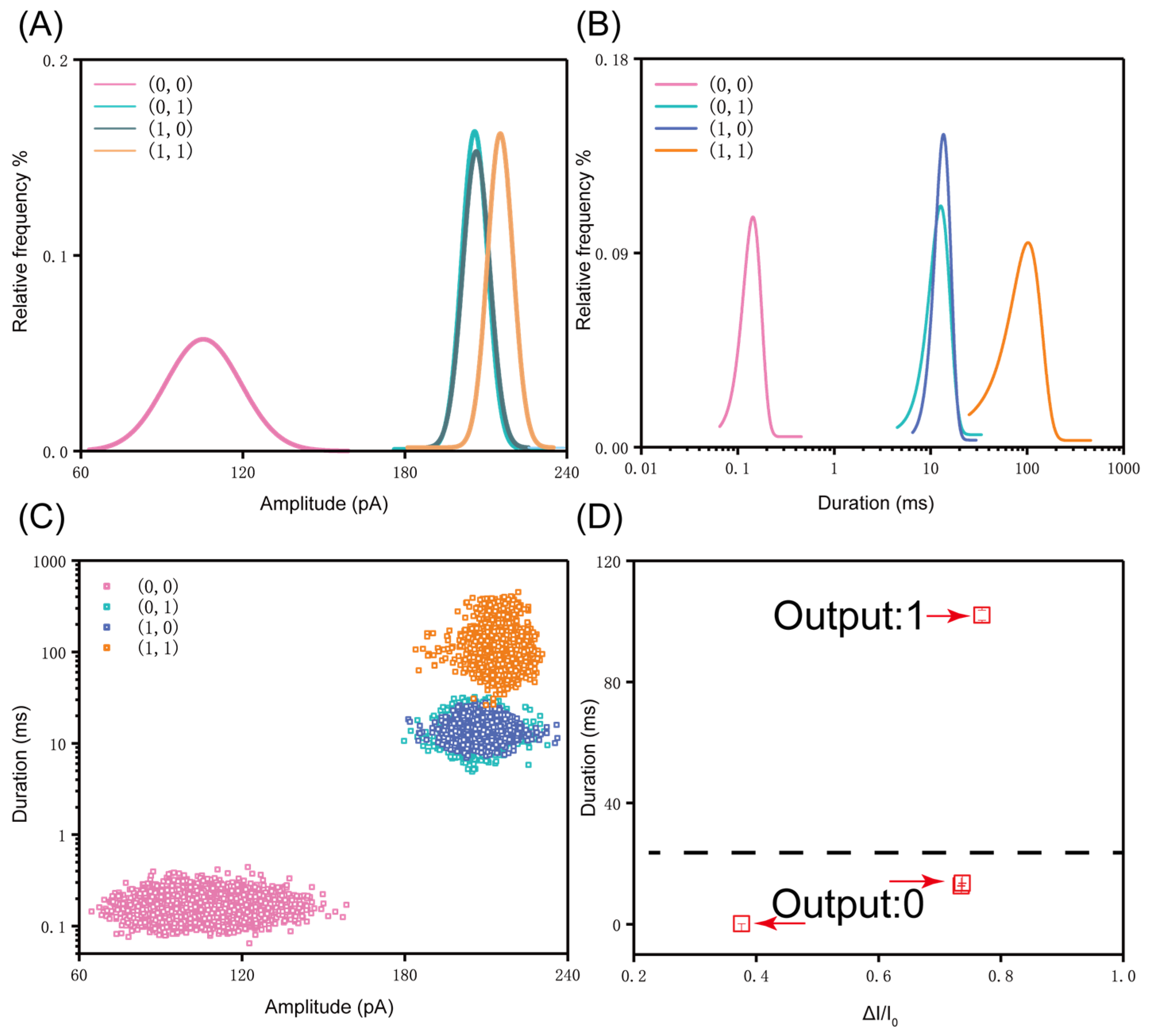
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Arugula, M.A.; Halamek, J.; Pita, M.; Katz, E. Enzyme-Based NAND and NOR logic gates with modular design. J. Phys. Chem. B 2009, 113, 16065–16070. [Google Scholar] [CrossRef]
- De Silva, P.A.; Gunaratne, N.H.Q.; McCoy, C.P. A molecular photoionic and gate based on fluorescent signalling. Nat. Cell Biol. 1993, 364, 42–44. [Google Scholar] [CrossRef]
- Privman, M.; Tam, T.K.; Pita, M.; Katz, E. Switchable electrode controlled by enzyme logic network system: approaching physiologically regulated bioelectronics. J. Am. Chem. Soc. 2009, 131, 1314–1321. [Google Scholar] [CrossRef]
- Amir, L.; Tam, T.K.; Pita, M.; Meijler, M.M.; Alfonta, L.; Katz, E. Biofuel cell controlled by enzyme logic systems. J. Am. Chem. Soc. 2009, 131, 826–832. [Google Scholar] [CrossRef]
- Szaciłowski, K.; Macyk, W.; Stochel, G. Light-Driven or and Xor programmable chemical logic gates. J. Am. Chem. Soc. 2006, 128, 4550–4551. [Google Scholar] [CrossRef] [PubMed]
- Weizmann, Y.; Elnathan, R.; Lioubashevski, O.; Willner, I. Endonuclease-Based logic gates and sensors using magnetic force-amplified readout of DNA scission on cantilevers. J. Am. Chem. Soc. 2005, 127, 12666–12672. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Ami, T.; Fujimoto, K. Autonomous DNA computing machine based on photochemical gate transition. J. Am. Chem. Soc. 2008, 130, 10050–10051. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Kyoi, Y.; Fujimoto, K. Nonenzymatic parallel DNA logic circuits. ChemBioChem 2007, 8, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, J.; Wang, Z.G.; Orbach, R.; Willner, I. Ph-Stimulated concurrent mechanical activation of two DNA “Tweezers”. A “Set-Reset” Logic Gate System. Nano Lett. 2009, 9, 4510–4514. [Google Scholar] [CrossRef] [PubMed]
- Saghatelian, A.; Voelcker, N.H.; Guckian, K.M.; Lin, A.V.S.-Y.; Ghadiri, M.R. DNA-Based photonic logic gates: And, Nand, and Inhibit. J. Am. Chem. Soc. 2003, 125, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Voelcker, N.H.; Guckian, K.M.; Saghatelian, A.; Ghadiri, M.R. Sequence-Addressable DNA logic. Small 2008, 4, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.N.; Mitchell, T.E.; Stefanovic, D. Deoxyribozyme-Based logic gates. J. Am. Chem. Soc. 2002, 124, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, R.P.; Hsing, M.; Sporer-Tuhten, C.S.; Sen, D. Duplex pinching: A structural switch suitable for contractile DNA nanoconstructions. Nano Lett. 2003, 3, 1073–1078. [Google Scholar] [CrossRef]
- Benenson, Y.; Paz-Elizur, T.; Adar, R.; Keinan, E.; Livneh, Z.; Shapiro, E. Programmable and autonomous computing machine made of biomolecules. Nat. Cell Biol. 2001, 414, 430–434. [Google Scholar] [CrossRef]
- Jian, Z.; Zhang, Z.Z.; Shi, Y.Y.; Li, X.X.; He, L. Linearly programmed DNA-Based molecular computer operated on magnetic particle surface in test-tube. Chin. Sci. Bull. 2004, 49, 17–22. [Google Scholar]
- Harding, B.I.; Pollak, N.M.; Stefanovic, D.; Macdonald, J. Repeated reuse of Deoxyribozyme-Based logic gates. Nano Lett. 2019, 19, 7655–7661. [Google Scholar] [CrossRef]
- Jiao, K.; Zhu, B.; Guo, L.; Zhou, H.; Wang, F.; Zhang, X.; Shi, J.; Li, Q.; Wang, L.; Li, J.; et al. Programming switchable transcription of topologically constrained DNA. J. Am. Chem. Soc. 2020, 142, 10739–10746. [Google Scholar] [CrossRef]
- Nakama, T.; Takezawa, Y.; Sasaki, D.; Shionoya, M. Allosteric regulation of DNAzyme activities through intrastrand transformation induced by Cu(II)-mediated artificial base pairing. J. Am. Chem. Soc. 2020, 142, 10153–10162. [Google Scholar] [CrossRef]
- Barnoy, E.A.; Popovtzer, R.; Fixler, D. Fluorescence for biological logic gates. J. Biophotonics 2020, 13, e202000158. [Google Scholar] [CrossRef]
- Goldsworthy, V.; LaForce, G.; Abels, S.; Khisamutdinov, E.F. Fluorogenic RNA Aptamers: A nano-platform for fabrication of simple and combinatorial logic gates. Nanomaterials 2018, 8, 984. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, S.; Ono, H.; Kawasaki, S.; Kuang, Y.; Fujita, Y.; Saito, H. Synthetic RNA-based logic computation in mammalian cells. Nat. Commun. 2018, 9, 4847. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Jian, J.; Hua, Y.; Yang, D.; Gao, Y.; You, J.; Wang, Z.; Chang, Y.; Yuan, K.; Bao, Z.; et al. DNA colorimetric logic gate in microfluidic chip based on unmodified gold nanoparticles and molecular recognition. Sens. Actuators B Chem. 2018, 273, 559–565. [Google Scholar] [CrossRef]
- Cao, C.; Ying, Y.-L.; Hu, Z.-L.; Liao, D.-F.; Tian, H.; Long, Y.-T. Discrimination of oligonucleotides of different lengths with a wild-type aerolysin nanopore. Nat. Nanotechnol. 2016, 11, 713–718. [Google Scholar] [CrossRef]
- Bandarkar, P.; Yang, H.; Henley, R.; Wanunu, M.; Whitford, P.C. How nanopore translocation experiments can measure RNA unfolding. Biophys. J. 2020, 118, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ying, Y.-L.; Liu, S.-C.; Hu, Y.-X.; Long, Y.-T. Measuring temperature effects on nanobubble nucleation via a solid-state nanopore. Analyst 2020, 145, 2510–2514. [Google Scholar] [CrossRef]
- Ying, Y.-L.; Yu, R.-J.; Hu, Y.-X.; Gao, R.; Long, Y.-T. Single antibody–antigen interactions monitored via transient ionic current recording using nanopore sensors. Chem. Commun. 2017, 53, 8620–8623. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wang, Y.-Q.; Ying, Y.-L.; Long, Y.-T. Revealing the transient conformations of a single flavin adenine dinucleotide using an aerolysin nanopore. Chem. Sci. 2019, 10, 10400–10404. [Google Scholar] [CrossRef]
- Yu, H.; Li, Z.; Tao, Y.; Sha, J.; Chen, Y. Thermal bubble nucleation in graphene nanochannels. J. Phys. Chem. C 2019, 123, 3482–3490. [Google Scholar] [CrossRef]
- Sun, K.; Zhao, C.; Zeng, X.; Chen, Y.; Jiang, X.; Ding, X.; Gou, L.; Xie, H.; Li, X.; Zhang, X.; et al. Active DNA unwinding and transport by a membrane-adapted helicase nanopore. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Roozbahani, G.M.; Guan, X. Salt-Mediated nanopore detection of ADAM-17. ACS Appl. Bio Mater. 2019, 2, 504–509. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhou, S.; Roozbahani, G.M.; Zhang, Y.; Wang, D.; Guan, X. Displacement chemistry-based nanopore analysis of nucleic acids in complicated matrices. Chem. Commun. 2018, 54, 13977–13980. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, D.V.; Pud, S.; Shi, X.; De Angelis, L.; Kuipers, L.; Dekker, C. Label-Free optical detection of DNA translocations through plasmonic nanopores. ACS Nano 2019, 13, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, K.; Bulushev, R.D.; Khlybov, S.; Dumcenco, D.; Kis, A.; Radenovic, A. Identification of single nucleotides in MoS2 nanopores. Nat. Nanotechnol. 2015, 10, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Yusko, E.C.; Bruhn, B.R.; Eggenberger, O.M.; Houghtaling, J.; Rollings, R.; Walsh, N.C.; Nandivada, S.; Pindrus, M.; Hall, A.R.; Sept, D.; et al. Real-time shape approximation and fingerprinting of single proteins using a nanopore. Nat. Nanotechnol. 2017, 12, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Duan, X.; Li, Q.; Wu, R.; Wang, Y.; Li, B. Low-Noise nanopore enables in-situ and label-free tracking of a trigger-induced DNA molecular machine at the single-molecular level. J. Am. Chem. Soc. 2020, 142, 4481–4492. [Google Scholar] [CrossRef]
- Yuan, B.; Li, S.; Ying, Y.-L.; Long, Y.-T. The analysis of single cysteine molecules with an aerolysin nanopore. Analyst 2020, 145, 1179–1183. [Google Scholar] [CrossRef]
- Nazari, M.; Li, X.; Alibakhshi, M.A.; Yang, H.; Souza, K.; Gillespie, C.; Gummuluru, S.; Hong, M.K.; Reinhard, B.M.; Korolev, K.S.; et al. Femtosecond photonic viral inactivation probed using solid-state nanopores. Nano Futur. 2018, 2, 045005. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wu, X.-Y.; Ying, Y.-L.; Long, Y.-T. Simultaneous single-molecule discrimination of cysteine and homocysteine with a protein nanopore. Chem. Commun. 2019, 55, 9311–9314. [Google Scholar] [CrossRef]
- Yu, R.J.; Lu, S.M.; Xu, S.W.; Li, Y.J.; Xu, Q.; Ying, Y.L.; Long, Y.T. Single molecule sensing of amyloid-beta aggregation by confined glass nanopores. Chem. Sci. 2019, 10, 10728–10732. [Google Scholar] [CrossRef] [Green Version]
- Restrepo-Pérez, L.; Wong, C.H.; Maglia, G.; Dekker, C.; Joo, C. Label-Free detection of post-translational modifications with a nanopore. Nano Lett. 2019, 19, 7957–7964. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.-N.; Ying, Y.-L.; Yang, J.; Long, Y.-T. A wild-type nanopore sensor for protein kinase activity. Anal. Chem. 2019, 91, 9910–9915. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ying, Y.-L.; Liu, S.-C.; Lin, Y.; Long, Y.-T. Detection of single proteins with a general nanopore sensor. ACS Sens. 2019, 4, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Zhang, Y.; Wu, G.; Kan, Y.; Zhang, Y.; Sha, J.; Chen, Y. Discrimination of protein amino acid or its protonated state at single-residue resolution by graphene nanopores. Small 2019, 15, e1900036. [Google Scholar] [CrossRef] [PubMed]
- Grinstaff, M.W.; Song, J.; Meller, A.; Grinstaff, M.W. Single-molecule protein sensing in a nanopore: A tutorial. Chem. Soc. Rev. 2018, 47, 8512–8524. [Google Scholar] [CrossRef]
- Sha, J.; Si, W.; Xu, B.; Zhang, S.; Li, K.; Lin, K.; Shi, H.; Chen, Y. Identification of spherical and nonspherical proteins by a solid-state nanopore. Anal. Chem. 2018, 90, 13826–13831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, M.; Ying, C.; Eggenberger, O.M.; Fennouri, A.; Nandivada, S.; Acharjee, M.; Li, J.; Hall, A.R.; Mayer, M. Estimation of shape, volume, and dipole moment of individual proteins freely transiting a synthetic nanopore. ACS Nano 2019, 13, 5231–5242. [Google Scholar] [CrossRef]
- Restrepo-Pérez, L.; Huang, G.; Bohländer, P.R.; Worp, N.; Eelkema, R.; Maglia, G.; Joo, C.; Dekker, C. Correction to resolving chemical modifications to a single amino acid within a peptide using a biological nanopore. ACS Nano 2020, 13, 13668–13676. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Nandivada, S.; Acharjee, M.C.; McNabb, D.S.; Li, J.L. Estimating Rna polymerase protein binding sites on lambda DNA using solid-state nanopores. ACS Sens. 2019, 4, 100–109. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Y.-Q.; Cao, C.; Li, D.; Long, Y.-T. Monitoring disulfide bonds making and breaking in biological nanopore at single molecule level. Sci. China Ser. B Chem. 2018, 61, 1385–1388. [Google Scholar] [CrossRef]
- Karmi, A.; Sakala, G.P.; Rotem, D.; Reches, M.; Porath, D. Durable, stable, and functional nanopores decorated by self-assembled dipeptides. ACS Appl. Mater. Interfaces 2020, 12, 14563–14568. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Hu, Y.; Chen, X.; Dou, R.; Liu, K.; Cui, C.; Liu, H.; Li, Q.; Pan, D.; et al. Translocation of tetrahedral DNA nanostructures through a solid-state nanopore. Nanoscale 2019, 11, 6263–6269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, Y.; Ali, I.; Liu, L.; Wu, H.; Lu, Z.; Liu, Q. Solid-State nanopore single-molecule sensing of DNAzyme cleavage reaction assisted with nucleic acid nanostructure. ACS Appl. Mater. Interfaces 2018, 10, 26555–26565. [Google Scholar] [CrossRef] [PubMed]
- Alibakhshi, M.A.; Halman, J.R.; Wilson, J.; Aksimentiev, A.; Afonin, K.A.; Wanunu, M. Picomolar fingerprinting of nucleic acid nanoparticles using solid-state nanopores. ACS Nano 2017, 11, 9701–9710. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, M.; Kawano, R. DNA logic operation with nanopore decoding to recognize MicroRNA patterns in small cell lung cancer. Anal. Chem. 2018, 90, 8531–8537. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, J.; Bošković, F.; Keyser, U.F. Nanopore-Based DNA hard drives for rewritable and secure data storage. Nano Lett. 2020, 20, 3754–3760. [Google Scholar] [CrossRef]
- Roether, J.; Chu, K.-Y.; Willenbacher, N.; Shen, A.Q.; Bhalla, N. Real-time monitoring of DNA immobilization and detection of DNA polymerase activity by a microfluidic nanoplasmonic platform. Biosens. Bioelectron. 2019, 142, 111528. [Google Scholar] [CrossRef]
- Kim, D.M.; Yoo, S.M. DNA-modifying enzyme reaction-based biosensors for disease diagnostics: Recent biotechnological advances and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 787–803. [Google Scholar] [CrossRef]
- Lei, S.; Liu, Z.; Xu, L.L.; Zou, L.N.; Li, G.P.; Ye, B.X. A “signal-on” electrochemical biosensor based on dnazyme-driven bipedal DNA walkers and Tdt-mediated cascade signal amplification strategy. Anal. Chim. Acta 2020, 1100, 40–46. [Google Scholar] [CrossRef]
- Feng, Q.-M.; Zhou, Z.; Li, M.-X.; Zhao, W.; Xu, J.-J.; Chen, H.-Y. DNA tetrahedral scaffolds-based platform for the construction of electrochemiluminescence biosensor. Biosens. Bioelectron. 2017, 90, 251–257. [Google Scholar] [CrossRef]
- Zhou, G.; Lin, M.; Song, P.; Chen, X.; Chao, J.; Wang, L.; Huang, Q.; Huang, W.; Fan, C.; Zuo, X. Multivalent capture and detection of cancer cells with DNA nanostructured biosensors and multibranched hybridization chain reaction amplification. Anal. Chem. 2014, 86, 7843–7848. [Google Scholar] [CrossRef]
- Ge, Z.-L.; Lin, M.; Wang, P.; Pei, H.; Yan, J.; Shi, J.; Huang, Q.; He, D.; Fan, C.; Zuo, X. Hybridization chain reaction amplification of MicroRNA detection with a tetrahedral DNA nanostructure-based electrochemical biosensor. Anal. Chem. 2014, 86, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Pei, H.; Wang, L.; Huang, Q.; Fan, C. Construction of functional DNA nanostructures for theranostic applications. Adv. Theranostic Mater. 2014, 47, 550–559. [Google Scholar] [CrossRef]
- Pei, H.; Lu, N.; Wen, Y.; Song, S.; Liu, Y.; Yan, H.; Fan, C. A DNA nanostructure-based biomolecular probe carrier platform for electrochemical biosensing. Adv. Mater. 2010, 22, 4754–4758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.H.; Wang, J.J.; Zhou, G.B.; Wang, J.B.; Wu, N.; Lu, J.X.; Gao, J.M.; Chen, X.Q.; Shi, J.Y.; Zuo, X.L.; et al. Programmable engineering of a biosensing interface with tetrahedral DNA nanostructures for ultrasensitive DNA detection. Angewandte Chemie-Int. Ed. 2015, 54, 2151–2155. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Liang, L.; Yao, G.B.; Li, J.; Huang, Q.; Fan, C.H. Reconfigurable three-dimensional DNA nanostructures for the construction of intracellular logic sensors. Angewandte Chemie-Int. Ed. 2012, 51, 9020–9024. [Google Scholar]
- Chen, Q.; Liu, H.; Lee, W.; Sun, Y.; Zhu, D.; Pei, H.; Fan, C.; Fan, X. Self-assembled DNA tetrahedral optofluidic lasers with precise and tunable gain control. Lab. A Chip 2013, 13, 3351–3354. [Google Scholar] [CrossRef]
- Kwok, H.; Briggs, K.; Tabard-Cossa, V. Nanopore fabrication by controlled dielectric breakdown. PLoS ONE 2014, 9, e92880. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Zhang, Z.; Weng, T.; Zhu, L.; Zhang, P.; Wang, D.; Liu, Q. Recognition of Bimolecular Logic Operation Pattern Based on a Solid-State Nanopore. Sensors 2021, 21, 33. https://doi.org/10.3390/s21010033
Yan H, Zhang Z, Weng T, Zhu L, Zhang P, Wang D, Liu Q. Recognition of Bimolecular Logic Operation Pattern Based on a Solid-State Nanopore. Sensors. 2021; 21(1):33. https://doi.org/10.3390/s21010033
Chicago/Turabian StyleYan, Han, Zhen Zhang, Ting Weng, Libo Zhu, Pang Zhang, Deqiang Wang, and Quanjun Liu. 2021. "Recognition of Bimolecular Logic Operation Pattern Based on a Solid-State Nanopore" Sensors 21, no. 1: 33. https://doi.org/10.3390/s21010033



