Analysis of Trace Metals in Human Hair by Laser-Induced Breakdown Spectroscopy with a Compact Microchip Laser
Abstract
:1. Introduction
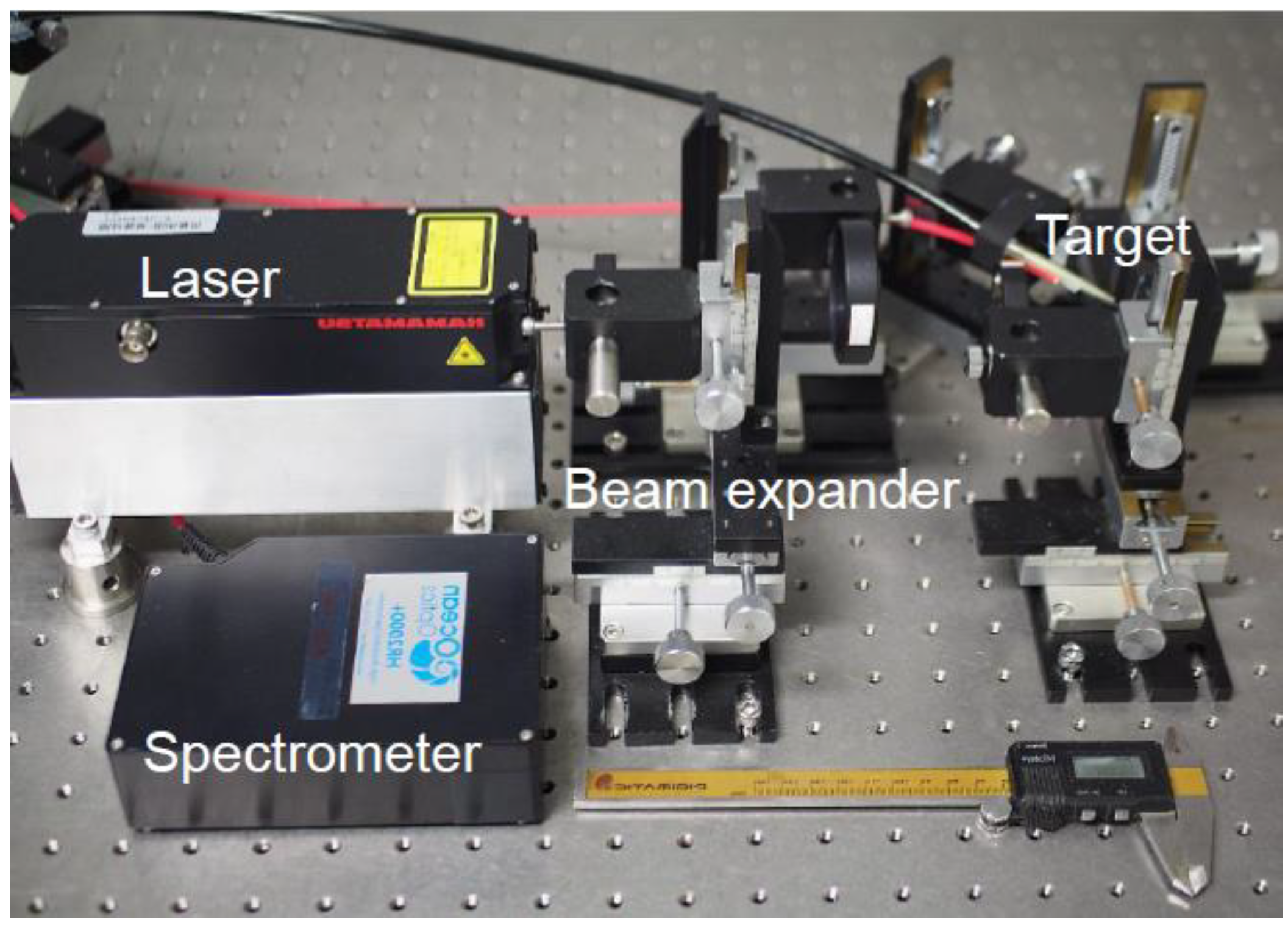
2. Materials and Methods
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons: Wes Sussex, UK, 2006. [Google Scholar] [CrossRef]
- Musazzi, S.; Perini, U. Laser-Induced Breakdown Spectroscopy: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Zayhowski, J.J. Passively Q-switched Nd: YAG microchip lasers and applications. J. Alloy. Compd. 2000, 46, 393–400. [Google Scholar] [CrossRef]
- Sakai, H.; Kan, H.; Taira, T. 1 MW peak power single-mode high-brightness passively Q-switched Nd3+: YAG microchip laser. Opt. Express 2008, 16, 19891–19899. [Google Scholar] [CrossRef]
- Merten, J.A.; Ewusi-Annan, E.; Smith, B.W.; Omenetto, N. Optimizing gated detection in high-jitter kilohertz powerchip laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2014, 29, 571–577. [Google Scholar] [CrossRef]
- Merten, J.A.; Smith, B.W.; Omenetto, N. Local thermodynamic equilibrium considerations in powerchip laser-induced plasmas. Spectrochim. Acta Part B At. Spectrosc. 2013, 83–84, 50–55. [Google Scholar] [CrossRef]
- Singh, J.P.; Thakur, S.N. Laser-Induced Breakdown Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2007; pp. 173–196. [Google Scholar] [CrossRef]
- Amponsah-Manager, K.; Omenetto, N.; Smith, B.W.; Gornushkin, I.B.; Winefordner, J.D. Microchip laser ablation of metals: Investigation of the ablation process in view of its application to laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2005, 20, 544–551. [Google Scholar] [CrossRef]
- Gornushkin, I.B.; Amponsah-Manager, K.; Smith, B.W.; Omenetto, N.; Winefordner, J.D. Microchip laser-induced breakdown spectroscopy: A preliminary feasibility investigation. Appl. Spectrosc. 2004, 58, 762–769. [Google Scholar] [CrossRef]
- Lopez-Moreno, C.; Amponsah-Manager, K.; Smith, B.W.; Gornushkin, I.B.; Omenetto, N.; Palanco, S.; Lasernab, J.J.; Winefordner, J.D. Quantitative analysis of low-alloy steel by microchip laser induced breakdown spectroscopy. J. Anal. At. Spectrom. 2005, 20, 552–556. [Google Scholar] [CrossRef]
- Gonzaga, F.B.; Pasquini, C. A compact and low cost laser induced breakdown spectroscopic system: Application for simultaneous determination of chromium and nickel in steel using multivariate calibration. Spectrochim. Acta Pt. B At. Spec. 2012, 69, 20–24. [Google Scholar] [CrossRef]
- Freedman, A.; Iannarilli, F.J.; Wormhoudt, J.C. Aluminum alloy analysis using microchip-laser induced breakdown spectroscopy. Spectrochim. Acta Pt. B At. Spec. 2005, 60, 1076–1082. [Google Scholar] [CrossRef]
- Cristoforetti, G.; Legnaioli, S.; Palleschi, V.; Salvetti, A.; Tognoni, E.; Benedetti, P.A.; Brioschi, F.; Ferrario, F. Quantitative analysis of aluminium alloys by low-energy, high-repetition rate laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2006, 21, 697–702. [Google Scholar] [CrossRef]
- Gaudiuso, R.; Melikechi, N.; Abdel-Salam, Z.A.; Harith, M.A.; Palleschi, V.; Motto-Ros, V.; Busser, B. Laser-induced breakdown spectroscopy for human and animal health: A review. Spectrochim. Acta Pt. B At. Spec. 2019, 152, 123–148. [Google Scholar] [CrossRef]
- Thompson, M.; Walsh, J.N. Handbook of Inductively Coupled Plasma Spectrometry, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar] [CrossRef]
- Vanhoe, H. A review of the capabilities of ICP-MS for trace element analysis in body fluids and tissues. J. Trace Elem. Electrol. Health Dis. 1993, 7, 131–139. Available online: http://europepmc.org/abstract/MED/8155984 (accessed on 9 December 2020).
- Bahreini, M.; Hosseinimakarem, Z.; Tavassoli, S.H. A study of association between fingernail elements and osteoporosis by laser-induced breakdown spectroscopy. J. Appl. Phys. 2012, 112, 054701. [Google Scholar] [CrossRef]
- Hosseinimakarem, Z.; Tavassoli, S.H. Analysis of human nails by laser-induced breakdown spectroscopy. J. Biomed. Opt. 2011, 16, 057002. [Google Scholar] [CrossRef]
- Hamzaoui, S.; Khleifia, R.; Jaïdane, N.; Lakhdar, Z.B. Quantitative analysis of pathological nails using laser-induced breakdown spectroscopy (LIBS) technique. Lasers Med. Sci. 2011, 26, 79–83. [Google Scholar] [CrossRef]
- Bahreini, M.; Ashrafkhani, B.; Tavassoli, S.H. Discrimination of patients with diabetes mellitus and healthy subjects based on laser-induced breakdown spectroscopy of their fingernails. J. Biomed. Opt. 2013, 18, 107006. [Google Scholar] [CrossRef]
- Haruna, M.; Ohmi, M.; Nakamura, M.; Morimoto, S. Calcium detection of human hair and nail by the nanosecond time-gated spectroscopy of laser-ablation plume. In SPIE 3917, Optical Biopsy II, Proceedings of the BiOS 2000 The International Symposium on Biomedical Optics, San Jose, CA, USA, 22 January 2000; SPIE Press: Bellingham, DC, USA; pp. 87–92. [CrossRef]
- Corsi, M.; Cristoforetti, G.; Hidalgo, M.; Legnaioli, S.; Palleschi, V.; Salvetti, A.; Tognoni, E.; Vallebona, C. Application of laser-induced breakdown spectroscopy technique to hair tissue mineral analysis. Appl. Opt. 2003, 42, 6133–6137. [Google Scholar] [CrossRef]
- Emara, E.M.; Imam, H.; Hassan, M.A.; Elnaby, S.H. Biological application of laser induced breakdown spectroscopy technique for determination of trace elements in hair. Talanta 2013, 117, 176–183. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, Y.; Ma, S.; Chen, F.; Zhang, D.; Hu, Z.; Zhang, Z.; Jin, H.; Guo, L. Highly accurate determination of Zn and Cu in human hair by ultrasound-assisted alkali dissolution combined with laser-induced breakdown spectroscopy. Microchem. J. 2020, 157, 105018. [Google Scholar] [CrossRef]
- Araki, S.; Hirai, S.; Mitou, A. Quantification of elements in hair standard data by neutron activation analysis. Bunseki Kagaku (Anal. Chem.) 1994, 43, 845–850. (In Japanese) [Google Scholar]
- NIST: Atomic Spectra Database Lines Form. Available online: https://physics.nist.gov/PhysRefData/ASD/LIBS/libs-form.html (accessed on 15 December 2020).
- Delaware State University. LIBS Database–Optical Science Center for Applied Research. Available online: https://oscar.desu.edu/libs/ (accessed on 18 January 2021).
- Farid, N.; Bashir, S.; Mahmood, K. Effect of ambient gas conditions on laser-induced copper plasma and surface morphology. Phys. Scr. 2012, 85, 015702. [Google Scholar] [CrossRef]
- Kyorin Preventive Medicine Institute, Hair Mineral Analysis. Available online: https://kyorin-yobou.net/hair_analysis/ (accessed on 12 December 2020). (In Japanese).
- Lazic, V.; Fantoni, R.; Colao, F.; Santagata, A.; Morone, A.; Spizzichino, V. Quantitative laser induced breakdown spectroscopy analysis of ancient marbles and corrections for the variability of plasma parameters and of ablation rate. J. Anal. At. Spectrom. 2004, 19, 429–436. [Google Scholar] [CrossRef]









| Element | Ca | Zn | Mg | Fe | Al | K | Na |
|---|---|---|---|---|---|---|---|
| Concentration (ppm) | 810 | 179 | 164 | 144 | 130 | 70 | 65 |
| Subject | 1 | 2 | #3 | 4 | 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element X | Mg | Zn | Ca | Mg | Zn | Ca | Mg | Zn | Ca | Mg | Zn | Ca | Mg | Zn | Ca | |
| CV | X | 0.126 | 0.350 | 0.134 | 0.159 | 0386 | 0.209 | 0.249 | 0.573 | 0.285 | 0.034 | 0.053 | 0.141 | 0.107 | 0.151 | 0.157 |
| X/C | 0.043 | 0.189 | 0.037 | 0.102 | 0.212 | 0.046 | 0.152 | 0.505 | 0.055 | 0.044 | 0.022 | 0.082 | 0.023 | 0.170 | 0.073 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, M.; Matsuura, Y. Analysis of Trace Metals in Human Hair by Laser-Induced Breakdown Spectroscopy with a Compact Microchip Laser. Sensors 2021, 21, 3752. https://doi.org/10.3390/s21113752
Nakagawa M, Matsuura Y. Analysis of Trace Metals in Human Hair by Laser-Induced Breakdown Spectroscopy with a Compact Microchip Laser. Sensors. 2021; 21(11):3752. https://doi.org/10.3390/s21113752
Chicago/Turabian StyleNakagawa, Makoto, and Yuji Matsuura. 2021. "Analysis of Trace Metals in Human Hair by Laser-Induced Breakdown Spectroscopy with a Compact Microchip Laser" Sensors 21, no. 11: 3752. https://doi.org/10.3390/s21113752
APA StyleNakagawa, M., & Matsuura, Y. (2021). Analysis of Trace Metals in Human Hair by Laser-Induced Breakdown Spectroscopy with a Compact Microchip Laser. Sensors, 21(11), 3752. https://doi.org/10.3390/s21113752






