Estimation of the Respiratory Rate from Localised ECG at Different Auscultation Sites
Abstract
:1. Introduction
2. Methodology
2.1. Subjects
2.2. Experimental Setup
2.3. Signal Processing
2.3.1. Signal Filtering
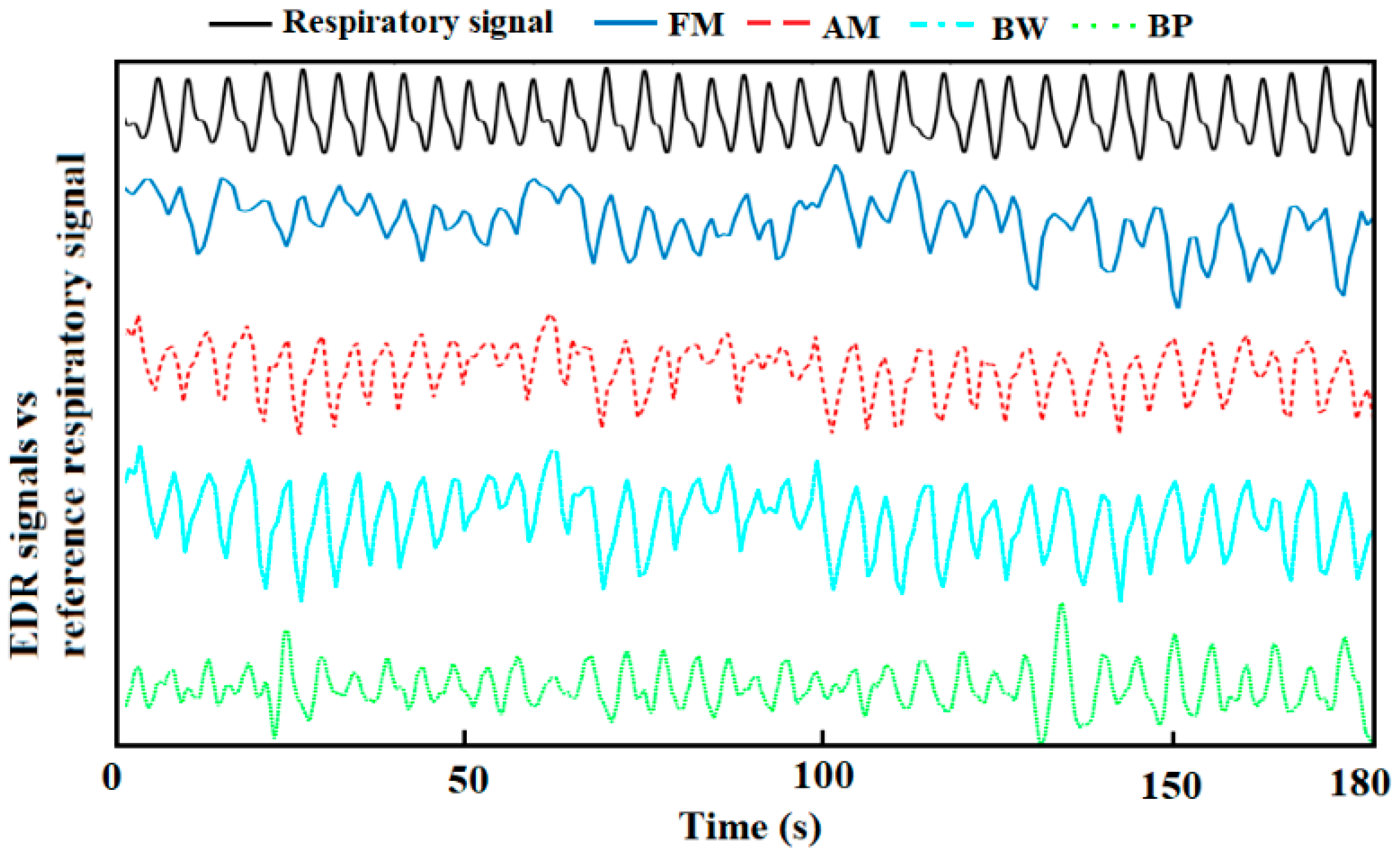
2.3.2. EDR Signals Extraction
- AM algorithm: The amplitude changes due to the respiration in the ECG signals was obtained by connecting the captured R-peaks.
- BW algorithm: Based on the R-peaks, Q points were found using the gradient descent method. Then, the baseline wander could be generated by connecting the middle points between R-peaks and Q points [32].
- FM algorithm: The intervals between the R peaks were calculated. The resulting signal was the frequency modulation caused by respiratory sinus arrhythmia.
- 4.
- BP algorithm: A band-pass filter (0.1–0.5 Hz) was used to capture the EDR signals. Although the normal RR for a healthy adult ranges between 0.2–0.35 Hz at rest, in our processing, we appropriately expanded the range to enable it to respond to special situations, such as the subjects’ occasional deep or rapid breaths. Besides, a wider band can help to further analyse the frequency components when there are no dominant peaks.
2.3.3. Respiratory Rate Estimation
2.4. Statistical Analysis
3. Results
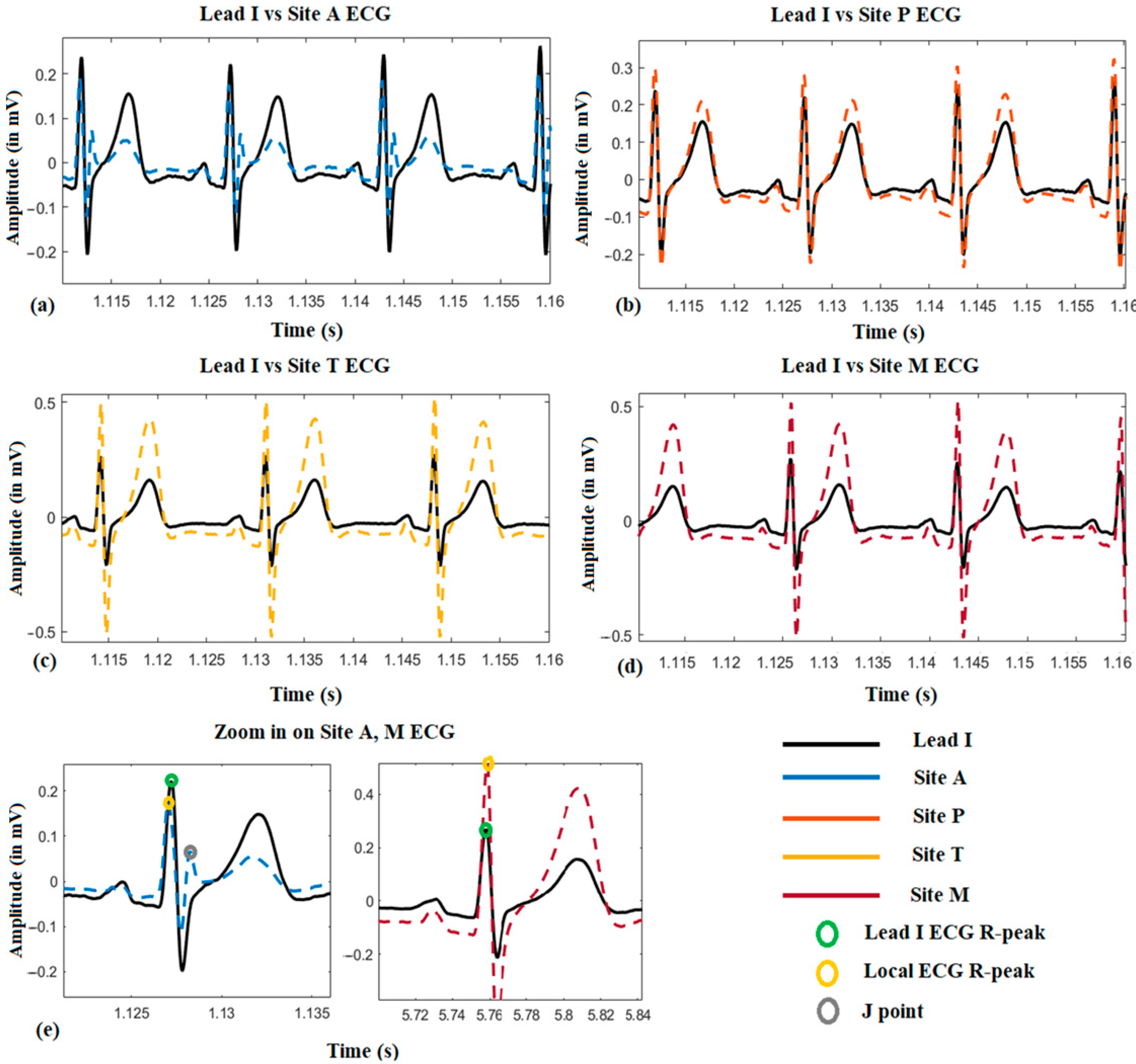
3.1. ECG Morphological Variation among the Auscultation Sites
3.2. Location Effect on EDR among the Auscultation Sites
3.3. The Performance of the EDR Algorithms
3.4. Time vs. Frequency Domain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flenady, T.; Dwyer, T.; Applegarth, J. Accurate respiratory rates count: So should you! Australas. Emerg. Nurs. J. 2017, 20, 45–47. [Google Scholar] [CrossRef] [Green Version]
- Fleming, S.; Thompson, M.; Stevens, R.; Heneghan, C.; Plüddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011, 377, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- McFadden, J.P.; Price, R.C.; Eastwood, H.D.; Briggs, R.S. Raised respiratory rate in elderly patients: A valuable physical sign. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 626–627. [Google Scholar] [CrossRef] [Green Version]
- Park, S.B.; Khattar, D. Tachypnea. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Brochard, L.; Martin, G.S.; Blanch, L.; Pelosi, P.; Belda, F.J.; Jubran, A.; Gattinoni, L.; Mancebo, J.; Ranieri, V.M.; Richard, J.C.M.; et al. Clinical review: Respiratory monitoring in the ICU-a consensus of 16. Crit. Care 2012, 16, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheatley, I. Respiratory rate 3: How to take an accurate measurement. Nurs. Times 2018, 114, 21–22. [Google Scholar]
- Charlton, P.H.; Villarroel, M.; Salguiero, F. Waveform analysis to estimate respiratory rate. In Secondary Analysis of Electronic Health Records; Springer: Cham, Switzerland, 2016; pp. 377–390. [Google Scholar]
- Ansermino, J.M.; Dumont, G.; Ginsburg, A.S. How uncertain is our reference standard for respiratory rate measurement? Am. J. Respir. Crit. Care Med. 2019, 199, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.M.; Ferdosi, N.; Narasimhan, R. Ambulatory respiratory rate detection using ECG and a triaxial accelerometer. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 4058–4061. [Google Scholar]
- Yabuki, S.; Toyama, H.; Takei, Y.; Wagatsuma, T.; Yabuki, H.; Yamauchi, M. Influences of environmental noise level and respiration rate on the accuracy of acoustic respiration rate monitoring. J. Clin. Monit. Comput. 2018, 32, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Einthoven, W.; Fahr, G.; De Waart, A. On the direction and manifest size of the variations of potential in the human heart and on the influence of the position of the heart on the form of the electrocardiogram. Am. Heart J. 1950, 40, 163. [Google Scholar] [CrossRef]
- Flaherty, J.T.; Blumenschein, S.D.; Alexander, A.W.; Gentzler, R.D.; Gallie, T.M.; Boineau, J.P.; Spach, M.S. Influence of respiration on recording cardiac potentials: Isopotential surface-mapping and vectorcardiographic studies. Am. J. Cardiol. 1967, 20, 21–28. [Google Scholar] [CrossRef]
- Riekkinen, H.; Rautaharju, P. Body position, electrode level, and respiration effects on the Frank lead electrocardiogram. Circulation 1976, 53, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, M.; Camm, A.J. Heart rate variability. Clin. Cardiol. 1990, 13, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Travaglini, A.; Lamberti, C.; DeBie, J.; Ferri, M. Respiratory signal derived from eight-lead ECG. In Proceedings of the Computers in Cardiology (Cat. No. 98CH36292), Cleveland, OH, USA, 13–16 September 1998; IEEE: Piscataway, NJ, USA, 1998; Volume 25, pp. 65–68. [Google Scholar]
- Boyle, J.; Bidargaddi, N.; Sarela, A.; Karunanithi, M. Automatic detection of respiration rate from ambulatory single-lead ECG. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Pinciroli, F.; Rossi, R.; Vergani, L. Detection of electrical axis variation for the extraction of respiratory information. Comput. Cardiol. 1985, 2, 499–502. [Google Scholar]
- Mazzanti, B.; Lamberti, C.; De Bie, J. Validation of an ECG-derived respiration monitoring method. In Proceedings of the Computers in Cardiology, Thessaloniki Chalkidiki, Greece, 21–24 September 2003; IEEE: Piscataway, NJ, USA, 2003; pp. 613–616. [Google Scholar]
- Zhao, L.; Reisman, S.; Findley, T. Derivation of Respiration from Electrocardiogram during Heart Rate Variability Studies; IEEE: Piscataway, NJ, USA, 1994; pp. 53–56. [Google Scholar]
- Behbehani, K.; Vijendra, S.; Burk, J.R.; Lucas, E.A. An investigation of the mean electrical axis angle and respiration during sleep. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Engineering in Medicine and Biology, Houston, TX, USA, 23–26 October 2002; IEEE: Piscataway, NJ, USA, 2002; Volume 2, pp. 1550–1551. [Google Scholar]
- Dobrev, D.; Daskalov, I. Two-electrode telemetric instrument for infant heart rate and apnea monitoring. Med. Eng. Phys. 1999, 20, 729–734. [Google Scholar] [CrossRef]
- Sakai, M.; Sekine, R.; Zhu, X. Single-channel ECG suitable for ECG-derived respiration. Biomed. Phys. Eng. Express 2019, 5, 055002. [Google Scholar] [CrossRef]
- Mason, C.L.; Tarassenko, L. Quantitative assessment of respiratory derivation algorithms. In Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001; IEEE: Piscataway, NJ, USA, 2001; Volume 2, pp. 1998–2001. [Google Scholar]
- De Chazal, P.; Heneghan, C.; Sheridan, E.; Reilly, R.; Nolan, P.; O’Malley, M. Automated processing of the single-lead electrocardiogram for the detection of obstructive sleep apnoea. IEEE Trans. Biomed. Eng. 2003, 50, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.S.; Laciar, E.; Torres, A.; Jane, R. Performance evaluation of three methods for respiratory signal estimation from the electrocardiogram. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 4760–4763. [Google Scholar]
- Widjaja, D.; Taelman, J.; Vandeput, S.; Braeken, M.A.; Otte, R.A.; Van den Bergh, B.R.; Van Huffel, S. ECG-derived respiration: Comparison and new measures for respiratory variability. In Proceedings of the 2010 Computing in Cardiology, Belfast, UK, 26–29 September 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 149–152. [Google Scholar]
- Campolo, M.; Labate, D.; La Foresta, F.; Morabito, F.C.; Lay-Ekuakille, A.; Vergallo, P. ECG-derived respiratory signal using empirical mode decomposition. In Proceedings of the 2011 IEEE International Symposium on Medical Measurements and Applications, Bari, Italy, 30–31 May 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 399–403. [Google Scholar]
- Bao, X.; Gall, N.; Deng, Y.; Kamavuako, E.N. Analysis of ECG and PCG Time Delay around Auscultation Sites. In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies, Valletta, Malta, 24–26 February 2020; Volume 4, pp. 206–213. [Google Scholar]
- Ricciardi, D.; Cavallari, I.; Creta, A.; Giovanni, G.D.; Calabrese, V.; Belardino, N.D.; Mega, S.; Colaiori, I.; Ragni, L.; Proscia, C.; et al. Impact of the high-frequency cutoff of bandpass filtering on ecg quality and clinical interpretation: A comparison between 40hz and 150hz cutoff in a surgical preoperative adult outpatient population. J. Electrocardiol. 2016, 49, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Laguna, P.; Jane, R.; Caminal, P. Adaptive filtering of ecg baseline wander. In Proceedings of the 1992 14th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Paris, France, 29 October–1 November 1992; Volume 2, pp. 508–509. [Google Scholar]
- Pan, J.; Tompkins, W.J. A real-time qrs detection algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Charlton, P.H.; Birrenkott, D.A.; Bonnici, T.; Pimentel, M.A.; Johnson, A.E.; Alastruey, J.; Tarassenko, L.; Watkinson, P.J.; Beale, R.; Clifton, D.A. Breathing rate estimation from the electrocardiogram and photoplethysmogram: A review. IEEE Rev. Biomed. Eng. 2017, 11, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, L. Signal Processing Methods for Non-Invasive Respiration Monitoring; University of Oxford: Oxford, UK, 2002. [Google Scholar]
- Kitney, R.I.; Rompelman, O. The Study of Heart-Rate Variability (No. 3); Oxford University Press: Oxford, UK, 1980. [Google Scholar]
- Bao, X.; Howard, M.; Niazi, I.K.; Kamavuako, E.N. Comparison between Embroidered and Gel Electrodes on ECG-Derived Respiration Rate. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 2622–2625. [Google Scholar]




| A | P | T | M | Lead I | |
|---|---|---|---|---|---|
| Subject1 | 0.16 | 0.06 | 0.14 | 0.12 | 0.08 |
| Subject2 | 2.27 | 3.74 | 1.77 | 0.86 | 2.00 |
| Subject3 | 1.88 | 1.37 | 3.17 | 2.15 | 2.60 |
| Subject4 | 0.50 | 1.69 | 0.54 | 0.82 | 0.83 |
| Subject5 | 1.27 | 1.10 | 0.28 | 0.14 | 0.47 |
| Subject6 | 5.79 | 4.61 | 6.91 | 3.23 | 5.45 |
| Subject7 | 1.60 | 2.60 | 0.38 | 2.60 | 2.24 |
| Subject8 | 2.54 | 5.41 | 3.16 | 3.47 | 2.24 |
| Subject9 | 0.73 | 0.20 | 0.67 | 0.53 | 1.16 |
| Subject10 | 0.36 | 1.62 | 1.69 | 1.25 | 1.11 |
| Subject11 | 0.85 | 2.10 | 1.03 | 1.46 | 1.45 |
| Subject12 | 1.93 | 3.06 | 1.07 | 0.99 | 2.39 |
| Mean | 1.66 | 2.30 | 1.73 | 1.47 | 1.83 |
| BW | AM | FM | BP | |
|---|---|---|---|---|
| Subject1 | 1.61 | 1.92 | 5.86 | 1.93 |
| Subject2 | 1.65 | 2.88 | 3.26 | 2.45 |
| Subject3 | 1.33 | 1.82 | 2.04 | 0.47 |
| Subject4 | 2.19 | 1.99 | 4.06 | 2.19 |
| Subject5 | 0.91 | 0.92 | 1.54 | 2.93 |
| Subject6 | 0.49 | 0.41 | 3.55 | 3.67 |
| Subject7 | 0.34 | 0.66 | 0.73 | 0.86 |
| Subject8 | 0.76 | 0.75 | 3.23 | 1.19 |
| Subject9 | 3.98 | 3.54 | 11.93 | 6.75 |
| Subject10 | 0.45 | 0.38 | 2.08 | 0.38 |
| Subject11 | 1.81 | 2.02 | 3.36 | 3.59 |
| Subject12 | 1.84 | 1.78 | 4.61 | 5.89 |
| Mean | 1.45 | 1.59 | 3.85 | 2.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Abdala, A.K.; Kamavuako, E.N. Estimation of the Respiratory Rate from Localised ECG at Different Auscultation Sites. Sensors 2021, 21, 78. https://doi.org/10.3390/s21010078
Bao X, Abdala AK, Kamavuako EN. Estimation of the Respiratory Rate from Localised ECG at Different Auscultation Sites. Sensors. 2021; 21(1):78. https://doi.org/10.3390/s21010078
Chicago/Turabian StyleBao, Xinqi, Aimé Kingwengwe Abdala, and Ernest Nlandu Kamavuako. 2021. "Estimation of the Respiratory Rate from Localised ECG at Different Auscultation Sites" Sensors 21, no. 1: 78. https://doi.org/10.3390/s21010078
APA StyleBao, X., Abdala, A. K., & Kamavuako, E. N. (2021). Estimation of the Respiratory Rate from Localised ECG at Different Auscultation Sites. Sensors, 21(1), 78. https://doi.org/10.3390/s21010078






