Novel Stable Capacitive Electrocardiogram Measurement System
Abstract
1. Introduction
2. Materials and Methods
2.1. Active Electrode Design
2.1.1. High-Input Impedance Amplifier
2.1.2. Input Resistor
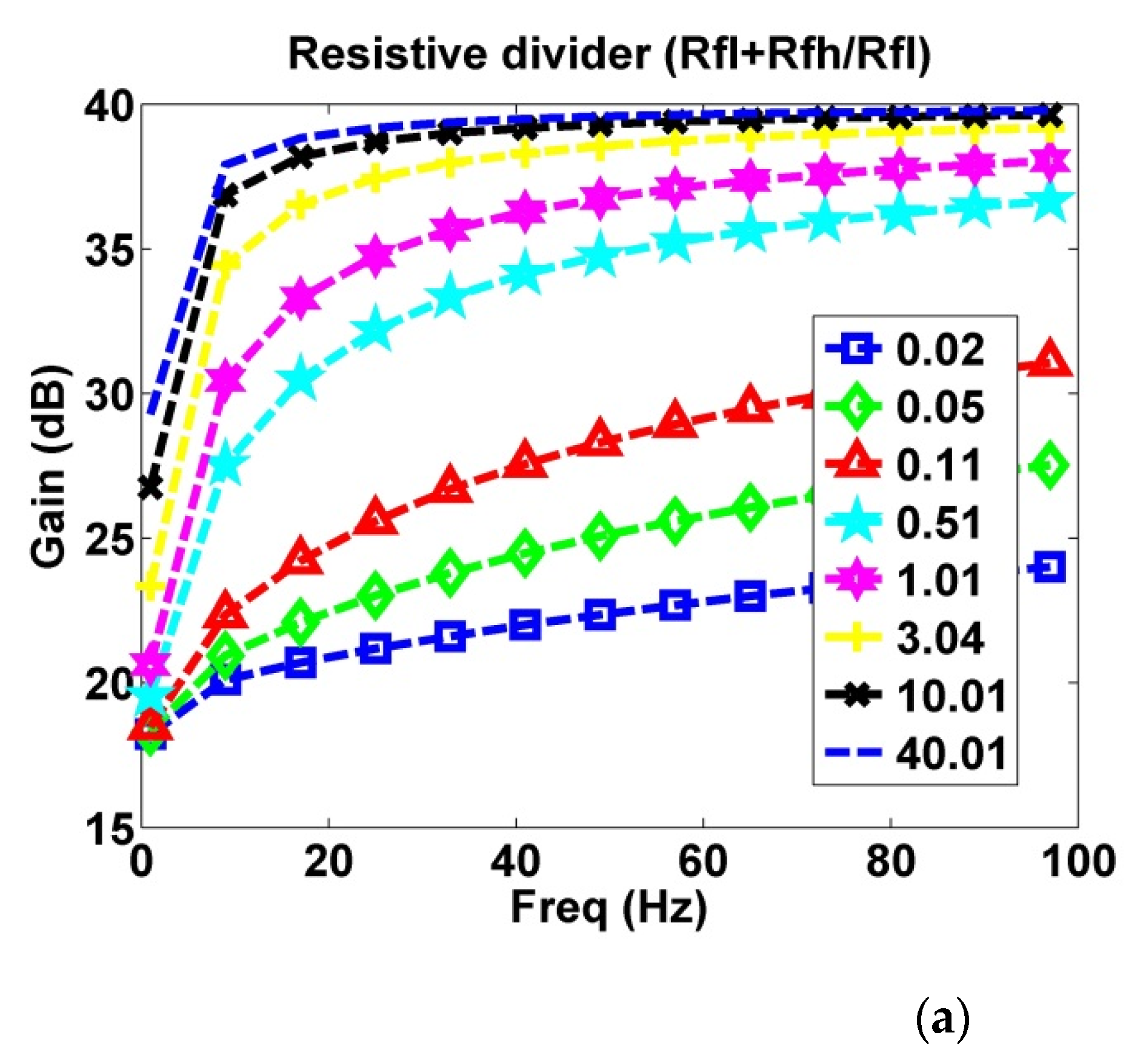
2.1.3. Output Divider Feedback
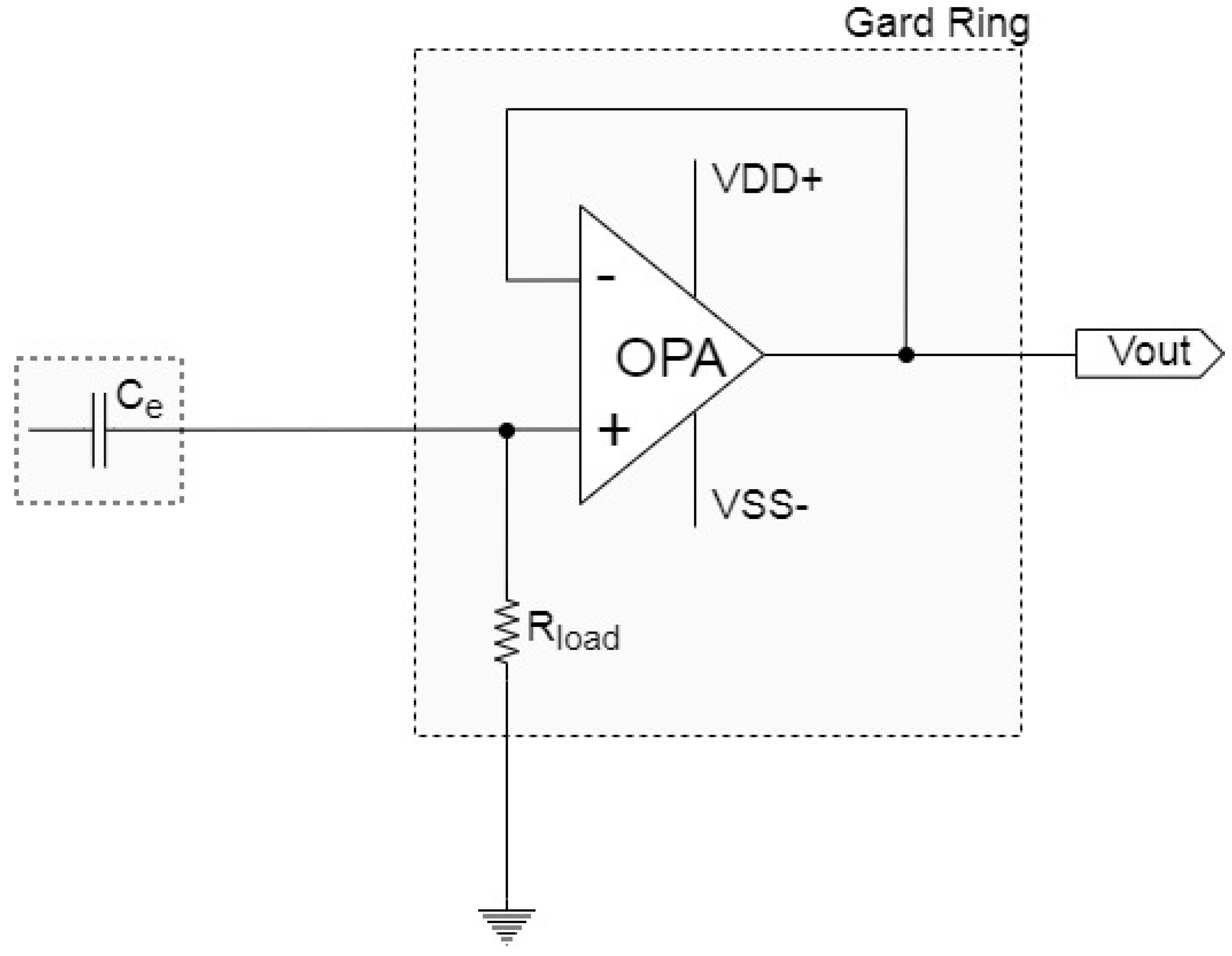
2.1.4. Ground Guard Ring
2.2. Constructing the CECG Measurement System
2.2.1. CDRL
2.2.2. Signal Processing Circuit
2.2.3. Data Acquisition
2.3. Experiments
2.3.1. Simulated Testing Experiment
- (1)
- Simulated Testing System Development
- (2)
- Interference Simulation Experiment
2.3.2. ECG Measurement Experiment
- (1)
- ECG Measurement System Development
- (2)
- ECG Measurement Experiment Interference Methods
3. Results
3.1. Simulated Measurement Results
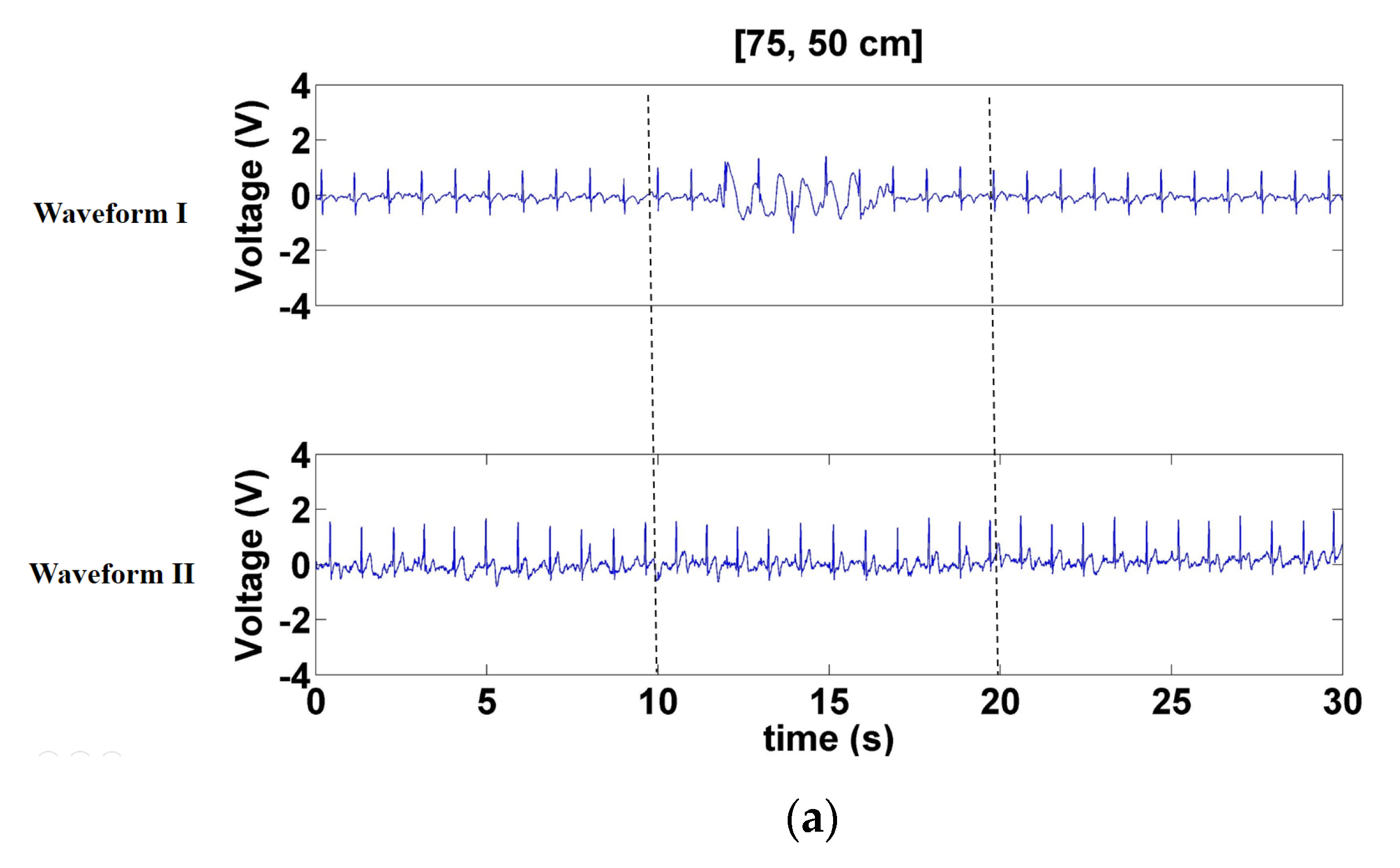
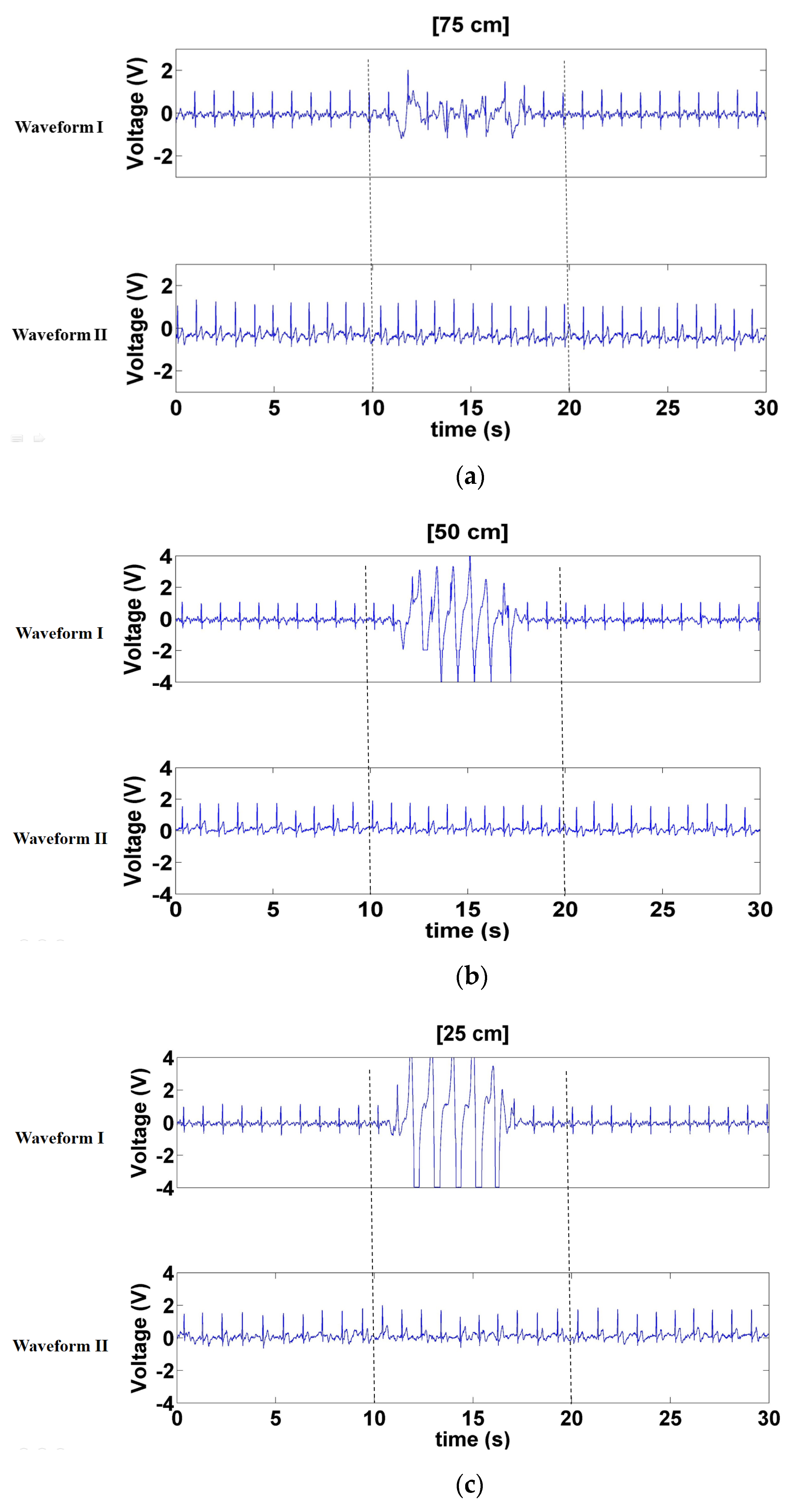
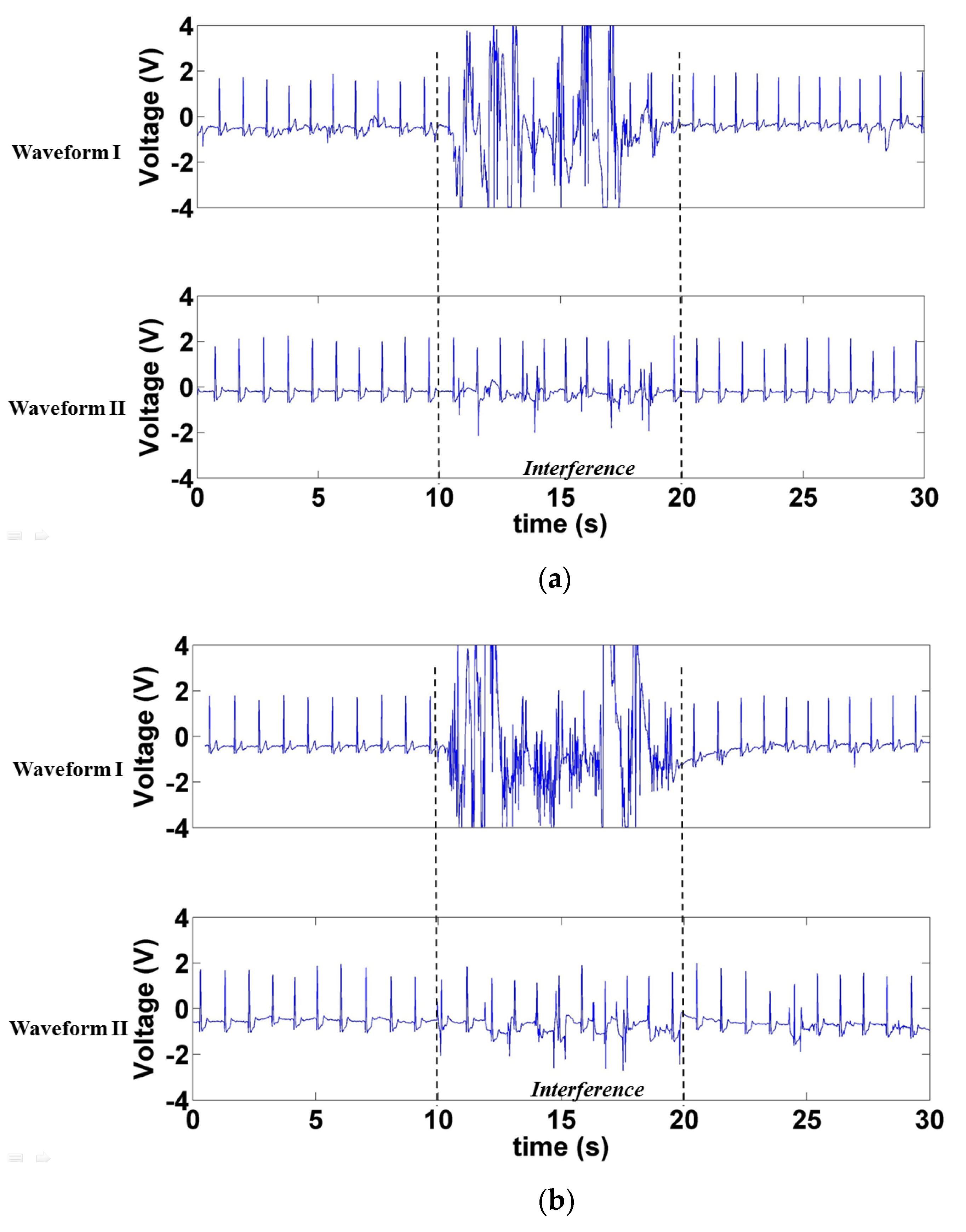
3.1.1. Signals Measured when Forward- and Backward-Moving Interference Was Positioned at the Front of the Electrode
3.1.2. Signals Measured When Left- and Right-Moving Interference Was Positioned at the Front of the Electrode
3.1.3. Signals Measured When Left- and Right-Moving Interference Was at the Sides of the Electrode
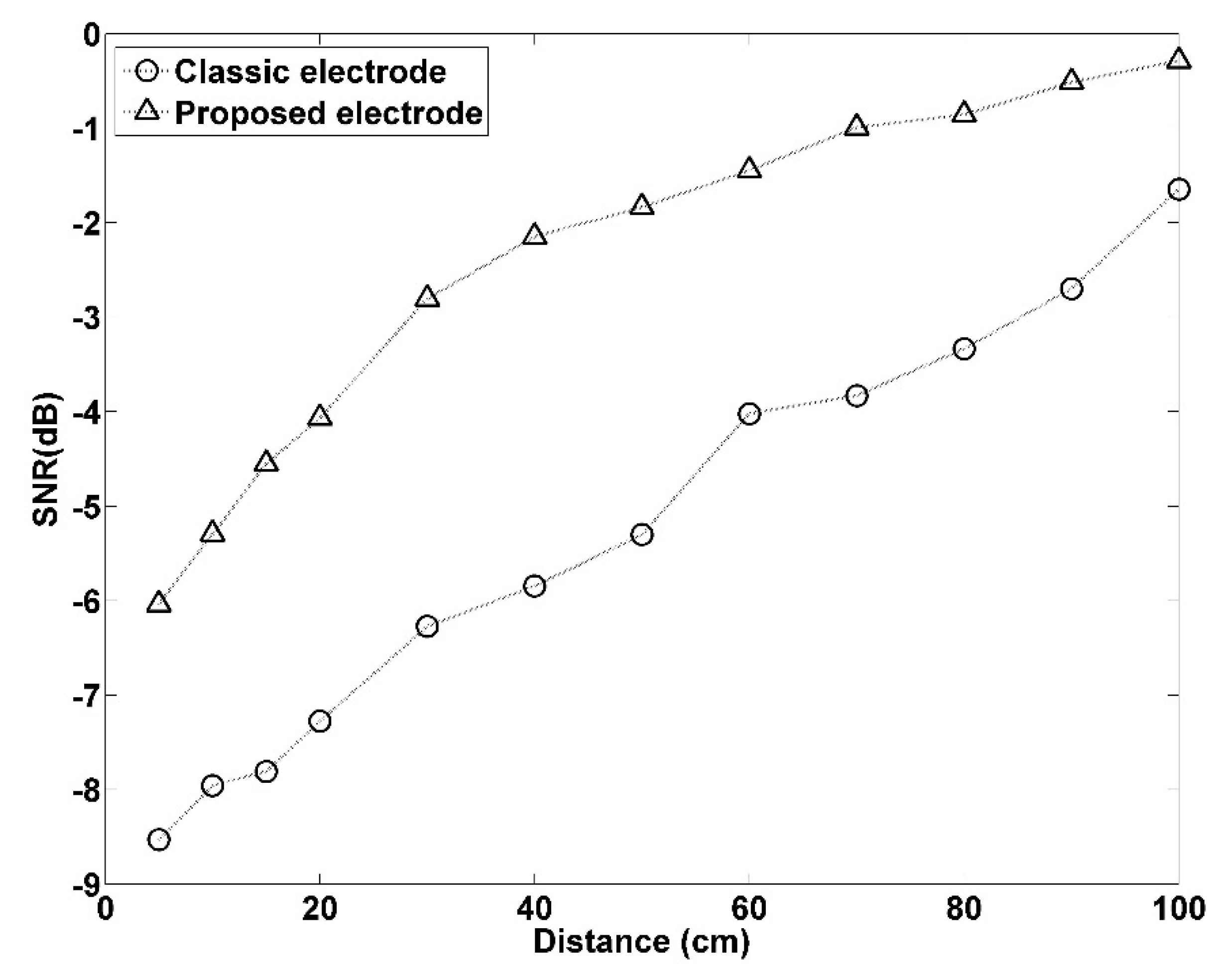
3.1.4. SNR under Interference at Different Distances
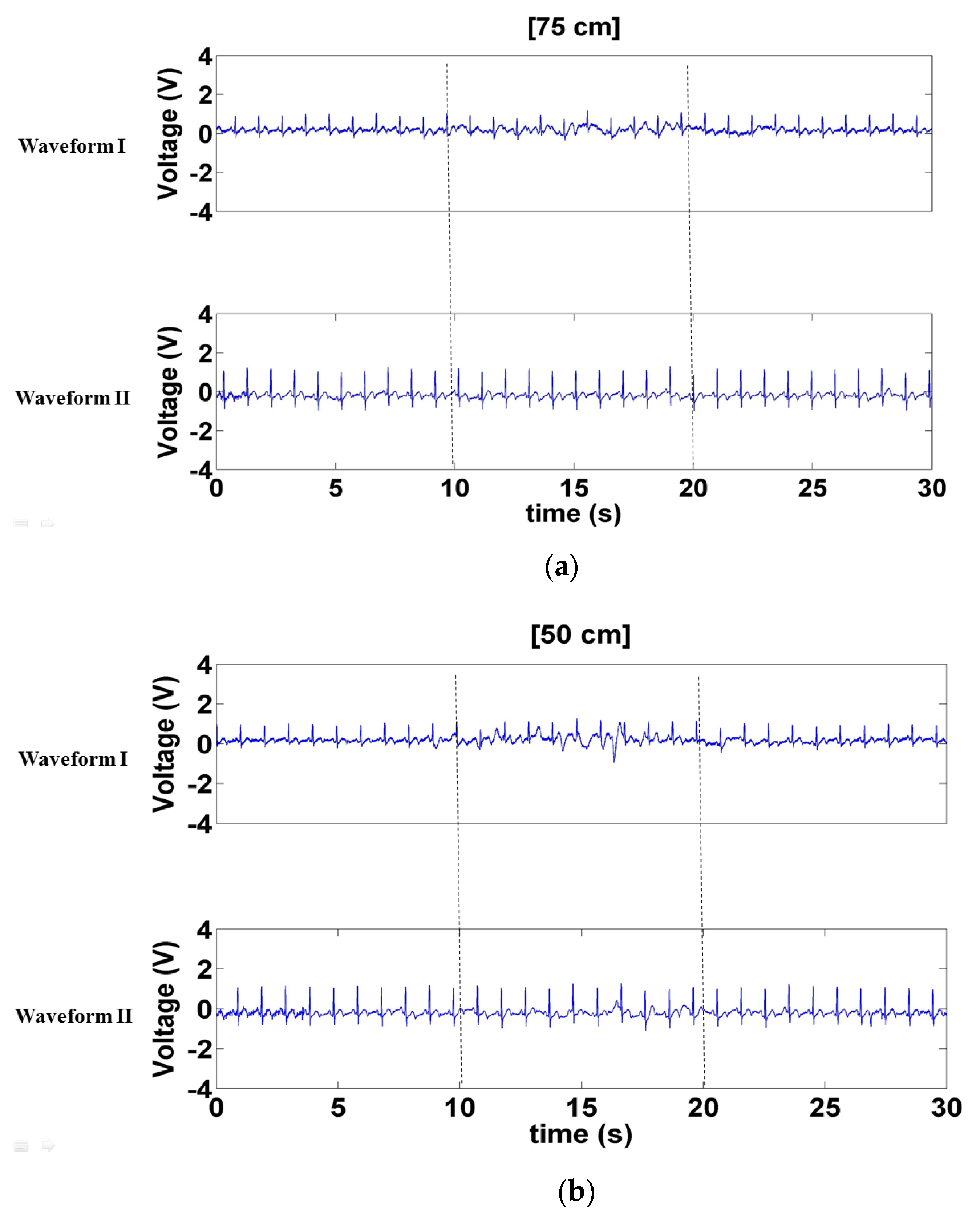
3.2. Human Measurement Results
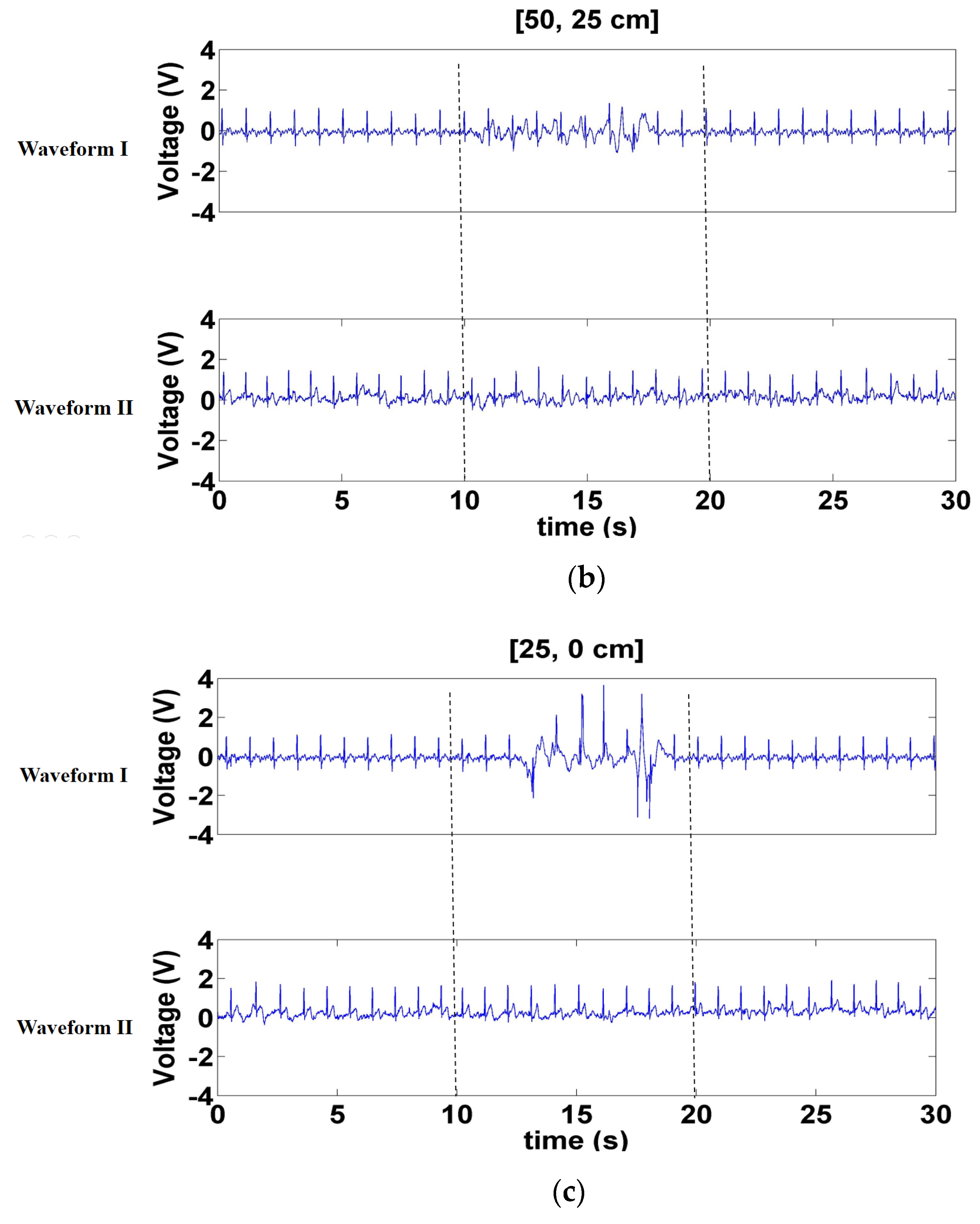
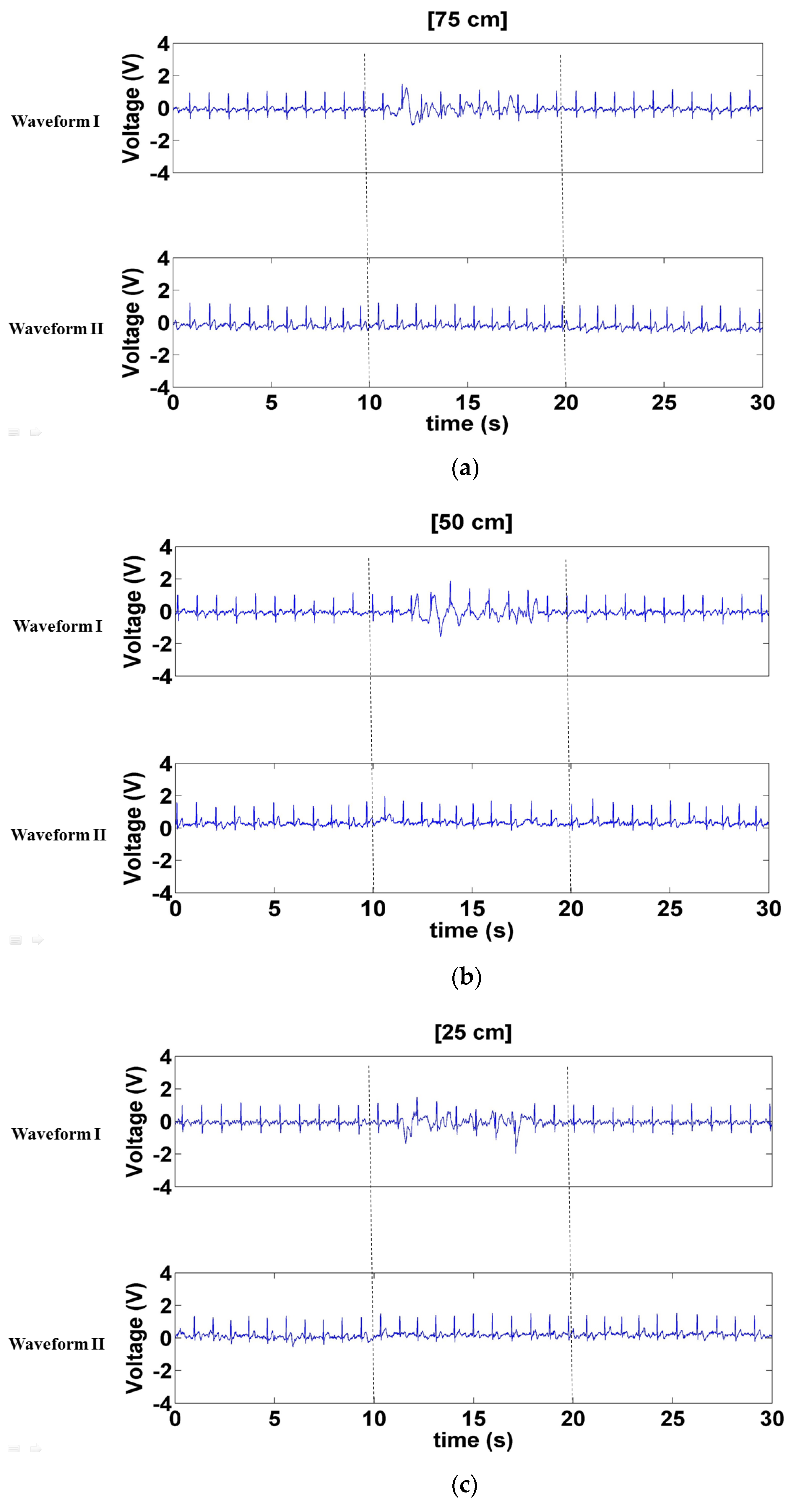
3.2.1. Signals Measured with a Forward- and Backward-Moving Interferer in Front of the Participant
3.2.2. Signals Measured with a Left- and Right-Moving Interferer in Front of the Participant
3.2.3. Signals Measured when a Left- and Right-Moving Interferer Was at the Sides of the Participant
3.2.4. Signals Measured When Interference Was Caused by the Participant’s Body Movement
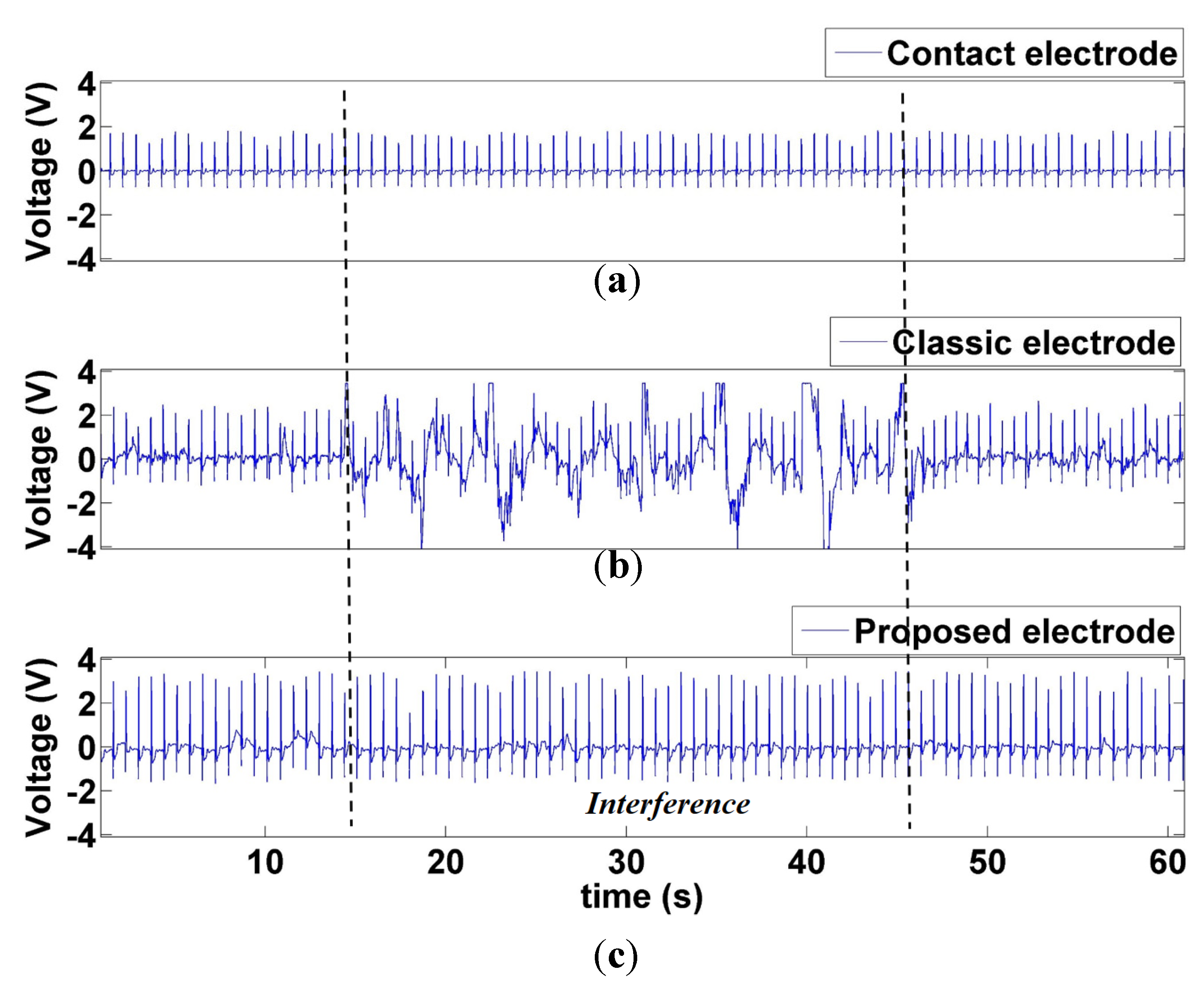
3.2.5. Comparison between ECG Signals Measured by Contact and Noncontact Electrodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, A.; Rehman, S.U.; Tu, S.S.; Mehmood, R.M.; Fawad; Ehatisham-ul-haq, M. A Hybrid Deep CNN Model for Abnormal Arrhythmia Detection Based on Cardiac ECG Signal. Sensors 2021, 21, 951. [Google Scholar] [CrossRef] [PubMed]
- Mathunjwa, B.M.; Lin, Y.T.; Lin, C.H.; Abbod, M.F.; Shieh, J.S. ECG arrhythmia classification by using a recurrence plot and convolutional neural network. Biomed. Signal Process. Control 2021, 64, 102262. [Google Scholar] [CrossRef]
- Adam, M.; Oh, S.L.; Sudarshan, V.K.; Koh, J.E.W.; Hagiwara, Y.; Tan, J.H.; Tan, R.S.; Acharya, U.R. Automated characterization of cardiovascular diseases using relative wavelet nonlinear features extracted from ECG signals. Comput. Methods Progr. Biomed. 2018, 161, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.Q.; Wang, C.; Tang, M.; Zheng, T.J. Extracting cardiac dynamics within ECG signal for human identification and cardiovascular diseases classification. Neural Netw. 2018, 100, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Bhaumik, B. An Energy Efficient ECG Signal Processor Detecting Cardiovascular Diseases on Smartphone. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 314–323. [Google Scholar] [CrossRef]
- Randazzo, V.; Ferretti, J.; Pasero, E. A Wearable Smart Device to Monitor Multiple Vital Parameters-VITAL ECG. Electronics 2020, 9, 300. [Google Scholar] [CrossRef]
- Choi, G.H.; Ko, H.; Pedrycz, W.; Singh, A.K.; Pan, S.B. Recognition System Using Fusion Normalization Based on Morphological Features of Post-Exercise ECG for Intelligent Biometrics. Sensors 2020, 20, 7130. [Google Scholar] [CrossRef]
- Abtahi, K.; Mastella, L.; Ryan, A. Evaluation of ECG Abnormalities in Obesity: Effects of Weight Loss and Aerobic Exercise Training. Obesity 2020, 28, 94. [Google Scholar]
- Wang, L.H.; Li, J.W.; Wang, Y.H. Modeling and Recognition of Driving Fatigue State Based on R-R Intervals of ECG Data. IEEE Access 2019, 7, 175584–175593. [Google Scholar] [CrossRef]
- Huang, S.T.; Li, J.; Zhang, P.Z.; Zhang, W.Q. Detection of mental fatigue state with wearable ECG devices. Int. J. Med. Informat. 2018, 119, 39–46. [Google Scholar] [CrossRef]
- Seo, W.; Kim, N.; Kim, S.; Lee, C.; Park, S.M. Deep ECG-Respiration Network (DeepER Net) for Recognizing Mental Stress. Sensors 2019, 19, 3021. [Google Scholar] [CrossRef]
- Chiang, H.S. ECG-based Mental Stress Assessment Using Fuzzy Computing and Associative Petri Net. J. Med. Biol. Eng. 2015, 35, 833–844. [Google Scholar] [CrossRef]
- Gao, Y.; Soman, V.V.; Lombardi, J.P.; Rajbhandari, P.P.; Dhakal, T.P.; Wilson, D.G.; Poliks, M.D.; Ghose, K.; Turner, J.N.; Jin, Z.P. Heart Monitor Using Flexible Capacitive ECG Electrodes. IEEE Trans. Instrum. Meas. 2020, 69, 4314–4323. [Google Scholar] [CrossRef]
- Kido, K.; Tamura, T.; Ono, N.; Altaf-Ul-Amin, M.; Sekine, M.; Kanaya, S.; Huang, M. A Novel CNN-Based Framework for Classification of Signal Quality and Sleep Position from a Capacitive ECG Measurement. Sensors 2019, 19, 1731. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chang, R.H.; Zhang, L.M.; Yan, F.; Ma, H.W.; Bu, X.F. Electrode Humidification Design for Artifact Reduction in Capacitive ECG Measurements. Sensors 2020, 20, 3449. [Google Scholar] [CrossRef] [PubMed]
- Uguz, D.U.; Tufan, T.B.; Uzun, A.; Leonhardt, S.; Antink, C.H. Physiological Motion Artifacts in Capacitive ECG: Ballistocardiographic Impedance Distortions. IEEE Trans. Instrum. Meas. 2020, 69, 3297–3307. [Google Scholar] [CrossRef]
- Wang, T.W.; Zhang, H.; Lin, S.F. Influence of Capacitive Coupling on High-Fidelity Non-Contact ECG Measurement. IEEE Sens. J. 2020, 20, 9265–9273. [Google Scholar] [CrossRef]
- Seo, M.; Choi, M.; Lee, J.S.; Kim, S.W. Adaptive Noise Reduction Algorithm to Improve R Peak Detection in ECG Measured by Capacitive ECG Sensors. Sensors 2018, 18, 2086. [Google Scholar] [CrossRef]
- Wannenburg, J.; Malekian, R.; Hancke, G.P. Wireless Capacitive-Based ECG Sensing for Feature Extraction and Mobile Health Monitoring. IEEE Sens. J. 2018, 18, 6023–6032. [Google Scholar] [CrossRef]
- Richardson, P. The insulated electrode: A pasteless electrocardiographic technique. In Proceedings of the 20th Annual Conference on Engineering in Medicine and Biology, Boston, MA, USA, 5–6 September 1967; pp. 15–17. [Google Scholar]
- David, R.M.; Portnoy, W.M. Insulated electrocardiogram electrodes. Med. Biol. Eng. 1972, 10, 742–751. [Google Scholar] [CrossRef]
- Lagow, C.H.; Sladek, K.J.; Richardson, P.C. Anodic insulated tantalum oxide electrocardiograph electrodes. IEEE Trans. Biomed. Eng. 1971, 18, 162–164. [Google Scholar] [CrossRef]
- Lopez, A.; Richardson, P.C. Capacitive Electrocardiographic and Bioelectric Electrodes. IEEE Trans. Biomed. Eng. 1969, BME-16, 99. [Google Scholar] [CrossRef]
- Prance, R.J.; Debray, A.; Clark, T.D.; Prance, H.; Nock, M.; Harland, C.J.; Clippingdale, A.J. An ultra-low-noise electrical-potential probe for human-body scanning. Meas. Sci. Technol. 2000, 11, 291–297. [Google Scholar] [CrossRef]
- Harland, C.J.; Clark, T.D.; Prance, R.J. Electric potential probes—New directions in the remote sensing of the human body. Meas. Sci. Technol. 2001, 13, 163–169. [Google Scholar] [CrossRef]
- Oehler, M.; Ling, V.; Melhorn, K.; Schilling, M. A multichannel portable ECG system with capacitive sensors. Physiol. Meas. 2008, 29, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.G.; Kim, K.K.; Park, K.S. ECC recording on a bed during steep without direct skin-contact. IEEE Trans. Biomed. Eng. 2007, 54, 718–725. [Google Scholar] [CrossRef]
- Baek, H.J.; Chung, G.S.; Kim, K.K.; Park, K.S. A Smart Health Monitoring Chair for Nonintrusive Measurement of Biological Signals. IEEE Trans. Informat. Technol. Biomed. 2012, 16, 150–158. [Google Scholar] [CrossRef]
- Nemati, E.; Deen, M.J.; Mondal, T. A Wireless Wearable ECG Sensor for Long-Term Applications. IEEE Commun. Mag. 2012, 50, 36–43. [Google Scholar] [CrossRef]
- Uguz, D.U.; Dettori, R.; Napp, A.; Walter, M.; Marx, N.; Leonhardt, S.; Antink, C.H. Car Seats with Capacitive ECG Electrodes Can Detect Cardiac Pacemaker Spikes. Sensors 2020, 20, 6288. [Google Scholar] [CrossRef]
- Liu, S.H.; Hsieh, C.H.; Chen, W.X.; Tan, T.H. ECG Noise Cancellation Based on Grey Spectral Noise Estimation. Sensors 2019, 19, 798. [Google Scholar] [CrossRef]
- Xu, X.W.; Liang, Y.; He, P.; Yang, J.L. Adaptive Motion Artifact Reduction Based on Empirical Wavelet Transform and Wavelet Thresholding for the Non-Contact ECG Monitoring Systems. Sensors 2019, 19, 2916. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, C.W.; Hsieh, C.W. Noise-Resistant CECG Using Novel Capacitive Electrodes. Sensors 2020, 20, 2577. [Google Scholar] [CrossRef]
- Lee, J.S.; Heo, J.; Lee, W.K.; Lim, Y.G.; Kim, Y.H.; Park, K.S. Flexible Capacitive Electrodes for Minimizing Motion Artifacts in Ambulatory Electrocardiograms. Sensors 2014, 14, 14732–14743. [Google Scholar] [CrossRef] [PubMed]
- Serteyn, A.; Vullings, R.; Meftah, M.; Bergmans, J.W.M. Motion Artifacts in Capacitive ECG Measurements: Reducing the Combined Effect of DC Voltages and Capacitance Changes Using an Injection Signal. IEEE Trans. Biomed. Eng. 2015, 62, 264–273. [Google Scholar] [CrossRef]
- Eilebrecht, B.; Wartzek, T.; Willkomm, J.; Schommartz, A.; Walter, M.; Leonhardt, S. Motion Artifact Removal from Capacitive ECG Measurements by Means of Adaptive Filtering. In Proceedings of the 5th European Conference of the International Federation for Medical and Biological Engineering, Budapest, Hungary, 14–18 September 2011; pp. 902–905. [Google Scholar]
- Guermandi, M.; Scarselli, E.F.; Guerrieri, R. A Driving Right Leg Circuit (DgRL) for Improved Common Mode Rejection in Bio-Potential Acquisition Systems. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Haberman, M.A.; Spinelli, E.M.; Garcia, P.A.; Guerrero, F.N. Capacitive driven-right-leg circuit design. Int. J. Biomed. Eng. Technol. 2015, 17, 115–126. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Balasubramanian, V. Viability of Cardiac Parameters Measured Unobtrusively Using Capacitive Coupled Electrocardiography (cECG) to Estimate Driver Performance. IEEE Sens. J. 2019, 19, 4321–4330. [Google Scholar] [CrossRef]
- Sakuma, J.; Anzai, D.; Wang, J.Q. Performance of human body communication-based wearable ECG with capacitive coupling electrodes. Healthc. Technol. Lett. 2016, 3, 222–225. [Google Scholar] [CrossRef] [PubMed]
- IEC 60601-2-47: Medical Electrical Equipment—Part 2–47: Particular Requirements for the Basic Safety and Essential Performance of Ambulatory Electrocardiographic Systems; IEC: Geneva, Switzerland, 2012; pp. 1–140.











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Lin, S.-Y.; Chang, W.-Y. Novel Stable Capacitive Electrocardiogram Measurement System. Sensors 2021, 21, 3668. https://doi.org/10.3390/s21113668
Chen C-C, Lin S-Y, Chang W-Y. Novel Stable Capacitive Electrocardiogram Measurement System. Sensors. 2021; 21(11):3668. https://doi.org/10.3390/s21113668
Chicago/Turabian StyleChen, Chi-Chun, Shu-Yu Lin, and Wen-Ying Chang. 2021. "Novel Stable Capacitive Electrocardiogram Measurement System" Sensors 21, no. 11: 3668. https://doi.org/10.3390/s21113668
APA StyleChen, C.-C., Lin, S.-Y., & Chang, W.-Y. (2021). Novel Stable Capacitive Electrocardiogram Measurement System. Sensors, 21(11), 3668. https://doi.org/10.3390/s21113668





