Recent Advances on Detection of Insecticides Using Optical Sensors
Abstract
:1. Introduction
2. Optical Sensors
2.1. Fluorescence
2.2. Colorimetric
2.3. Surface Enhanced Raman Scattering
2.4. Surface Plasmon Resonance
2.5. Chemiluminescence
2.6. Others
3. Recognition Elements
3.1. Enzymes
3.2. Antibodies
3.3. Aptamers
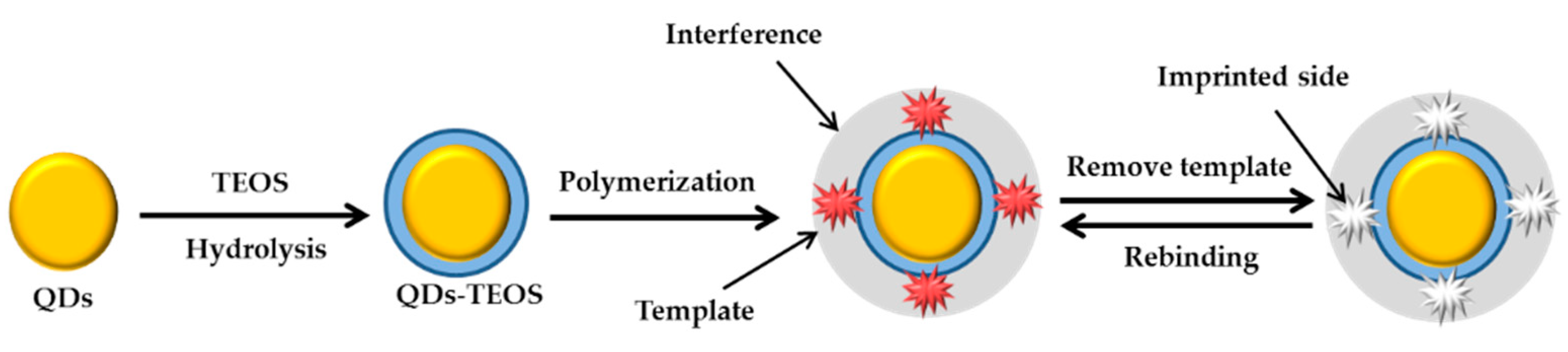
3.4. Molecularly-Imprinted Polymers
3.5. Others
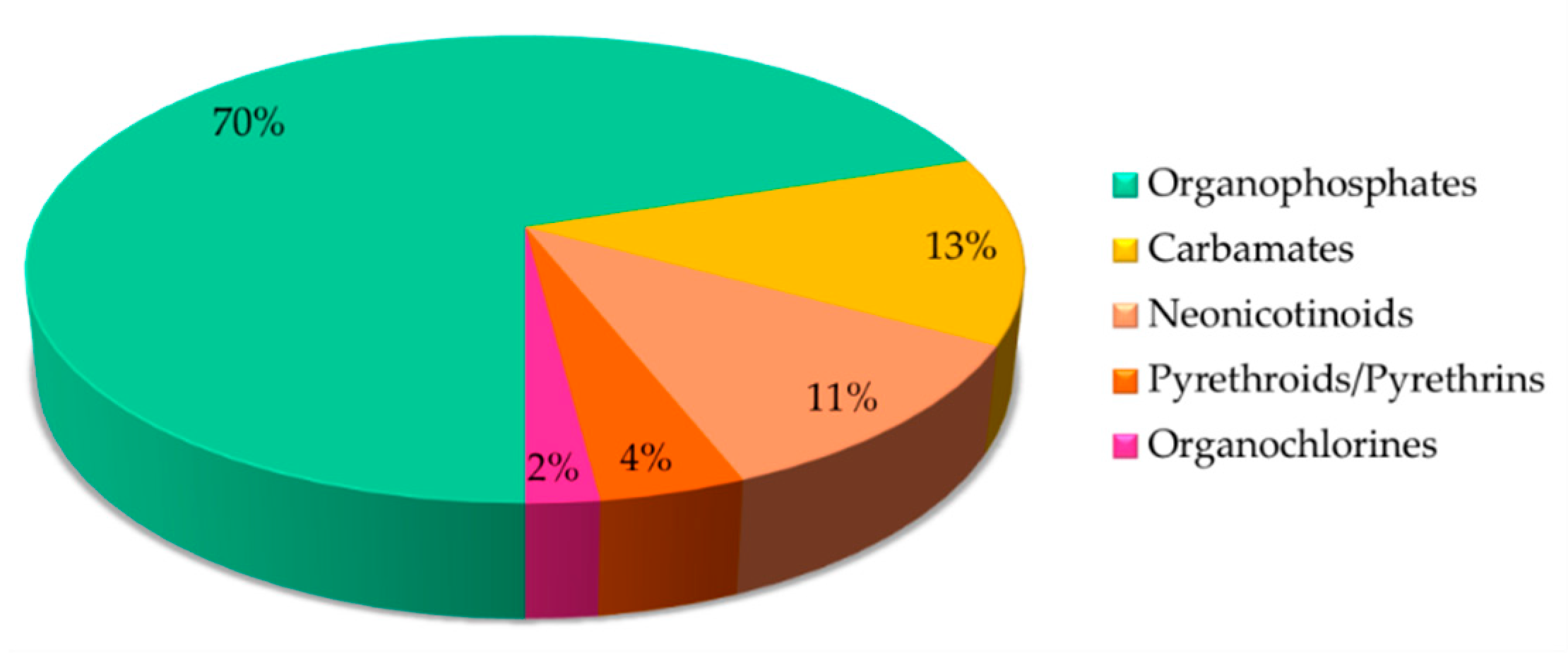
4. Various Classes of Insecticides Detection by Optical Sensors
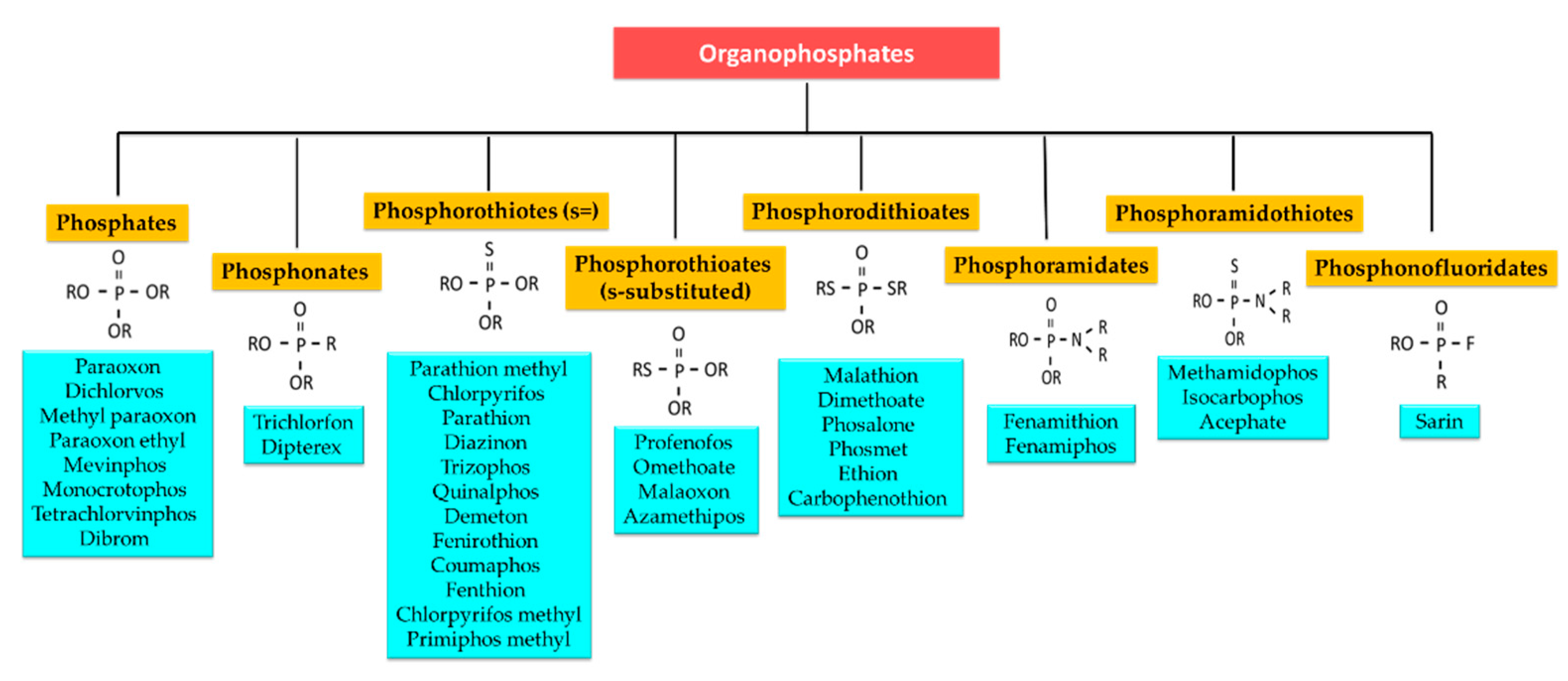
4.1. Organophosphates
4.1.1. Phosphates
4.1.2. Phosphonates
4.1.3. Phosphorothiotes (s =)
4.1.4. Phosphorothioates (S-Substituted)
4.1.5. Phosphorodithioates
4.1.6. Phosphoramidates
4.1.7. Phosphoramidothioates
4.1.8. Phosphonofluoridates
4.2. Carbamates
4.3. Neonicotinoids
4.4. Pyrethroids/Pyrethrins
4.5. Organochlorines
5. Analysis and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernieri, T.; Rodrigues, D.; Barbosa, I.R.; Ardenghi, G.P.; Silva, L.B. Occupational exposure to pesticides and thyroid function in Brazilian soybean farmers. Chemosphere 2019, 218, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Horsak, R.D.; Bedient, P.B.; Hamilton, M.C.; Thomas, F.B. Environmental Forensics: Contaminant Specific Guide, 1st ed.; Elsevier: Cambridge, MA, USA, 2010; pp. 144–163. [Google Scholar]
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides classification and its impact on environment. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Dalmolin, S.P.; Dreon, D.B.; Thiesen, F.V.; Dallegrave, E. Biomarkers of occupational exposure to pesticides: Systematic review of insecticides. Environ. Toxicol. Pharmacol. 2019, 75, 103304. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Mukherjee, I.R.M.; Malik, J.K.; Doss, R.B.; Dettbarn, W.; Milatovic, D. Chapter 26- Insecticides. In Biomarkers Toxicology, 2nd ed.; Elsevier: Cambridge, MA, USA, 2019; pp. 455–475. [Google Scholar]
- Soares, S.; Rosado, T.; Barroso, M.; Vieira, D.N.; Gallardo, E. Organophosphorus pesticide determination in biological specimens: Bioanalytical and toxicological aspects. Int. J. Legal Med. 2019, 133, 1763–1784. [Google Scholar] [CrossRef]
- Leung, M.C.K.; Meyer, J.N. Mitochondria as a target of organophosphate and carbamate pesticides: Revisiting common mechanisms of action with new approach methodologies. Reprod. Toxicol. 2019, 89, 83–92. [Google Scholar] [CrossRef]
- Lotti, M.; Vale, A. Chapter 10- Organophosphorus and carbamate insecticide poisoning. Handb. Clin. Neurol. 2015, 131, 149–168. [Google Scholar] [CrossRef]
- Buszewski, B.; Bukowska, M.; Ligor, M.; Staneczko-baranowska, I. A holistic study of neonicotinoids neuroactive insecticides-properties, applications, occurrence and analysis. Environ. Sci. Pollut. Res. 2019, 26, 34723–34740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, D.; Morrissey, C.; Minneau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Aponte, A.; Penilla, R.P.; Rodríguez, A.D.; Ocampo, C.B. Mechanisms of pyrethroid resistance in aedes (Stegomyia) aegypti from Colombia. Acta Trop. 2019, 191, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Costa, L.G. Chapter 9- The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. Critical Review DDT, Pyrethrins, Pyrethroids and Insect Sodium Channels. Life 2007, 59, 151–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolzonella, C.; Lucchetta, M.; Teo, G.; Boatto, V.; Zanella, A. Is there a way to rate insecticides that is less detrimental to human and environmental health? Glob. Ecol. Conserv. 2019, 20, e00699. [Google Scholar] [CrossRef]
- Oaya, C.S.; Malgwi, A.M.; Degri, M.M.; Samaila, A.E. Impact of synthetic pesticides utilization on humans and the environment: An overview. J. Agric. Sci. Technol. 2019, 11, 279–286. [Google Scholar] [CrossRef]
- Kim, K.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2016, 575, 525–535. [Google Scholar] [CrossRef]
- Marrs, T.C. Organophosphate poisoning. Pharmacol. Ther. 1993, 58, 51–66. [Google Scholar] [CrossRef]
- Moreno-Bondi, M.C. Biomimetic recognition elements for sensing applications. Anal. Bioanal. Chem. 2012, 402, 3019–3020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigdeli, A.; Ghasemi, F.; Golmohammadi, H.; Abbasi-Moayed, S.; Nezhad, M.A.F.; Fahimi-Kashani, N.; Jafarinejad, S.; Shahrajabian, M.; Hormoni-Nezhad, M.R. Nanoparticle-based optical sensor arrays. Nanoscale 2017, 9, 16546–16563. [Google Scholar] [CrossRef]
- Farré, M.; Barceló, D. Food Toxicants Analysis, 1st ed.; Elsevier Science: València, Spain, 2007; pp. 599–636. [Google Scholar]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Córdova, M.L.F.; Ruiz-Medina, A. Trends in flow-based analytical methods applied to pesticide detection: A review. Anal. Chim. Acta 2011, 684, 30–39. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [Green Version]
- Hiremath, S.D.; Priyadarshi, B.; Banerjee, M.; Chatterjee, A. Carbon dots-MnO2 based turn-on fluorescent probe for rapid and sensitive detection of hydrazine in water. J. Photochem. Photobiol. A Chem. 2019, 389, 112258. [Google Scholar] [CrossRef]
- Ratajczak, K.; Stobiecka, M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review. Carbohydr. Polym. 2019, 229, 115463. [Google Scholar] [CrossRef]
- Tafreshi, F.A.; Fatahi, Z.; Ghasemi, S.F. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Fan, Z. RecJf exonuclease-assisted fluorescent self-assembly aptasensor for supersensitive detection of pesticides in food. J. Lumin. 2020, 226, 117469. [Google Scholar] [CrossRef]
- Han, Y.; Yang, W.; Luo, X.; He, X.; Zhao, H.; Tang, W.; Yue, T.; Li, Z. Carbon dots based ratiometric fluorescent sensing platform for food safety. Crit. Rev. Food Sci. Nutr. 2020, 1–17. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yao, J.; Zhuang, Z.; Ni, C.; Yao, H.; Su, D.; Zhou, J.; Zhao, Z. AEE-active conjugated polymers based on di (naphthalen-2-yl)-1,2-diphenylethene for sensitive fluorescence detection of picric acid. Dyes Pigm. 2019, 174, 108041. [Google Scholar] [CrossRef]
- Chen, L.; Tian, X.; Li, Y.; Lu, L.; Nie, Y.; Wang, Y. Broad-spectrum pesticide screening by multiple cholinesterases and thiocholine sensors assembled high-throughput optical array system. J. Hazard. Mater. 2021, 402, 123830. [Google Scholar] [CrossRef]
- Korram, J.; Dewangan, L.; Karbhal, I.; Nagwanshi, R.; Vaishanav, S.K.; Ghosh, K.K.; Satnami, M.L. CdTe QD-based inhibition and reactivation assay of acetylcholinesterase for the detection of organophosphorus pesticides. RSC Adv. 2020, 10, 24190–24202. [Google Scholar] [CrossRef]
- Cai, Y.; Qiu, Z.; Lin, X.; Zeng, W.; Cao, Y.; Liu, W.; Liu, Y. Self-Assembled nanomaterials based on aggregation-induced emission of AuNCs: Fluorescence and colorimetric dual-mode biosensing of organophosphorus pesticides. Sens. Actuators B Chem. 2020, 321, 128481. [Google Scholar] [CrossRef]
- Li, W.; Feng, J.; Ma, Z. Nitrogen, sulfur, boron and flavonoid moiety co-incorporated carbon dots for sensitive fluorescence detection of pesticides. Carbon 2020, 161, 685–693. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Zheng, Z.; Li, G. Application of upconversion rare earth fluorescent nanoparticles in biomedical drug delivery system. J. Lumin. 2020, 223, 117226. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, J.; Wang, K.; Zhao, R.; Yang, S. Ingenious as partic acid-functionalized gold nanoparticles by one-pot protocol for the sensitive detection of chromium (III) ions. Microchem. J. 2020, 159, 105359. [Google Scholar] [CrossRef]
- Davidson, C.E.; Dixon, M.M.; Williams, B.R.; Kilper, G.K.; Lim, S.H.; Martino, R.A.; Rhodes, P.; Hulet, M.S.; Miles, R.W.; Samuels, A.C.; et al. Detection of chemical warfare agents by colorimetric sensor arrays. ACS Sens. 2020, 5, 1102–1109. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Binyam, A.; Liu, M.; Wu, Y.; Li, F. Discovering the enzyme mimetic activity of metal-organic framework (MOF) for label-free and colorimetric sensing of biomolecules. Biosens. Bioelectron. 2016, 86, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, J. Plasmon-Based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sens. 2017, 7, 857–875. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.; Wei, T.; Ma, X.; Zheng, X.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Halvorson, R.A.; Vikesland, P.J. Surface-Enhanced Raman Spectroscopy (SERS) for environmental analyses. Environ. Sci. Technol. 2010, 44, 7749–7755. [Google Scholar] [CrossRef]
- Shan, B.; Pu, Y.; Chen, Y.; Liao, M.; Li, M. Novel SERS labels: Rational design, functional integration and biomedical applications. Coord. Chem. Rev. 2018, 371, 11–37. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A. Analysis of Pb(II) ion sensing by crosslinked chitosan thin film using surface plasmon resonance spectroscopy. Optik 2013, 124, 126–133. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Zulholinda, M. Structural and optical properties of chitosan-poly(amidoamine) dendrimer composite thin film for potential sensing Pb2+ using an optical spectroscopy. Optik 2018, 185, 351–358. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric (III) ion. Opt. Laser Technol. 2019, 120, 105724. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Abdullah, J.; Daniyal, W.M.E.M.M.; Saleviter, S. Detection of phenol by incorporation of gold modified-enzyme based graphene oxide thin film with surface plasmon resonance technique. Opt. Express 2020, 28, 9738–9752. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Kamil, Y.M.; Fauzi, N.I.M.; Hashim, H.S.; Mahdi, M.A. Quantitative and selective surface plasmon resonance response based on a reduced graphene oxide–polyamidoamine nanocomposite for detection of dengue virus E-Proteins. Nanomaterials 2019, 10, 569. [Google Scholar] [CrossRef] [Green Version]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef]
- Daniyal, W.M.; Fen, Y.W.; Anas, N.A.; Omar, N.A.; Ramdzan, N.S.; Nakajima, H.; Mahdi, M.A. Enhancing the sensitivity of a surface plasmon resonance-based optical sensor for zinc ion detection by the modification of a gold thin film. RSC Adv. 2019, 9, 41729–41736. [Google Scholar] [CrossRef] [Green Version]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Optical properties of cross-linked chitosan thin film for copper ion detection using surface plasmon resonance technique. Opt. Appl. 2011, 41, 999–1013. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M.; Moksin, M.M.; Talib, Z.A.; Yusof, N.A. Surface plasmon resonance optical sensor for mercury ion detection by crosslinked chitosan thin film. J. Optoelectron. Adv. Mater. 2011, 13, 279–285. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix[4]arene-tetrakis in chitosan thin film as an active layer. Sens. Actuators B Chem. 2012, 171, 287–293. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Detection of mercury and copper ions using surface plasmon resonance optical sensor. Sens. Mater. 2011, 23, 325–334. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 305–314. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Utilization of chitosan-based sensor thin films for the detection of lead ion by surface plasmon resonance optical sensor. IEEE Sens. J. 2012, 13, 1413–1418. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Development of surface plasmon resonance sensor for determining zinc ion using novel active nanolayers as probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A.; Ishak, N.S.; Omar, N.A.S.; Zainudin, A.A. Preparation, characterization and optical properties of ionophore doped chitosan biopolymer thin film and its potential application for sensing metal ion. Optik 2015, 126, 4688–4692. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, optical and sensing properties of ionophore doped graphene based bionanocomposite thin film. Optik 2017, 144, 308–315. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of surface plasmon resonance with novel valinomycin doped chitosan-graphene oxide thin film for sensing potassium ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 111–115. [Google Scholar] [CrossRef]
- Rosddi, N.N.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M. Cationically Modified Nanocrystalline Cellulose/Carboxyl-Functionalized Graphene Quantum Dots Nanocomposite Thin Film: Characterization and Potential Sensing. Crystals 2020, 10, 875. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M. Investigating the properties of cetyltrimethylammonium bromide/hydroxylated graphene quantum dots thin film for potential optical detection of heavy metal ions. Materials 2020, 13, 2591. [Google Scholar] [CrossRef]
- Omar, N.A.; Fen, Y.W.; Saleviter, S.; Kamil, Y.M.; Daniyal, W.M.; Abdullah, J.; Mahdi, M.A. Experimental evaluation on surface plasmon resonance sensor performance based on sensitive hyperbranched polymer nanocomposite thin films. Sens. Actuator A Phys. 2020, 303, 111830. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Abdullah, J.; Sadrolhosseini, A.R.; Omar, N.A.S. Design and analysis of surface plasmon resonance optical sensor for determining cobalt ion based on chitosan-graphene oxide decorated quantum dots-modified gold active. Opt. Express 2019, 27, 32294–32307. [Google Scholar] [CrossRef]
- Daniyal, W.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A. Label-free optical spectroscopy for characterizing binding properties of highly sensitive nanocrystalline cellulose-graphene oxide based nanocomposite towards nickel ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 212, 25–31. [Google Scholar] [CrossRef]
- Daniyal, W.M.; Fen, Y.W.; Fauzi, N.I.; Hashim, H.S.; Ramdzan, N.S.; Omar, N.A. Recent advances in surface plasmon resonance optical sensors for potential application in environmental monitoring. Sens. Mater. 2020, 32, 4191–4200. [Google Scholar]
- Ramdzan, N.S.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Saleviter, S. Development of biopolymer and conducting polymer-based optical sensors for heavy metal ion detection. Molecules 2020, 25, 2548. [Google Scholar] [CrossRef]
- Zainuddin, N.H.; Fen, Y.W.; Alwahib, A.A.; Yaacob, M.H.; Bidin, N.; Omar, N.A.S.; Mahdi, M.A. Detection of adulterated honey by surface plasmon resonance optical sensor. Optik 2018, 168, 134–139. [Google Scholar] [CrossRef]
- Omar, N.A.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.; Daniyal, W.M.; Mahdi, M.A. Sensitive surface plasmon resonance performance of cadmium sulfide quantum dots-amine functionalized graphene oxide based thin film towards dengue virus E-protein. Opt. Laser Technol. 2019, 114, 204–208. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Zainudin, A.A.; Yusof, N.A. Development of optical sensor for determination of Co (II) based on surface plasmon resonance phenomenon. Sens. Lett. 2017, 15, 862–867. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W. Recent development of SPR spectroscopy as potential method for diagnosis of dengue virus E-protein. Sens. Rev. 2018, 38, 106–116. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Zainudin, A.A.; Daniyal, W.M.E.M.M. Optical and structural characterization of immobilized 4-(2-pyridylazo) resorcinol in chitosan-graphene oxide composite thin film and its potential for Co2+ sensing using surface plasmon resonance technique. Results Phys. 2018, 11, 118–122. [Google Scholar] [CrossRef]
- Omar, N.A.; Fen, Y.W.; Abdullah, J.; Chik, C.E.; Mahdi, M.A. Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Bio-Sens. Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Abdullah, J.; Zaid, M.H.M. Structural and optical studies of cadmium sulfide quantum dot-graphene oxide-chitosan nanocomposite thin film as a novel SPR spectroscopy active layer. J. Nanomater. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M. Development of a graphene-based surface plasmon resonance optical sensor chip for potential biomedical application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniyal, W.M.E.M.M.; Saleviter, S.; Fen, Y.W. Development of surface plasmon resonance spectroscopy for metal ion detection. Sens. Mater. 2018, 30, 2023–2038. [Google Scholar] [CrossRef] [Green Version]
- Al-Rekabi, S.H.; Kamil, Y.M.; Bakar, M.H.A.; Fen, Y.W.; Lim, H.N.; Kanagesan, S.; Mahdi, M.A. Hydrous ferric oxide-magnetite-reduced graphene oxide nanocomposite for optical detection of arsenic using surface plasmon resonance. Opt. Laser Technol. 2019, 111, 417–423. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Seong, K.C.; Ahmed, A.Y.; Ferrell, T.L.; Fen, Y.W.; Sadrolhosseini, A.R.; Ayodele, O.B.; Meriaudeau, F.; Saidu, A. Enhanced sensitivity of surface plasmon resonance biosensor functionalized with doped polyaniline composites for the detection of low-concentration acetone vapour. J. Sens. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Eddin, F.B.K.; Fen, Y.W. The principle of nanomaterials based surface plasmon resonance biosensors and its potential for dopamine detection. Molecules 2020, 25, 2769. [Google Scholar] [CrossRef] [PubMed]
- Eddin, F.B.K.; Fen, Y.W. Recent advances in electrochemical and optical sensing of dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Lin, Z.; Lin, J. A review on applications of chemiluminescence detection in food analysis. Anal. Chim. Acta 2010, 670, 1–10. [Google Scholar] [CrossRef]
- Xiao, Q.; Lin, J. Advances and applications of chemiluminescence immunoassay in clinical diagnosis and foods safety. Chin. J. Anal. Chem. 2015, 43, 929–938. [Google Scholar] [CrossRef]
- Iqbal, S.S.; Mayo, M.W.; Bruno, J.G.; Bronk, B.V.; Batt, C.A.; Chambers, J.P. A review of molecular recognition technologies for detection of biological threat agents. Biosens. Bioelectron. 2000, 15, 549–578. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance-a review. Int. J. Food Sci. Technol. 2012, 49, 383–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.K.; Singh, P. Enzyme based optical biosensors for organophosphate class of pesticide detection. Phys. Chem. Chem. Phys. 2020, 22, 15105–15119. [Google Scholar] [CrossRef]
- Amine, A.; Arduini, F.; Moscone, D.; Palleschi, G. Recent advances in biosensors based on enzyme inhibition. Biosens. Bioelectron. 2015, 76, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Chen, J. Development of biosensor technologies for analysis of environmental contaminants. Trends Environ. Anal. Chem. 2014, 2, 25–32. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent Advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Petr, S. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Beier, R.C.; Lei, H.; Gee, S.; Bruce, D.; Wang, H.; Wang, Z.; Sun, X.; Shen, Y.; et al. Immunochemical techniques for multianalyte analysis of chemical residues in food and the environment: A review. Trends Anal. Chem. 2016, 88, 25–40. [Google Scholar] [CrossRef]
- Fu, X.; Chen, L.; Choo, J. Optical nanoprobes for ultrasensitive immunoassay. Anal. Chem. 2016, 89, 124–137. [Google Scholar] [CrossRef]
- Jia, M.; Zhai, F.; Bing, X. Rapid multi-residue detection methods for pesticides and veterinary drugs. Molecules 2020, 25, 3590. [Google Scholar] [CrossRef]
- Song, K.; Lee, S.; Ban, C. Aptamers and their biological applications. Sensors 2012, 12, 612–631. [Google Scholar] [CrossRef] [Green Version]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent progress in nanomaterial-based optical aptamer assay for the detection of food chemical contaminants. ACS Appl. Mater. Interfaces 2017, 9, 23287–23301. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Zhu, T.; Qi, Y.; Zhao, Y.; Xia, H.; Fu, W. Development of a quartz crystal microbalance biosensor with aptamers as bio-recognition element. Sensors 2010, 10, 5859–5871. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Wu, S.; Dai, S.; Gu, H.; Hao, L.; Ye, H.; Wang, Z. Advances in aptasensors for the detection of food contaminants. Analyst 2016, 141, 3942–3961. [Google Scholar] [CrossRef]
- Elskens, J.P.; Elskens, J.M.; Madder, A. Chemical modification of aptamers for increased binding affinity in diagnostic applications: Current status and future prospects. Int. J. Mol. Sci. 2020, 21, 4522. [Google Scholar] [CrossRef]
- Volkert, A.A.; Haes, A.J. Advancements in nanosensors using plastic antibodies. Analyst 2014, 139, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Drioli, E.; Guzzo, L.; Donato, L. Bio-mimetic sensors based on molecularly imprinted membranes. Sensors 2014, 14, 13863–13912. [Google Scholar] [CrossRef] [Green Version]
- Schirhagl, R. Bioapplications for molecularly imprinted polymers. Anal. Chem. 2014, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Yuste, A.R.; Carrasco, S. Molecularly imprinted polymer-based hybrid materials for the development of optical sensors. Polymers 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dissanayake, N.M.; Arachchilage, J.S.; Samuels, T.A.; Obare, S.O. Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence detection of pesticides using quantum dot materials—A review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.F.; Ho, K.Y.F.; Gong, C.B. Gong. Synthesis of a new bimetallic Re(I)-NCS-Pt(II) complex as chemodosimetric ensemble for the selective detection of mercapto-containing pesticides. Anal. Chem. 2015, 87, 6112–6118. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Barquet, A.; Freed, V.H.; Haque, R.; Morgade, C.; Robert, E.; Vaclavek, C. Human pesticide poisonings by a fat-soluble organophosphate insecticide. Arch. Environ. Health 1975, 30, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Classification and uses of organophosphates and carbamates. Uses Abus. Epidemiol. 2006, 5–24. [Google Scholar] [CrossRef]
- Pope, C.; Karanth, S.; Liu, J. Pharmacology and toxicology of cholinesterase inhibitors: Uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005, 19, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.I.; Figueroa, R.; Rojas, M.; Millán, D.; Pavez, P.; Tapia, R.A. Dual function of amino acid ionic liquids (Bmim[AA]) on the degradation of the organophosphorus pesticide, Paraoxon. Org. Biomol. Chem. 2018, 16, 7446–7453. [Google Scholar] [CrossRef]
- Webb, R.E.; Argauer, R.J. Uptake of monocrotophos by chrysanthemum cultivars and resulting control of melon aphid. J. Econ. Entomol. 1972, 67, 251–252. [Google Scholar] [CrossRef]
- Rogers, K.R.; Wang, Y.; Mulchandani, A.; Mulchandani, P.; Chen, W. Organophosphorus hydrolase-based assay for organophosphate pesticides. Biotechnol. Prog. 1999, 15, 517–521. [Google Scholar] [CrossRef]
- Shankar, M.V.; Cheralathan, K.K.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Enhanced photocatalytic activity for the destruction of monocrotophos pesticide by TiO2/Hβ. J. Mol. Catal. A Chem. 2004, 223, 195–200. [Google Scholar] [CrossRef]
- Slusky, D.A.; Metayer, C.; Aldrich, A.C.; Ward, M.H.; Lea, C.S.; Selvin, S.; Buffler, P.A. Reliability of maternal-reports regarding the use of household pesticides: Experience from a case-control study of childhood leukemia. J. Cancer Epidemiol. 2012, 36, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Peterson, C.J.; Coats, J.R. Fumigation toxicity of monoterpenoids to several stored product insects. J. Stored Prod. Res. 2003, 39, 77–85. [Google Scholar] [CrossRef]
- Walker, A.I.T.; Brown, V.K.H.; Stevenson, D.E.; Thorpe, E. Toxicological Studies with the Insecticide. tetrachlorvinphos. Manag. Sci. 1972, 3, 517–525. [Google Scholar] [CrossRef]
- Morgan, M.B.; Snell, T.W. Characterizing stress gene expression in reef-building corals exposed to the mosquitoside dibrom. Mar. Pollut. Bull. 2002, 44, 1206–1218. [Google Scholar] [CrossRef]
- Luo, M.; Wei, J.; Zhao, Y.; Sun, Y.; Liang, H.; Wang, S.; Li, P. Fluorescent and visual detection of methyl-paraoxon by using boron-and nitrogen-doped carbon dots. Microchem. J. 2020, 154, 104547. [Google Scholar] [CrossRef]
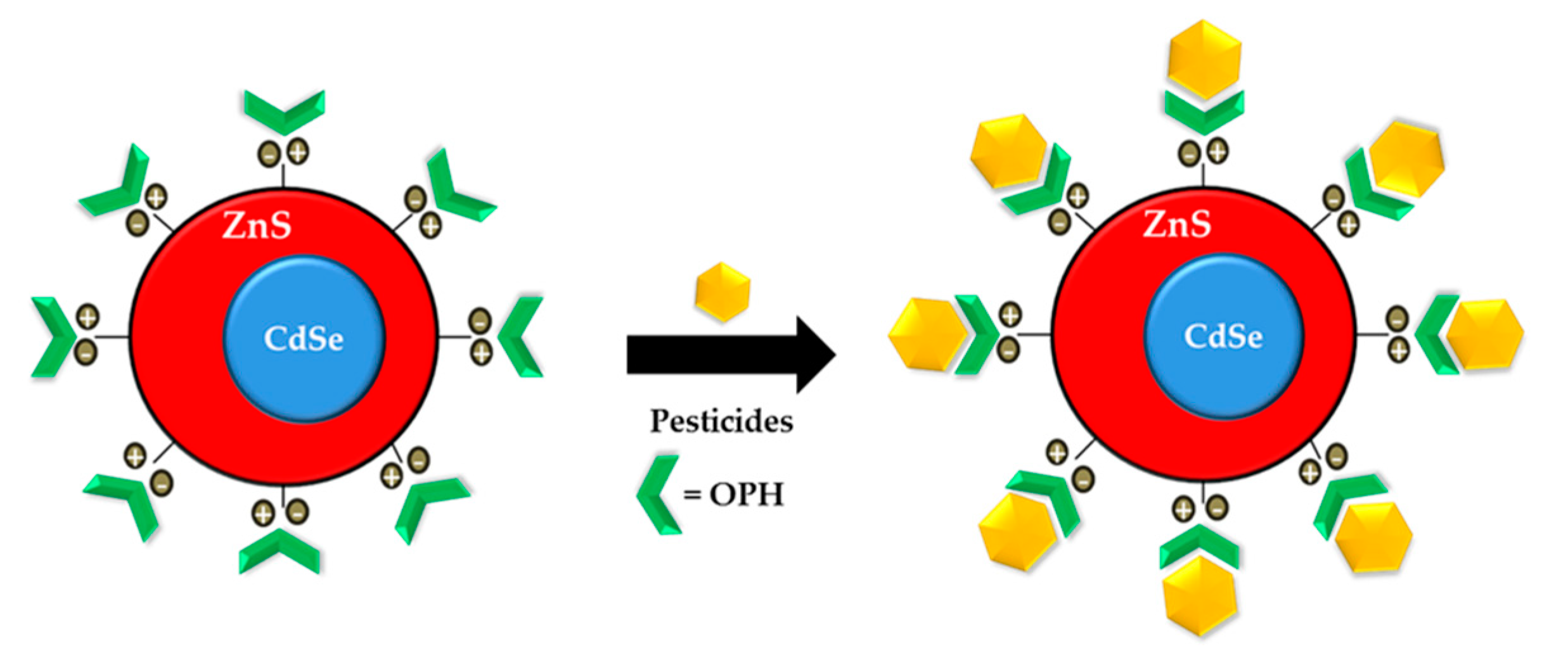
- Constantine, C.A.; Gattas-Asfura, K.M.; Mello, S.V.; Crespo, G.; Rastogi, V.; Cheng, T.; DeFrank, J.J.; Leblanc, R.M. Layer-by-Layer films of chitosan, organophosphorus hydrolase and thioglycolic acid-capped CdSe quantum dots for the detection of paraoxon. J. Phys. Chem. B 2003, 107, 13762–13764. [Google Scholar] [CrossRef]
- Ji, X.; Zheng, J.; Xu, J.; Rastogi, V.K.; Cheng, T.; DeFrank, J.J.; Leblanc, R.M. (CdSe) ZnS quantum dots and organophosphorus hydrolase bioconjugate as biosensors for detection of paraoxon. J. Phys. Chem. B 2005, 109, 3793–3799. [Google Scholar] [CrossRef]
- Hossain, S.Z.; Luckham, R.E.; McFadden, M.J.; Brennan, J.D. Reagentless bidirectional lateral flow bioactive paper sensors for detection of pesticides in beverage and food samples. Anal. Chem. 2009, 81, 9055–9064. [Google Scholar] [CrossRef] [PubMed]
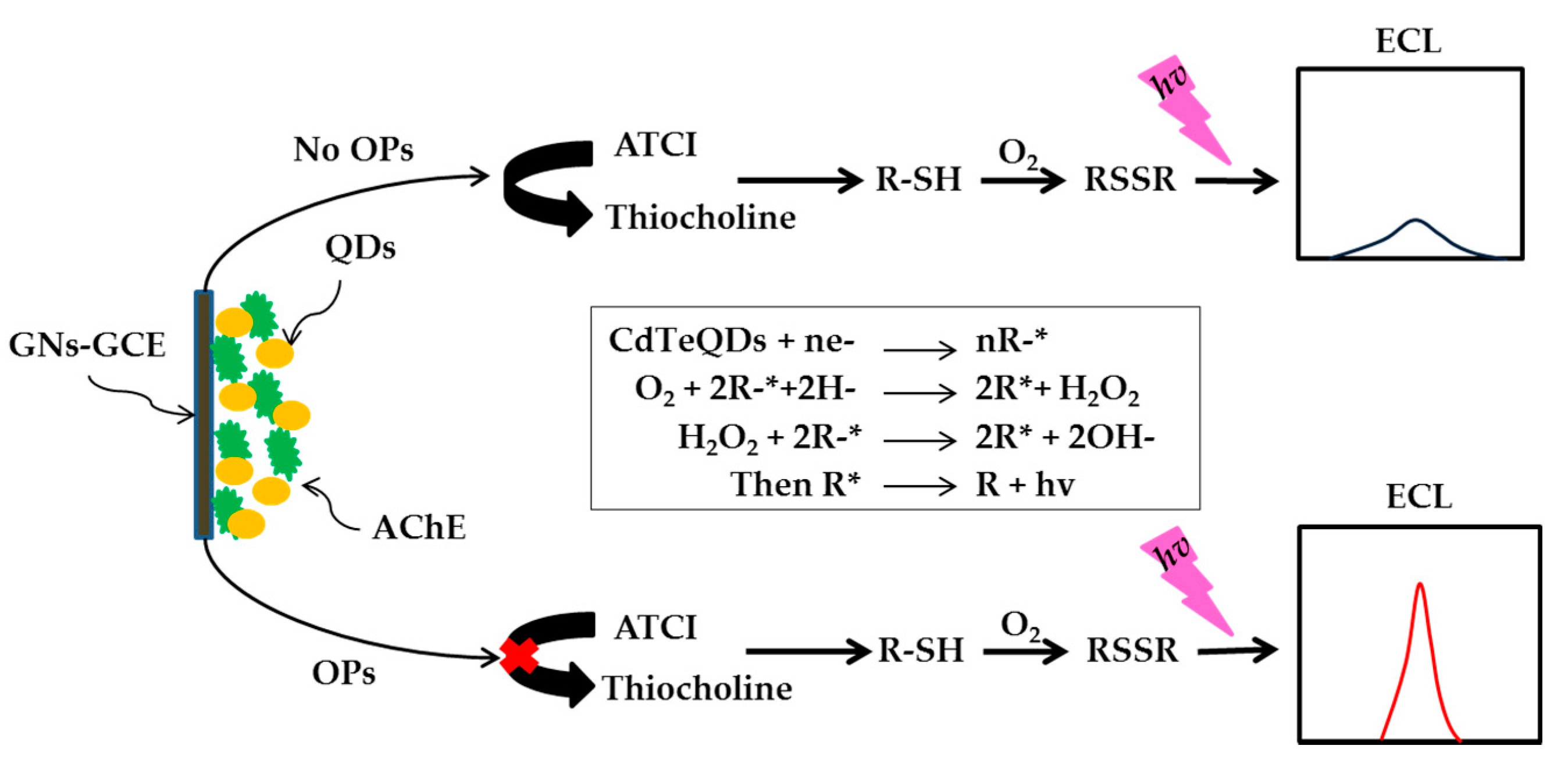
- Zheng, Z.; Zhou, Y.; Li, X.; Liua, S.; Tang, Z. Highly-sensitive organophosphorous pesticide biosensors based on nanostructured films of acetylcholinesterase and CdTe quantum dots. Biosens. Bioelectron. 2011, 26, 3081–3085. [Google Scholar] [CrossRef]
- Zhang, Y.; Hei, T.; Cai, Y.; Gao, Q.; Zhang, Q. Affinity binding-guided fluorescent nanobiosensor for acetylcholinesterase inhibitors via distance modulation between the fluorophore and metallic nanoparticle. Anal. Chem. 2012, 84, 2830–2836. [Google Scholar] [CrossRef]
- Gao, X.; Tang, G.; Su, X. Optical detection of organophosphorus compounds based on Mn-doped ZnSe d-dot enzymatic catalytic sensor. Biosens. Bioelectron. 2012, 36, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Chen, W.; Yue, X.; Jiang, X. Highly sensitive colorimetric detection of organophosphate pesticides using copper catalyzed click chemistry. Talanta 2013, 103, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ban, R.; Zhu, J.; Zhang, J. Manganese-doped ZnS quantum dots as a phosphorescent probe Manganese-doped ZnS quantum dots as a phosphorescent probe for use in the bi-enzymatic determination of organophosphorus pesticides. Microchim. Acta 2014, 181, 1591–1599. [Google Scholar] [CrossRef]
- Luan, E.; Zheng, Z.; Li, X.; Gu, H.; Liu, S. Inkjet-assisted layer-by-layer printing of quantum dot/enzyme microarrays for highly sensitive detection of organophosphorous pesticides. Anal. Chim. Acta 2016, 916, 77–83. [Google Scholar] [CrossRef]
- Wu, X.; Song, Y.; Yan, X.; Zhu, C.; Ma, Y.; Du, D.; Lin, Y. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens. Bioelectron. 2017, 94, 292–297. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Lu, G.; Su, X. Carbon dot-based bioplatform for dual colorimetric and fluorometric sensing of organophosphate pesticides. Sens. Actuators B. Chem. 2018, 260, 563–570. [Google Scholar] [CrossRef]
- Wu, X.; Wang, P.; Hou, S.; Wu, P.; Xue, J. Fluorescence sensor for facile and visual detection of organophosphorus pesticides using AIE fluorogens-SiO2-MnO2 sandwich nanocomposites. Talanta 2019, 198, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Yue, Z.; Bing, Z.; Yiwei, T.; Xiuying, L.; Jianrong, L. Highly-sensitive organophosphorus pesticides biosensensors based CdTe quantum dots bi-enzyme immobilized eggshell membranes. Analyst 2016, 141, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Yue, Z.; Bing, Z.; Yiwei, T.; Xiuying, L.; Jianrong, L. Sensitive fluorescence assay of organophosphorus pesticides based on the fluorescence resonance energy transfer between CdTe quantum dots and porphyrin. Analyst 2016, 141, 4941–4946. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Hu, T.; Su, X. A novel fluorimetric sensing platform for highly sensitive detection of organophosphorus pesticides by using egg white-encapsulated gold nanoclusters. Biosens. Bioelectron. 2017, 91, 232–237. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, X.; Dai, Z.; Liu, S.; Tang, Z. Detection of mixed organophosphorus pesticides in real samples using quantum dots/bi-enzyme assembly multilayers. J. Mater. Chem. 2011, 21, 16955–16962. [Google Scholar] [CrossRef]
- Han, Z.; Chi, C.; Bai, B.; Liu, G.; Rao, Q.; Peng, S.; Liu, H.; Zhao, Z.; Zhang, D.; Wu, A. Chromogenic platform based on recombinant Drosophila melanogaster acetylcholinesterase for visible unidirectional assay of organophosphate and carbamate insecticide residues. Anal. Chim. Acta 2012, 720, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wei, J.; Ren, X.; Ren, J.; Tang, F. A simple and sensitive fluorescence biosensor for detection of organophosphorus pesticides using H2O2-sensitive quantum dots/bi-enzyme. Biosens. Bioelectron. 2013, 47, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cao, J.; Hu, H.; Yang, Q.; Yang, F.; Su, H.; He, C.; Li, P.; Wu, A. Sensitive and selective detection of oxo-form organophosphorus pesticides based on CdSe/ZnS quantum dots. Molecules 2017, 22, 1421. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Uttl, L.; Pulkrabova, J.; Hajslova, J. Screening of carbamate and organophosphate pesticides in food matrices using an affordable and simple spectrophotometric acetylcholinesterase assay. Appl. Sci. 2020, 10, 565. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yin, Q.; Fan, Y.; Zhang, L.; Xu, Y.; Hu, O.; Guo, X.; Shi, Q.; Fu, H.; She, Y. Double quantum dots-nanoporphyrin fluorescence-visualized paper-based sensors for detecting organophosphorus pesticides. Talanta 2019, 199, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Fan, K.; Pan, Y.; Jiang, H.; Wang, F.; Yang, D.; Lu, D.; Feng, J.; Zhao, J.; Yang, L.; et al. Fe3O4 magnetic nanoparticle peroxidase mimetic-based colorimetric assay for the rapid detection of organophosphorus pesticide and nerve agent. Anal. Chem. 2013, 85, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xue, S.; Deng, J.; Zhang, M.; Shi, G.; Zhou, T. Polyacrylic acid-coated cerium oxide nanoparticles: An oxidase mimic applied for colorimetric assay to organophosphorus pesticides. Biosens. Bioelectron. 2016, 85, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Sahub, C.; Tuntulani, T.; Nhujak, T.; Tomapatanaget, B. Effective biosensor based on graphene quantum dots via enzymatic reaction for directly photoluminescence detection of organophosphate pesticide. Sens. Actuators B. Chem. 2018, 258, 88–97. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, T.; Liu, F.; Sun, M.; Yu, H.; Liu, B.; Zhang, Z.; Jiang, H.; Wang, S. Selective fluorescence turn-on and ratiometric detection of organophosphate using dual-emitting Mn-Doped ZnS nanocrystal probe. Anal. Chem. 2014, 86, 11727–11733. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, Y.; Wang, L.; Paulsen, M.D.; Simpson, C.; Liu, F.; Du, D.; Lin, Y. Simultaneous detection of dual biomarkers from humans exposed to organophosphorus pesticides by combination of immunochromatographic test strip and ellman assay. Biosens. Bioelectron. 2018, 104, 39–44. [Google Scholar] [CrossRef]
- Chang, M.F.F.; Ginjom, I.R.; Ng, S.M. Single-shot “turn-off” optical probe for rapid detection of paraoxon-ethyl pesticide on vegetable utilising fluorescence carbon dots. Sens. Actuators B Chem. 2017, 242, 1050–1056. [Google Scholar] [CrossRef]
- Chen, Q.; Fung, Y. Capillary electrophoresis with immobilized quantum dot fluorescence detection for rapid determination of organophosphorus pesticides in vegetables. Electrophoresis 2010, 31, 3107–3114. [Google Scholar] [CrossRef]
- Long, Q.; Li, H.; Zhang, Y.; Yao, S. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organophosphorus pesticides. Biosens. Bioelectron. 2015, 68, 168–174. [Google Scholar] [CrossRef]
- Marcos, S.; Callizo, E.; Mateos, E.; Gaiban, J. An optical sensor for pesticide determination based on the autoindicating optical properties of peroxidase. Talanta 2014, 122, 251–256. [Google Scholar] [CrossRef]
- Guo, G.; Li, B.; Huang, H.; Zhao, N.; Li, J.; Liu, Y.; Lv, X.; Zhang, M.; Cao, L.; Tai, Z. Radical-based advanced oxidation for trichlorfon degradation and phosphorus recovery: Process feasibility and reaction mechanism. J. Clean. Prod. 2020, 275, 122706. [Google Scholar] [CrossRef]
- De, J.J.V.C.D.I. Acute toxicity of Trichlorfon (Dipterex) to fry of Cichlasoma uwphthalmus Giinther. Aquac. Res. 1988, 19, 341–345. [Google Scholar] [CrossRef]
- He, Y.; Xu, B.; Li, W.; Yu, H. Silver nanoparticle-based chemiluminescent sensor array for pesticide discrimination. J. Agric. Food Chem. 2015, 63, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yan, F.; Huang, X.; Zhang, X.; Zhang, Y.; Zhang, C.; Jin, J.; Li, H.; Yao, S. A new water-soluble and colorimetric fluorescent probe for highly sensitive detection of organophosphorus pesticides. RSC Adv. 2016, 6, 88096–88103. [Google Scholar] [CrossRef]
- Dowgiallo, A.M.; Guenther, D.A. Determination of the limit of detection of multiple pesticides utilizing gold nanoparticles and surface-enhanced raman spectroscopy. J. Agric. Food Chem. 2019, 67, 12642–12651. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, T.; Zhu, F.; Ouyang, G. In vivo monitoring and exposure potency assessment of phase I metabolism of fenthion in vegetables. J. Hazard. Mater. 2020, 399, 123013. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J. Combined treatments of spinosad and chlorpyrifos-methyl for management of resistant psocid pests (Psocoptera: Liposcelididae) of stored grain. Pest Manag. Sci. 2007, 63, 104–109. [Google Scholar] [CrossRef]
- Govindarajan, D.; Chatterjee, C.; Shakambari, G.; Varalakshmi, P.; Jayakumar, K.; Balasubramaniem, A. Oxidative stress response, epigenetic and behavioral alterations in Caenorhabditis elegans exposed to organophosphorus pesticide quinalphos. Biocatal. Agric. Biotechnol. 2019, 17, 702–709. [Google Scholar] [CrossRef]
- Hicks, D.J. Census Demographics and Chlorpyrifos Use in California’s Central Valley, 2011–15: A Distributional Environmental Justice Analysis. Int. J. Environ. Res. Public Health 2020, 17, 2593. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Li, Y.; Ren, F.; Wang, R.; Pang, G. Toxicity, residue, degradation and detection methods of the insecticide triazophos. Environ. Chem. Lett. 2019, 17, 1769–1785. [Google Scholar] [CrossRef]
- Buffa, A.; Mandler, D. Adsorption and detection of organic pollutants by fixed bed carbon nanotube electrochemical membrane. Chem. Eng. J. 2019, 359, 130–137. [Google Scholar] [CrossRef]
- Wei, L.; Huang, X.; Zheng, L.; Wang, J.; Ya, Y.; Yan, F. Electrochemical sensor for the sensitive determination of parathion based on the synergistic effect of ZIF-8 and ionic liquid. Ionics 2019, 25, 5013–5021. [Google Scholar] [CrossRef]
- Tamura, H.; Maness, S.C.; Reischmann, K.; Dorman, D.C.; Gray, L.E.; Gaido, K.W. Androgen receptor antagonism by the organophosphate insecticide fenitrothion. Toxicol. Sci. 2001, 60, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pordel, M.A.; Maleki, A.; Ghanbari, R.; Rezaee, R.; Khamforoush, M.; Daraei, H.; Athar, S.D.; Shahmoradi, B.; Safari, M.; Ziaee, A.H.; et al. Evaluation of the effect of electrospun nanofibrous membrane on removal of diazinon from aqueous solutions. React. Funct. Polym. 2019, 139, 85–91. [Google Scholar] [CrossRef]
- Girbal, L.; Rols, J.; Lindley, N.D. Growth rate influences reductive biodegradation of the organophosphorus pesticide demeton by Corynebacterium glutamicum. Biodegradation 2000, 11, 371–376. [Google Scholar] [CrossRef]
- Li, A.Y.; Pruett, J.H.; Davey, R.B.; George, J.E. Toxicological and biochemical characterization of coumaphos resistance in the San Roman strain of Boophilus microplus (Acari: Ixodidae). Pestic. Biochem. Physiol. 2005, 81, 145–153. [Google Scholar] [CrossRef]
- Hemingway, J. Vectors: Recognising the challenge and reducing neglect. Int. Health 2019, 11, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Tran, C.; Vu, D.C.; Dieu, T.; Ung, T.; Nguyen, H.Y. Fabrication of fluorescence-based biosensors from functionalized CdSe and CdTe quantum dots for pesticide detection. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 1–4. [Google Scholar] [CrossRef]
- Hai, N.N.; Chinh, V.D.; Chi, T.K.; Thi, U.; Thuy, D.; Nghia, N.X.; Cao, D.T.; Nga, P.T. Optical detection of the pesticide by functionalized quantum dots as fluorescence-based biosensor. Key Eng. Mater. 2012, 495, 314–318. [Google Scholar] [CrossRef]
- Liang, H.; Song, D.; Gong, J. Signal-on electrochemiluminescence of biofunctional CdTe quantum dots for biosensing of organophosphate pesticides. Biosens. Bioelectron. 2014, 53, 363–369. [Google Scholar] [CrossRef]
- Lu, L.; Xia, Y. Enzymatic reaction modulated gold nanorod end-to-end self-assembly for ultrahigh sensitively colorimetric sensing of cholinesterase and organophosphate pesticides in human blood. Anal. Chem. 2015, 87, 1–30. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Wang, X.; Su, X. A novel fluorescence probing strategy for the determination of parathion-methyl. Talanta 2015, 131, 88–94. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Han, X.; Su, X. A ratiometric fluorescent quantum dots based biosensor for organophosphorus pesticides detection by inner-filter effect. Biosens. Bioelectron. 2015, 74, 277–283. [Google Scholar] [CrossRef]
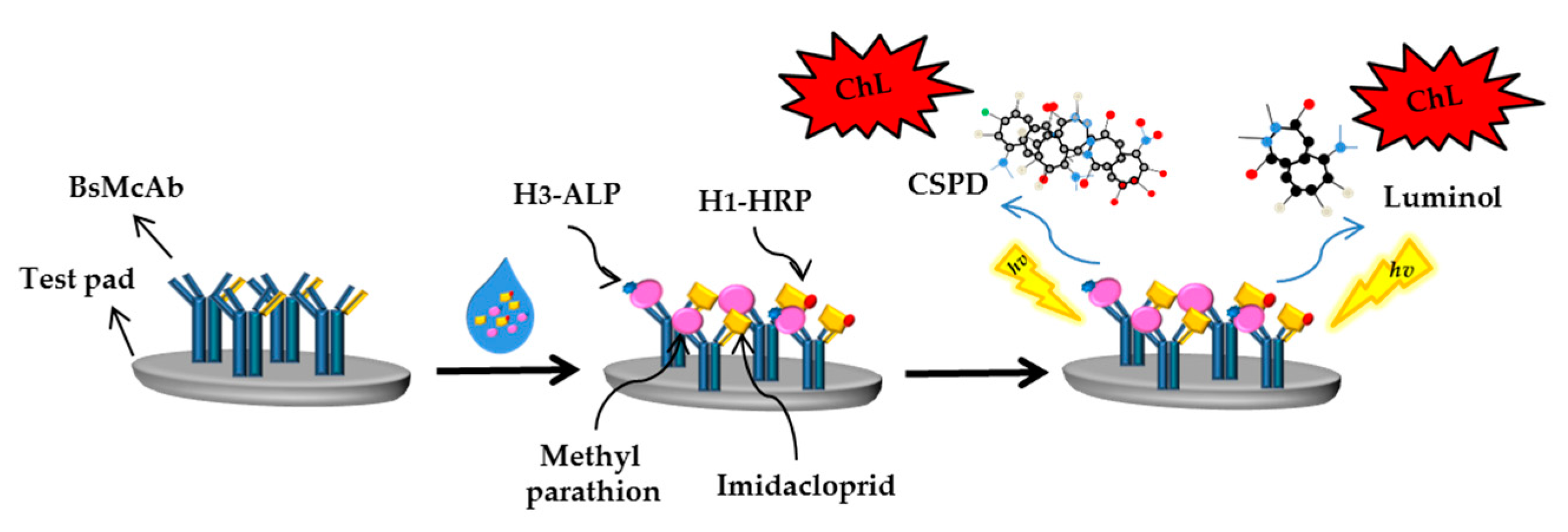
- Ouyang, H.; Wang, L.; Yang, S.; Wang, W.; Wang, L.; Liu, F.; Fu, Z. Chemiluminescence reaction kinetics-resolved multianalyte immunoassay strategy using a bispecific monoclonal antibody as the unique recognition reagent. Anal. Chem. 2015, 87, 2952–2958. [Google Scholar] [CrossRef]
- Shu, Q.; Wang, L.; Ouyang, H.; Wang, W.; Liu, F.; Fu, Z. Multiplexed immunochromatographic test strip for time-resolved chemiluminescent detection of pesticide residues using a bifunctional antibody. Biosens. Bioelectron. 2017, 87, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kong, L.; Guo, Z.; Liu, J. Synthesis of novel decorated one-dimensional gold nanoparticle and its application in ultrasensitive detection of insecticide. J. Mater. Chem. 2010, 20, 5271–5279. [Google Scholar] [CrossRef]
- Hou, J.; Dong, J.; Zhu, H.; Teng, X.; Ai, S.; Mang, M. A simple and sensitive fluorescent sensor for methyl parathion based on L-tyrosine methyl ester functionalized carbon dots. Biosens. Bioelectron. 2015, 68, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, H.; Yan, Y.; Su, X. Selective detection of parathion-methyl based on near-infrared CuInS2 quantum dots. Food Chem. 2015, 173, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Fahimi-Kashani, N.; Rashti, A.; Hormozi-Nezhad, M.R.; Mahdavi, V. MoS2 Quantum-dots as label-free fluorescent nanoprobe for highly selective detection of methyl parathion pesticide. Anal. Methods 2016, 9, 716–723. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Q.; Meng, G.; Wang, X.; Hu, X.; Han, F.; Lei, Y. Silver nanoparticle-assembled micro-bowl arrays for sensitive SERS detection of pesticide residue. Nanotechnology 2020, 31, 1–18. [Google Scholar] [CrossRef] [PubMed]
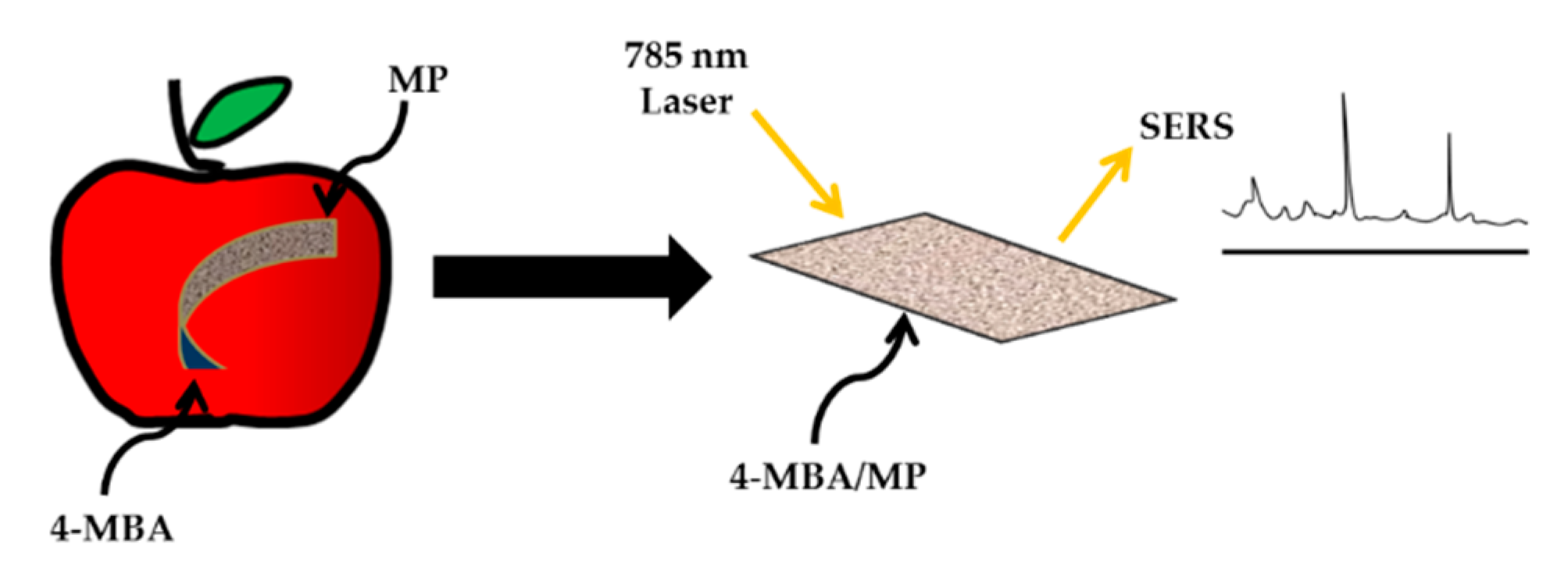
- Xie, J.; Li, L.; Khan, I.M.; Wang, Z.; Ma, X. Flexible paper-based SERS substrate strategy for rapid detection of methyl parathion on the surface of fruit. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118104. [Google Scholar] [CrossRef]
- Xie, H.; Bei, F.; Hou, J.; Ai, S. A highly sensitive dual-signaling assay via inner filter effect between g-C3N4 and gold nanoparticles for organophosphorus pesticides. Sens. Actuators B. Chem. 2018, 255, 2232–2239. [Google Scholar] [CrossRef]
- Zou, Z.; Du, D.; Wang, J.; Smith, J.N.; Timchalk, C.; Li, Y.; Lin, Y. Quantum dot-based immunochromatographic fluorescent biosensor for biomonitoring trichloropyridinol, a biomarker of exposure to chlorpyrifos. Anal. Chem. 2010, 82, 5125–5133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Mei, Q.; Guan, G.; Liu, B.; Wang, S.; Zhang, Z. Ligand replacement-induced fluorescence switch of quantum dots for ultrasensitive detection of organophosphorothioate pesticides. Anal. Chem. 2010, 82, 9579–9586. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, H.L.; Liu, N.; Sai, N.; Liu, X.; Liu, Z.; Gao, Z.; Ning, B.A. A fluoroimmunoassay based on quantum dot−streptavidin conjugate for the detection of chlorpyrifos. J. Agric. Food Chem. 2010, 58, 8895–8903. [Google Scholar] [CrossRef]
- Chen, Y.P.; Ning, B.; Liu, N.; Feng, Y.; Liu, Z.; Liu, X.; Gao, Z.X. A rapid and sensitive fluoroimmunoassay based on quantum dot for the detection of chlorpyrifos residue in drinking water. J. Environ. Sci. Health Part B 2010, 45, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, E.; Calle, A.; Manclús, J.J.; Montoya, A.; Lechuga, L.M. Multi-analyte SPR immunoassays for environmental biosensing of pesticides. Anal. Bioanal. Chem. 2007, 387, 1449–1458. [Google Scholar] [CrossRef]
- Yao, G.; Liang, R.; Huang, C.; Wang, Y.; Qiu, J. Surface Plasmon Resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal. Chem. 2013, 85, 11944–11951. [Google Scholar] [CrossRef] [PubMed]
- Lertvachirapaiboon, C.; Shinbo, K.; Kato, K.; Kaneko, F. Surface plasmon resonance-enhanced photoelectrochemical sensor for detection of an organophosphate pesticide chlorpyrifos. MRS Commun. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Li, Q.; Dou, X.; Zhang, L.; Zhao, X.; Luo, J.; Yang, M. Oriented assembly of surface plasmon resonance biosensor through staphylococcal protein A for the chlorpyrifos detection. Anal. Bioanal. Chem. 2019, 411, 6057–6066. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and adhesive surface enhance raman scattering active tape for rapid detection of pesticide residues in fruits and vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, X.; Xu, L.; Ying, Y.; Wu, Y.; Wen, Y.; Yang, H. Template-free synthesis of SERS-active gold nanopopcorn for rapid detection of chlorpyrifos residues. Sens. Actuators B Chem. 2017, 241, 1008–1013. [Google Scholar] [CrossRef]
- Ma, P.; Wang, L.; Xu, L.; Li, J.; Zhang, X.; Chen, H. Rapid quantitative determination of chlorpyrifos pesticide residues in tomatoes by surface-enhanced Raman spectroscopy. Eur. Food Res. Technol. 2019, 246, 239–251. [Google Scholar] [CrossRef]
- Ouyang, H.; Tu, X.; Fu, Z.; Wang, W.; Fu, S.; Zhu, C.; Du, D.; Lin, Y. Colorimetric and chemiluminescent dual-readout immunochromatographic assay for detection of pesticide residues utilizing g-C3N4/BiFeO3 nanocomposites. Biosens. Bioelectron. 2018, 106, 43–49. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.; Bansal, V.; Kumar, A.; Deep, A. Practical utilization of nanocrystalmetal organic framework biosensor for parathion specific recognition. Microchem. J. 2016, 128, 102–107. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Li, H.; Wang, L. Composite QDs@MIPNanospheres for specific recognition and direct fluorescent quantification of pesticides in aqueous media. Anal. Chem. 2012, 84, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhu, G.; Liu, C.; Huang, Y.; Zhang, Y.; Li, H.; Zhao, J.; Yao, S. A label-free silicon quantum dots-based photoluminescence sensor for ultrasensitive detection of pesticides. Anal. Chem. 2013, 85, 11464–11470. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Li, H.; Hou, T.; Li, F. Paper-based fluorescent sensor for rapid naked-eye detection of acetylcholinesterase activity and organophosphorus pesticides with high sensitivity and selectivity. Biosens. Bioelectron. 2016, 86, 971–977. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Hassan, M.; Guo, Z.; Zhang, Z.; Chen, Q. Fabricating an acetylcholinesterase modulated UCNPs- Cu2+ fluorescence biosensor for ultrasensitive detection of organophosphorus pesticides-diazinon in food. J. Agric. Food Chem. 2019, 67, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Guo, Y.; Zhao, Y.; Liu, Y.; Gui, W.; Zhu, G. Multi-residue detection of pesticides using a sensitive immunochip assay based on nanogold enhancement. Anal. Chim. Acta 2016, 938, 146–155. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Ganguly, P.; Kumar, S. Molecular assembly of 3-mercaptopropinonic acid and guanidine acetic acid on silver nanoparticles for selective colorimetric detection of triazophos in water and food samples. Sens. Actuators B. Chem. 2016, 233, 486–495. [Google Scholar] [CrossRef]
- Du, P.; Jin, M.; Chen, G.; Zhang, C.; Cui, X.; Zhang, Y.; Zhang, Y. Competitive colorimetric triazophos immunoassay employing magnetic microspheres and multi-labeled gold nanoparticles along with enzymatic signal enhancement. Microchim. Acta 2017, 184, 3705–3712. [Google Scholar] [CrossRef]
- Wang, L.; Cai, J.; Wang, Y.; Fang, Q. A bare-eye-based lateral flow immunoassay based on the use of gold nanoparticles for simultaneous detection of three pesticides. Microchim. Acta 2014, 181, 1565–1572. [Google Scholar] [CrossRef]
- Fahimi-kashani, N.; Hormozi-nezhad, M.R. Gold nanoparticle-based colorimetric sensor array gold nanoparticle-based colorimetric sensor array for discrimination of organophosphate pesticides. Anal. Chem. 2016, 88, 8099–8106. [Google Scholar] [CrossRef]
- Kant, R. Surface plasmon resonance based fiber–optic nanosensor for the pesticide fenitrothion utilizing Ta2O5 nanostructures sequestered onto a reduced graphene oxide matrix. Microchim. Acta 2020, 187, 2–11. [Google Scholar] [CrossRef]
- Bakas, I.; Oujji, N.B.; Moczko, E.; Istamboulie, G.; Piletsky, S.; Piletska, E.; Ait-addi, E.; Ait-ichou, I.; Noguer, T.; Rouillon, R. Computational and experimental investigation of molecular imprinted polymers for selective extraction of dimethoate and its metabolite omethoate from olive oil. J. Chromatogr. A 2013, 1274, 13–18. [Google Scholar] [CrossRef]
- Lakshmi, K.; Kadirvelu, K.; Mohan, P.S. Rare earth metal functionalized electrospun nano fi ber catalyst for effective photo-decontamination of profenofos toxin. J. Ind. Eng. Chem. 2019, 80, 182–189. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Mikhail, W.Z.A.; Sobhy, H.M.; Radwan, E.M.M.; Salaheldin, T.A. Impact of nanosilver-profenofos on cotton leafworm, Spodoptera littoralis (Boisd.) larvae. Bull. Natl. Res. Cent. 2019, 43, 46. [Google Scholar] [CrossRef]
- Roth, M.; Richards, R.H.; Dobson, D.P.; Rae, G.H. Field trials on the efficacy of the organophosphorus compound azamethiphos for the control of sea lice (Copepoda: Caligidael infestations of farmed Atlantic salmon (Salmo salar). Aquaculture 1996, 140, 217–239. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Ahmad, S. Management strategies for control of stored grain insect pests in farmer stores and public ware houses. World J. Agric. Sci. 2011, 7, 527–549. [Google Scholar]
- Li, W.; Yan, X.; Gao, C.; Duan, J.; Beecham, S. A consecutive chlorination and alkaline hydrolysis process for rapid degradation and detoxication of malathion in aqueous solution. Chem. Eng. J. 2019, 392, 123793. [Google Scholar] [CrossRef]
- Dou, X.; Chu, X.; Kong, W.; Luo, J.; Yang, M. A gold-based nanobeacon probe for fluorescence sensing of organophosphorus pesticides. Anal. Chim. Acta 2015, 891, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Y.; Yun, X.; Luo, B.; Chen, C.; Wang, S.; Min, D. Multicolor colorimetric sensor for detection of omethoate based on the inhibition of the enzyme-induced metallization of gold nanorods. ACS Appl. Nano Mater. 2020, 6, 5212–5219. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gao, N.; Peng, Y.; Guo, C.; Lv, Z.; Wang, Y.; Zhou, C.; Ning, B.; Liu, M.; Gao, Z. Surface plasmon resonance sensor for profenofos detection using molecularly imprinted thin film as recognition element. Food Control 2012, 25, 543–549. [Google Scholar] [CrossRef]
- Tang, T.; Deng, J.; Zhang, M.; Shi, G.; Zhou, T. Quantum dot-DNA aptamer conjugates coupled with capillary electrophoresis: A universal strategy for ratiometric detection of organophosphorus pesticides. Talanta 2016, 146, 55–61. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Fiber optic profenofos sensor based on surface plasmon resonance technique and molecular imprinting. Biosens. Bioelectron. 2016, 79, 150–157. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-naggar, M.; Taha, M.; Nabil, S.; Youssef, M.A.; Awwad, N.S.; El Sayed, M.T. Designing a sensitive luminescent probe for organophosphorus insecticides detection based on post-synthetic modification of IRMOF-3. J. Mol. Struct. 2020, 1199, 1–9. [Google Scholar] [CrossRef]
- Bhasin, A.K.K.; Raj, P.; Chauhan, P.; Mandal, S.K.; Chaudhary, S.; Singh, N.; Kaur, N. Design and synthesis of a novel coumarin-based framework as a potential chemomarkerofaneurotoxic insecticide, azamethiphos. New J. Chem. 2020, 44, 3341–3349. [Google Scholar] [CrossRef]
- Abdollahi, M.; Mostafalou, S.; Pournourmohammadi, S.; Shadnia, S. Oxidative stress and cholinesterase inhibition in saliva and plasma of rats following subchronic exposure to malathion. Comp. Biochem. Physiol. Part C 2004, 137, 29–34. [Google Scholar] [CrossRef]
- Ambrosi, D.; Kearney, P.C.; Macchia, J.A. Persistence and metabolism of phosalone in soil. J. Agric. Food Chem. 1977, 25, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Westlake, G.E.; Bunyan, P.J.; Stanley, P.I. Variation in the response of plasma enzyme activities in avian species dosed with carbophenothion. Ecotoxicol. Environ. Saf. 1977, 2, 151–159. [Google Scholar] [CrossRef]
- Dryden, M.W.; Rust, M.K. The cat flea: Biology, ecology and control. Vet. Parasitol. 1994, 52, 1–19. [Google Scholar] [CrossRef]
- Madsen, H.F.; Bailex, B.J. Control of the apple aphid and the rosy apple aphid with new spray chemicals. J. Econ. Entomol. 1958, 52, 493–496. [Google Scholar] [CrossRef]
- Boesten, J.J.T.I.; Pas, L.J.T.V.D. Movement of water, bromide and the pesticides ethoprophos and bentazone in a sandy soil: The Vredepeel data set. Agric. Water Manag. 2000, 44, 21–42. [Google Scholar] [CrossRef]
- Scalisi, E.M.; Pecoraro, R.; Salvaggio, A.; Corsaro, A.; Messina, G.; Ignoto, S.; Lombardo, B.M.; Brundo, M.V. Evaluation of dimethoate toxicity on fertilization and on embryonic development of Paracentrotus lividus (Lamarck, 1816). Toxicol. Res. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Wojeck, G.A.; Nigg, H.N.; Stamper, J.H.; Bradway, D.E. Worker exposure to ethion in Florida citrus. Arch. Environm. Contain. Toxicol. 1981, 10, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-contreras, E.; Reyes, M.; Barros, W.; Sauphanor, B. Evaluation of azinphos-methyl resistance and activity of detoxifying enzymes in codling moth (Lepidoptera: Tortricidae) from central chile. J. Econ. Entomol. 2007, 100, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Leandro, C.C.; Hancock, P.; Fussell, R.J.; Keely, B.J. Comparison of ultra-performance liquid chromatography and high-performance liquid chromatography for the determination of priority pesticides in baby foods by tandem quadrupole mass spectrometry. J. Chromatogr. A 2006, 1103, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Salles, N.A.; Fourcade, F.; Geneste, F.; Floner, D.; Amrane, A. Relevance of an electrochemical process prior to a biological treatment for the removal of an organophosphorous pesticide, phosmet. J. Hazard. Mater. 2010, 181, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Vorkamp, K.; Kellner, E.; Taube, J.; Kai, D.M.; Herrmann, R. Fate of methidathion residues in biological waste during anaerobic digestion. Chemosphere 2002, 48, 287–297. [Google Scholar] [CrossRef]
- Meng, X.; Schultz, C.W.; Cui, C.; Li, X.; Yu, H. On-site chip-based colorimetric quantitation of organophosphorus pesticides using an office scanner. Sens. Actuators B. Chem. 2015, 215, 577–583. [Google Scholar] [CrossRef]
- Biswas, S.; Tripathi, P.; Kumar, N.; Nara, S. Gold nanorods as peroxidase mimetics and its application for colorimetric biosensing of malathion. Sens. Actuators B. Chem. 2016, 231, 584–592. [Google Scholar] [CrossRef]
- Albuquerque, C.D.L.; Poppi, R.J. Detection of malathion in food peels by surface-enhanced Raman imaging spectroscopy and multivariate curve resolution. Anal. Chim. Acta 2015, 879, 24–33. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, P.; Kumar, N.; Nara, S. Colorimetric sensing of malathion using palladium-gold bimetallic nanozyme. Biosens. Bioelectron. 2017, 92, 280–286. [Google Scholar] [CrossRef]
- Lina, Z.; Yujuan, C.; Bixia, L.; Shuhua, S.; Ying, Y.; Lingling, S. In-situ visual and ultrasensitive detection of phosmet using a fluorescent immunoassay probe. Sens. Actuators B. Chem. 2017, 241, 915–922. [Google Scholar] [CrossRef]
- Çakır, O.; Baysal, Z. Pesticide analysis with molecularly imprinted nanofilms using surface plasmon resonance sensor and LC-MS/MS: Comparative study for environmental water samples. Sens. Actuators B Chem. 2019, 297, 126–764. [Google Scholar] [CrossRef]
- Oliver, D.P.; Kookana, R.S.; Salama, R.B. Land use effects on sorption of pesticides and their metabolites in sandy soils. I. Fenamiphos and two metabolites, fenamiphos sulfoxide and fenamiphos sulfone, and fenarimol and azinphos methyl. Aust. J. soil Res. 2003, 41, 847–860. [Google Scholar] [CrossRef]
- Williamsona, V.M.; Husseyb, R.S. Nematode pathogenesis and resistance in plants. Plant Cell 1996, 8, 1735–1745. [Google Scholar] [CrossRef] [Green Version]
- Qu, F.; Zhou, X.; Xu, J.; Li, H.; Xie, G. Luminescence switching of CdTe quantum dots in presence of p-sulfonatocalix[4]arene to detect pesticides in aqueous solution. Talanta 2009, 78, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Han, C.; Li, H. Dual-signal fenamithion probe by combining fluorescence with colorimetry based on Rhodamine B modified silver nanoparticles. Analyst 2011, 136, 1351–1356. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Battisti, A.D.; Ferro, S.; Reyna, S.; López, M.C.; Quiro, M.A. Removal of the pesticide methamidophos from aqueous solutions by electrooxidation using Pb/PbO2, Ti/SnO2, and Si/BDD electrodes. Environ. Sci. Technol. 2008, 42, 6929–6935. [Google Scholar] [CrossRef] [PubMed]
- Meerdink, C.L. Organophosphorus and carbamate insecticide poisoning in large animals. Vet. Clin. N. Am. Food Anim. Pract. 1989, 5, 375–389. [Google Scholar] [CrossRef]
- Deng, S.; Chen, Y.; Wang, D.; Shi, T.; Wu, X.; Ma, X.; Li, X.; Hua, R.; Tang, X.; Li, Q.X. Rapid biodegradation of organophosphorus pesticides by Stenotrophomonas sp. G1. J. Hazard. Mater. 2015, 297, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Raghu, P.; Reddy, T.M.; Reddaiah, K.; Swamy, B.E.K.; Sreedhar, M. Acetylcholinesterase based biosensor for monitoring of malathion and acephate in food samples: A voltammetric study. Food Chem. 2014, 142, 188–196. [Google Scholar] [CrossRef]
- Bencic-nagale, S.; Sternfeld, T.; Walt, D.R. Microbead chemical switches: An approach to detection of reactive organophosphate chemical warfare agent vapors. J. Am. Chem. Soc. 2006, 128, 5041–5048. [Google Scholar] [CrossRef]
- Hulse, E.J.; Davies, J.O.J.; Simpson, A.J.; Sciuto, A.M.; Eddleston, M. Respiratory Complications of organophosphorus nerve agent and insecticide poisoning -implications for respiratory and critical care. Am. J. Respir. Crit. Care Med. 2014, 190, 1342–1354. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, A.C.M.; Snider, T.H.; Babin, C.; Jr, G.E.P.; Jett, D.A.; Yeung, D.T. Evaluating the broad-spectrum efficacy of the acetylcholinesterase oximes reactivators MMB4 DMS, HLo-7 DMS, and 2-PAMCl against phorate oxon, sarin, and VX in the hartley guinea pig. Neurotoxicology 2018, 68, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, L.; Bao, Y.; Xie, J. A simple, label-free AuNPs-based colorimetric ultrasensitive detection of nerve agents and highly toxic organophosphate pesticide. Biosens. Bioelectron. 2011, 28, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S. Chapter 41- Cumulative effects of organophosphorus or carbamate pesticides. Acad. Press 2006, 607–615. [Google Scholar] [CrossRef]
- Chu, X.; Başağaoğlu, H.; Marino, M.A.; Volker, R.E. Aldicarb transport in subsurface environment: Comparison of models. J. Environ. Eng. 2000, 126, 121–129. [Google Scholar] [CrossRef]
- Wang, Q.; Lemley, A.T. Oxidation of Carbaryl in Aqueous Solution by membrane anodic fenton treatment. J. Agric. Food Chem. 2002, 50, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Walgenbach, D.D.; Sutter, G.R. Degradation rates of technical carbofuran and a granular formulation in four soils with known insecticide use history. Bull. Environ. Contam. Toxicol. 1979, 574, 572–574. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, D.; Wu, M.; Wang, L.; Yang, J. Induction of time- and dose-dependent oxidative stress of triazophos to brain and liver in zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C 2020, 228, 108640. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.H.; Brown, M.J. Determination of carbofuran and 3-hydroxycarbofuran residues in small fruits. J. Agric. Food Chem. 1973, 21, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, T.M.; Poole, C.F. Extraction of Thiabendazole and Carbendazim from foods using pressurized hot (subcritical) water for extraction: A Feasibility Study. J. Agric. Food Chem. 1998, 46, 3124–3132. [Google Scholar] [CrossRef]
- Adiguzel, C.; Kalender, Y. Bendiocarb-induced nephrotoxicity in rats and the protective role of vitamins C and E. Environ. Sci. Pollut. Res. 2020, 27, 6449–6458. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Wang, L.; Hu, B.; Li, P.; Liu, F. Effect of hapten structures on specific and sensitive enzyme-linked immunosorbent assays for N-methylcarbamate insecticide metolcarb. Anal. Chim. Acta 2008, 5, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, F. Synthesis of CdTe quantum dots in sol-gel-derived composite silica spheres coated with Calix[4]arene as luminescent probes for pesticides. Chem. Mater. 2007, 5, 4148–4154. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, S.; Gui, W.; Zhu, G. Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Anal. Biochem. 2009, 389, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, E.; Calle, A.; Abad, A.; Montoya, A.; Hildebrandt, A.; Barcel, D.; Lechuga, L.M. Determination of carbaryl in natural water samples by a surface plasmon resonance flow-through immunosensor. Biosens. Bioelectron. 2006, 21, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Cui, Z.; Li, H. p-Amino benzenesulfonic acid functionalized gold nanoparticles: Synthesis, colorimetric detection of carbaryl and mechanism study by zeta potential assays. Sens. Actuators B. Chem. 2013, 183, 297–302. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, H.; Cai, J.; Duan, Y.; Liu, Y. Development of fluorescence sensing material based on cdse/zns quantum dots and molecularly imprinted polymer for the detection of carbaryl in rice and chinese cabbage. J. Agric. Food Chem. 2015, 63, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Cervera-chiner, L.; March, C.; Arnau, A. Detection of DDT and carbaryl pesticides in honey by means of immunosensors based on High Fundamental Frequency Quartz Crystal Microbalance ( HFF-QCM ). J. Sci. Food Agric. 2020, 100, 2468–2472. [Google Scholar] [CrossRef]
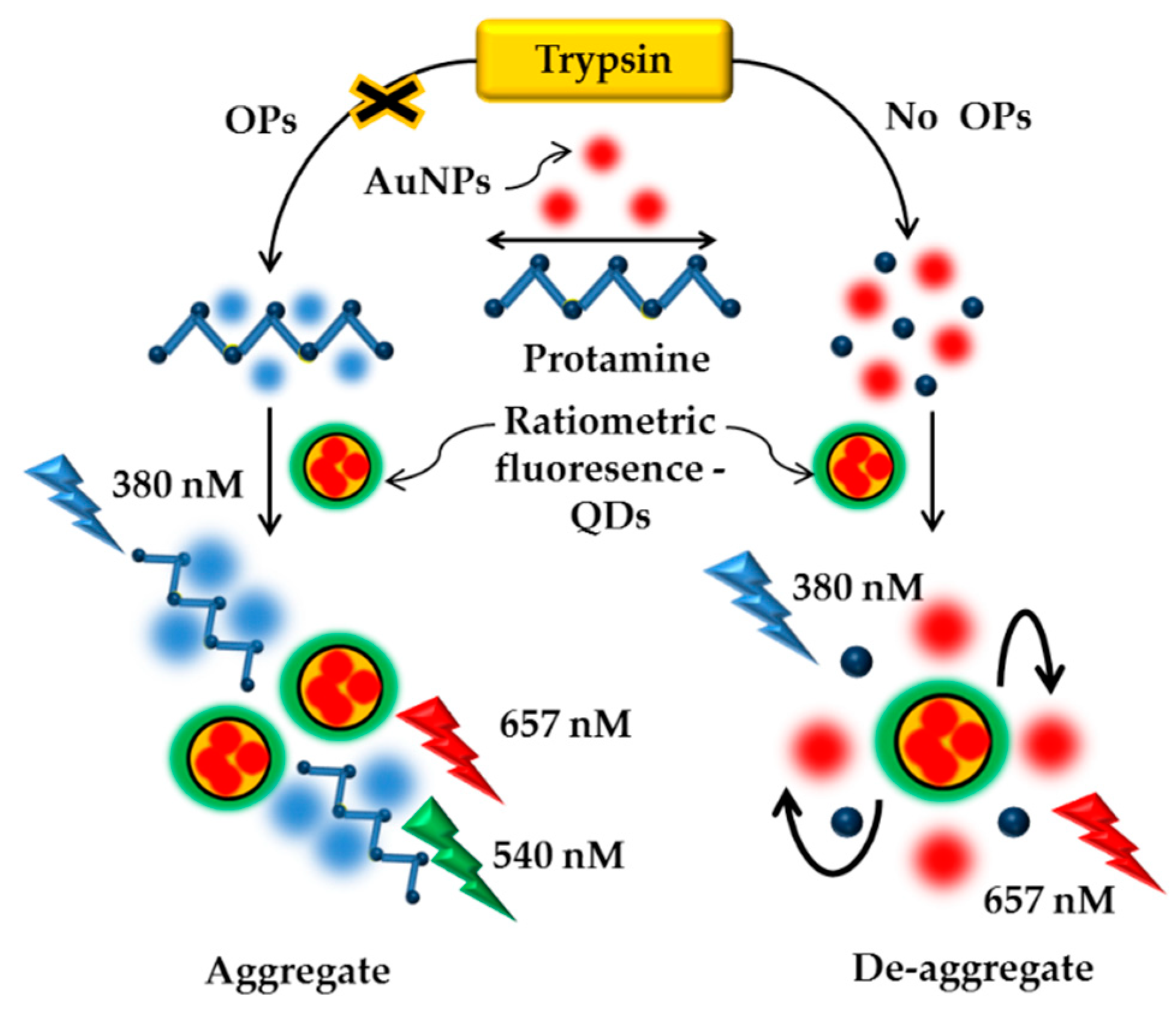
- Shahdost-fard, F.; Fahimi-Kashani, N.; Hormozi-nezhad, M.R. A Ratiometric fluorescence nanoprobe using CdTe QDs for fast detection of carbaryl insecticide in apple. Talanta 2020, 221, 121467. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, X.; Yuan, C.; Shi, R.; Wang, Y. Double responsive analysis of carbaryl pesticide based on carbon quantum dots and Au nanoparticles. Dyes Pigm. 2020, 181, 108529. [Google Scholar] [CrossRef]
- Minh, P.N.; Hoang, V.; Dinh, N.X.; Hoang, O.V.; Cuong, N.V.; Hop, D.T.B.; Tuan, T.Q.; Khi, N.T.; Huy, T.Q.; Le, A. Reduced graphene oxide-wrapped silver nanoparticles for applications to ultrasensitive colorimetric detection of Cr (VI) ions and carbaryl pesticide. New J. Chem. 2020, 44, 7611–7620. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, L.; Yang, L.; Cao, X.; Tian, D.; Li, H. Pesticide macroscopic recognition by a naphthol-appended calix[4]arene. Org. Lett. 2015, 17, 2976–2979. [Google Scholar] [CrossRef]
- Chen, X.; Lin, M.; Sun, L.; Xu, T.; Lai, K.; Huang, M.; Lin, H. Detection and quantification of carbendazim in Oolong tea by surface- enhanced Raman spectroscopy and gold nanoparticle substrates. Food Chem. 2019, 293, 271–277. [Google Scholar] [CrossRef]
- Li, Q.; Dou, X.; Zhao, X.; Zhang, L.; Luo, J.; Xing, X.; Yang, M. A gold/Fe3O4 nanocomposite for use in a surface plasmon resonance immunosensor for carbendazim. Microchim. Acta 2019, 186, 2–8. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoids insecticides toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2004, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Fogel, M.N.; Schneider, M.I.; González, B.; Desneux, N.; Ronco, A.E. Impact of the neonicotinoid acetamiprid on immature stages of the predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 2013, 22, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bishop, B.A.; Grafius, E.J. Inheritance and synergism of resistance to imidacloprid in the colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2000, 93, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Downing, H.F.; Delorenzo, M.E.; Fulton, M.H.; Scott, G.I.; Madden, C.J.; Kucklick, J.R. Effects of the agricultural pesticides atrazine, chlorothalonil, and endosulfan on south Florida microbial assemblages. Ecotoxicology 2004, 13, 245–260. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Shukla, R.; Sharma, T.K.; Bansal, V. Aptamer-controlled reversible inhibition of gold nanozyme activity for pesticide sensing. Anal. Chem. 2014, 86, 11937–11941. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, J.; Yang, X.; Jiang, D.; Du, X.; Wang, K.; Mao, H.; Wang, K. A facile label-free colorimetric aptasensor for acetamiprid based on the peroxidase-like activity of hemin-functionalized reduced graphene oxide. Biosens. Bioelectron. 2015, 65, 39–46. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Q.; Li, H.; Ouyang, Q.; Zhao, J. Fabricating a novel label-free aptasensor for acetamiprid by fluorescence resonance energy transfer between NH2-NaYF4:Yb, Ho@SiO2 and Au nanoparticles. Biosens. Bioelectron. 2016, 80, 398–404. [Google Scholar] [CrossRef]
- Lin, B.; Yu, Y.; Li, R.; Cao, Y.; Guo, M. Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer Bixia. Sens. Actuators B. Chem. 2016, 229, 100–109. [Google Scholar] [CrossRef]
- Xu, Q.; Du, S.; Jin, G.; Li, H.; Hu, X.Y. Determination of acetamiprid by a colorimetric method based on the aggregation of gold nanoparticles. Microchim. Acta 2011, 173, 323–329. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, G.; Liu, M.; Fan, L.; Cao, T. Aptamer-based colorimetric sensing of acetamiprid in soil samples: Sensitivity, selectivity and mechanism. J. Hazard. Mater. 2013, 260, 754–761. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Zheng, W.; Su, X. Visual and fluorescent detection of tyrosinase activity by using dual-emission ratiometric fluorescence probe. Anal. Chem. 2015, 87, 8904–8909. [Google Scholar] [CrossRef]
- Qi, Y.; Xiu, F.; Zheng, M.; Li, B. A simple and rapid chemiluminescence aptasensor for acetamiprid in contaminated samples: Sensitivity, selectivity and mechanism Yingying. Biosens. Bioelectron. 2016, 83, 243–249. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Sheng, Z.; Li, T.; Li, X. A colorimetric detection method of pesticide acetamiprid by fine-tuning aptamer length. Anal. Biochem. 2016, 513, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Chen, Y.; Xiu, F.; Hou, J. An aptamer-based colorimetric sensing of acetamiprid in environmental samples: Convenience, sensitivity and practicability. Sens. Actuators B. Chem. 2020, 304, 127359. [Google Scholar] [CrossRef]
- Hai, N.N.; Chinh, V.D.; Thuy, U.T.D.; Chi, T.K.; Yen, N.H.; Cao, D.T.; Liem, N.Q.; Nga, P.T. Detection of the pesticide by functionalised quantum dots as fluorescence-based biosensor. Int. J. Nanotechnol. 2013, 10, 137–145. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Lavaee, P.; Taghdisi, S.M. Aptamer based fluorometric acetamiprid assay using three kinds of nanoparticles for powerful signal amplification. Microchim. Acta 2016, 184, 81–90. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Shi, H.; Yang, X. Development of a bi-enzyme tracer competitive enzyme-linked immunosorbent assay for detection of thiacloprid and imidaclothiz in agricultural samples. Food Chem. 2014, 164, 166–172. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, Y.; Wang, M.; Chen, X.; Wang, B.; Li, Q.X. Ultrasensitive quantitation of imidacloprid in vegetables by colloidal gold and time-resolved fluorescent nanobead traced lateral fl ow immunoassays. Food Chem. 2020, 311, 126055. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Zhang, L.; Zhu, P.; Ge, S.; Yu, J. Paper-based SERS sensing platform based on 3D silver dendrites and molecularly imprinted identifier sandwich hybrid for neonicotinoids quantification. ACS Appl. Mater. Interfaces 2020, 12, 8845–8854. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef]
- Wengatz, I.; Stoutamire, D.W.; Gee, S.J.; Hammock, B.D. Development of an enzyme-linked immunosorbent assay for the detection of the pyrethroid insecticide fenpropathrin. J. Agric. Food Chem. 1998, 46, 2211–2221. [Google Scholar] [CrossRef]
- Fetoui, H.; Makni, M.; Garoui, E.M.; Zeghal, N. Toxic effects of lambda-cyhalothrin, a synthetic pyrethroid pesticide, on the rat kidney: Involvement of oxidative stress and protective role of ascorbic acid. Exp. Toxicol. Pathol. 2010, 62, 593–599. [Google Scholar] [CrossRef]
- Gammon, D.W.; Leggett, M.F.; Clark, J.M. Pyrethroid mode(s) of action in the context of food quality protection act (FQPA) regulation. J. Agric. Food Chem. 2011, 59, 2773–2785. [Google Scholar] [CrossRef]
- Shi, X.; Gu, A.; Ji, G.; Li, Y.; Di, J.; Jin, J.; Hu, F.; Long, Y.; Xia, Y.; Lu, C.; et al. Chemosphere developmental toxicity of cypermethrin in embryo-larval stages of zebrafish. Chemosphere 2011, 85, 1010–1016. [Google Scholar] [CrossRef]
- Ogoma, S.B.; Ngonyani, H.; Simfukwe, E.T.; Mseka, A.; Moore, J.; Killeen, G.F. Spatial repellency of transfluthrin-treated hessian strips against laboratory-reared Anopheles arabiensis mosquitoes in a semi-field tunnel cage. Parasites Vectors 2012, 54, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Y.; Cheng, J. Molecularly imprinted silica nanospheres embedded CdSe quantum dots for highly selective and sensitive optosensing of pyrethroids. Chem. Mater. 2010, 22, 2451–2457. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, H.; Shen, H.; Pan, J.; Dai, X.; Yan, Y.; Pan, G.; Sellergren, B. Molecularly imprinted fluorescent hollow nanoparticles as sensors for rapid and efficient detection λ-cyhalothrin in environmental water. Biosens. Bioelectron. 2016, 85, 387–394. [Google Scholar] [CrossRef]
- Wei, X.; Hao, T.; Xu, Y.; Lu, K.; Li, H.; Yan, Y.; Zhou, Z. Facile polymerizable surfactant inspired synthesis of fluorescent molecularly imprinted composite sensor via aqueous CdTe quantum dots for highly selective detection of λ-cyhalothrin. Sens. Actuators B. Chem. 2016, 224, 315–324. [Google Scholar] [CrossRef]
- Ren, X.; Chen, L. Quantum dots coated with molecularly imprinted polymer as fluorescence probe for detection of cyphenothrin. Biosens. Bioelectron. 2015, 64, 182–188. [Google Scholar] [CrossRef]
- Xiao, T.; Shi, X.; Jiao, H.; Sun, A.; Ding, H.; Zhang, R. Selective and sensitive determination of cypermethrin in fish via enzyme-linked immunosorbent assay-like method based on molecularly imprinted artificial antibody-quantum dot optosensing materials. Biosens. Bioelectron. 2016, 75, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Dyk, J.S.V.; Pletschke, B. Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere 2011, 82, 291–307. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Fukuto, T.R. DDT substitutes. Crit. Rev. Environ. Sci. Technol. 1973, 3, 25–29. [Google Scholar] [CrossRef]
- Bann, J.M.; Decino, T.J.; Earle, N.W.; Sun, Y.P. Pesticide metabolism, fate of aldrin and dieldrin in the animal body. J. Agric. Food Chem. 1956, 4, 937–941. [Google Scholar] [CrossRef]
- Magni, P.A.; Pazzi, M.; Vincenti, M.; Converso, V.; Dadour, I.R. Development and validation of a method for the detection of α- and β-Endosulfan (organochlorine insecticide) in Calliphora vomitoria (Diptera: Calliphoridae). J. Med. Entomol. 2018, 55, 51–58. [Google Scholar] [CrossRef]
- Matsumura, F.; Boush, G.M. Dieldrin: Degradation by soil microorganisms. Science 1967, 156, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, D.; Xu, X.; Ying, Y.; Li, Y.; Ye, Z.; Wang, J. Immunosensors for detection of pesticide residues. Biosens. Bioelectron. 2008, 23, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Kubackova, J.; Fabriciova, G.; Miskovsky, P.; Jancura, D.; Sanchez-Cortes, S. Sensitive Surface-Enhanced Raman Spectroscopy (SERS) detection of organochlorine pesticides by alkyl dithiol-functionalized metal nanoparticles-induced plasmonic hot spots. Anal. Chem. 2015, 87, 663–669. [Google Scholar] [CrossRef]


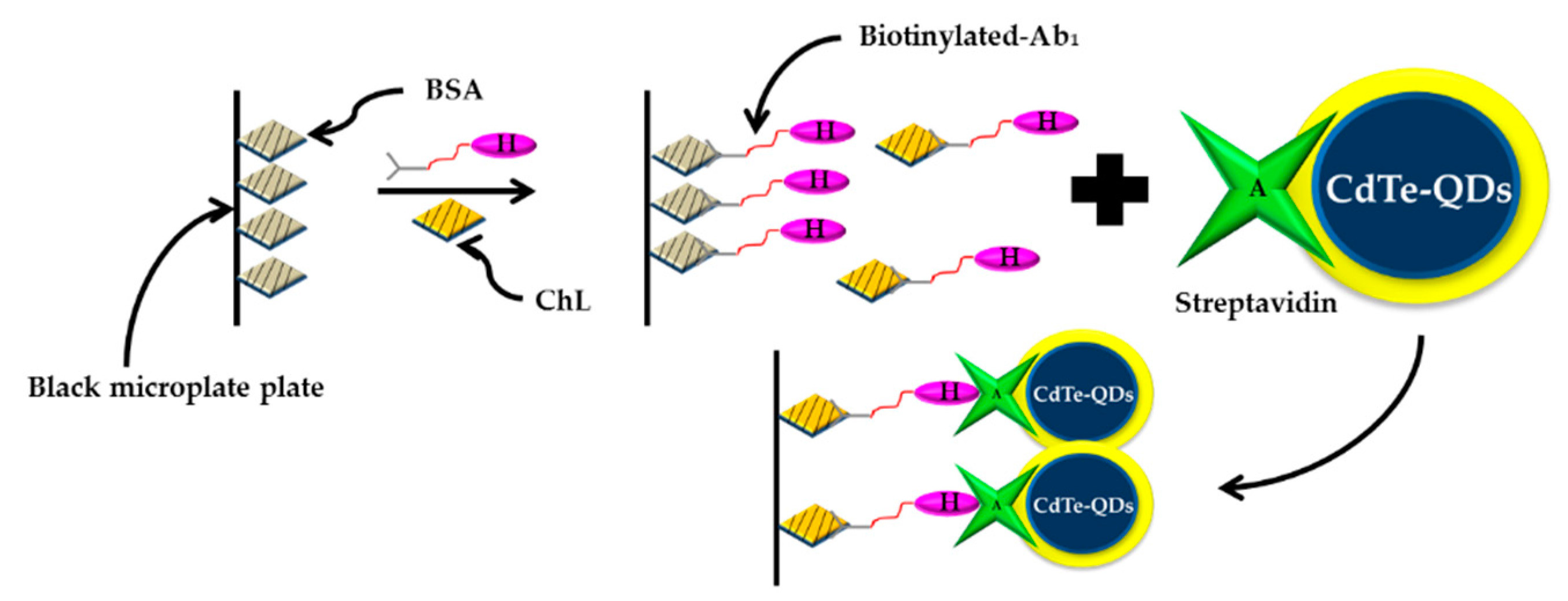

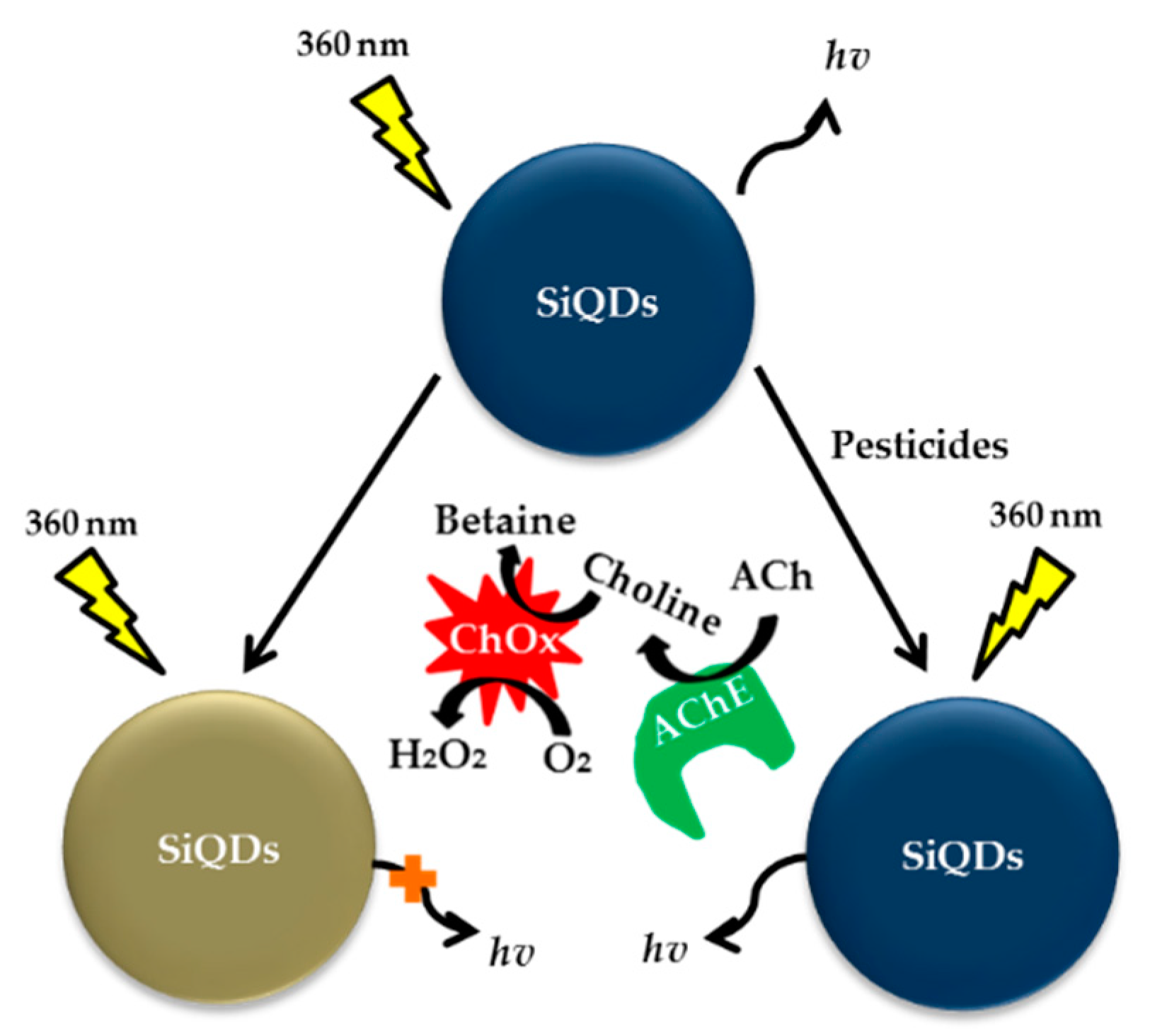
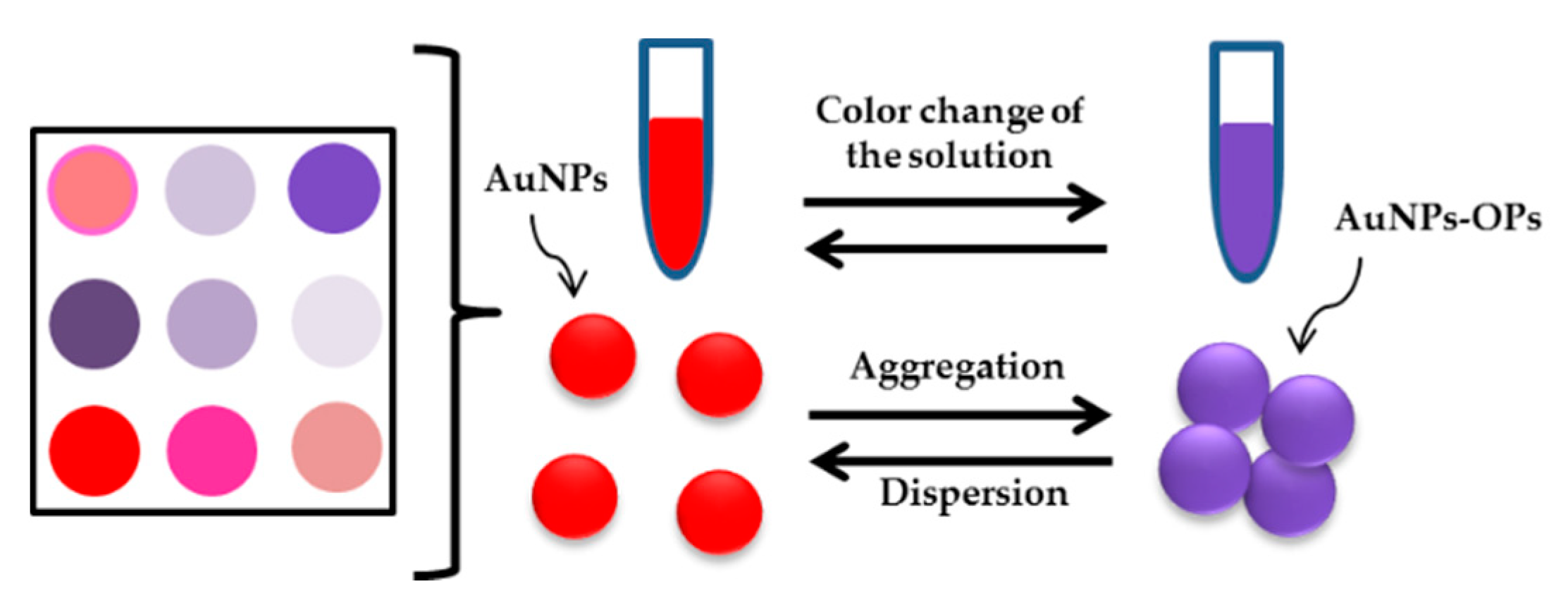


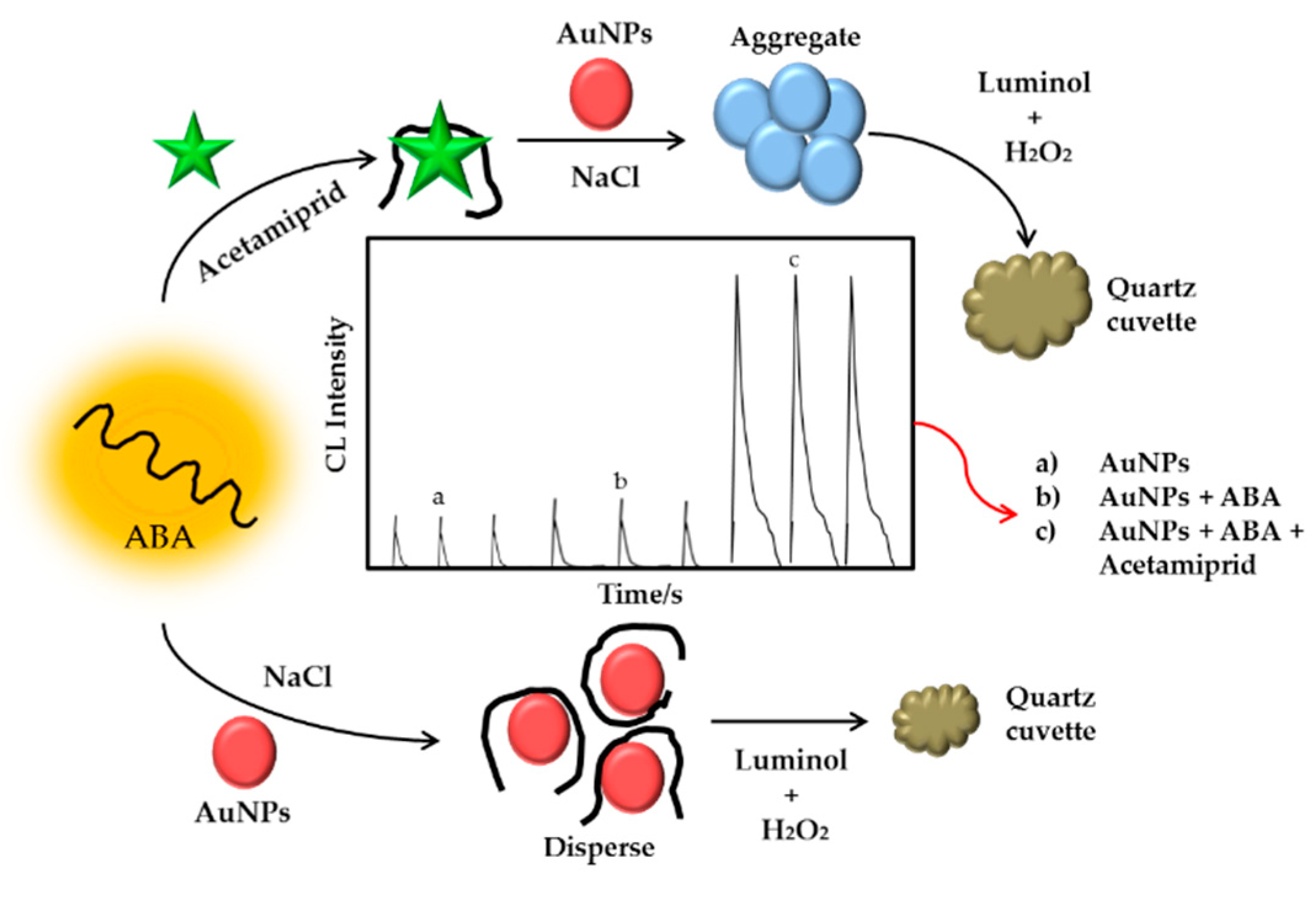
| Type of Phosphates | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Paraoxon | Photoluminescence | chitosan-CdSeQDs/OPH | 0–1.000 µM | 1.000 µM | 2003 | [119] |
| Paraoxon | Photoluminescence | (CdSe)ZnSQDs-OPH | 0.010–10.000 µM | 0.010 µM | 2005 | [120] |
| Paraoxon | Colorimetric | IPA/sol-gel derived silica inks-AChE | 0–10.000 µM | 1.000 nM | 2009 | [121] |
| Mevinphos | Fluorescence | CdTe/CdS coreshell QDs | 0.892–124.917 µM | 0.714 µM | 2010 | [146] |
| Paraoxon | Fluorescence | CdTeQDs-AChE | - | 2.750 pM | 2011 | [134] |
| Dichlorvos | 2.090 pM | |||||
| Paraoxon | Fluorescence | PAH/CdTeQDs-AChE | - | 0.011 nM | 2011 | [122] |
| Dichlorvos | Colorimetric | indoxyl acetate-R-DmAChE | 4.525 pM–0.453 µM | 0.136 µM | 2012 | [137] |
| Paraoxon | Fluorescence | AuNPs/DDAO-AChE | - | 0.400 μM | 2012 | [123] |
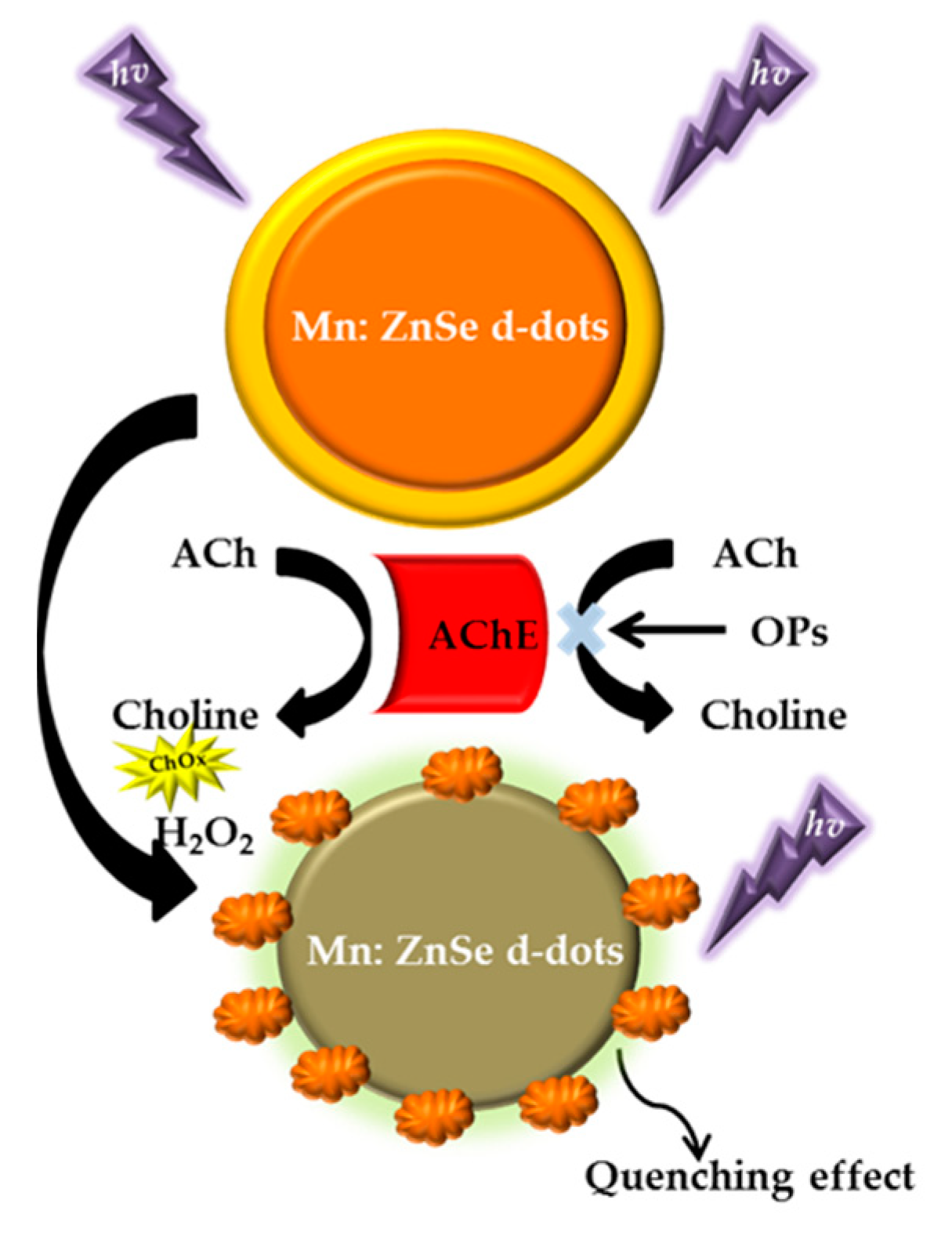
| Paraoxon | Phosphorescence | Mn:ZnSe d-dots–H2O2-AChE | - | 0.013 nM | 2012 | [124] |
| Methyl-paraoxon | Colorimetric | Fe3O4/MNP-AChE/CHOx | - | 10.000 nM | 2013 | [140] |
| Paraoxon | Colorimetric | ATCl/AuNPs-AChE | 3.634–363.376 nM | 3.634 nM | 2013 | [126] |
| Dichlorvos | Fluorescence | QDs-AChE/ChOx | 4.490–6780 nM | 4.490 nM | 2013 | [135] |
| Tetrachlorvinphos | Colorimetric | oxyferryl-HRP | - | 0.200 µM | 2014 | [147] |
| Paraoxon-ethyl | Fluorescence | Mn-ZnS nanocrystal/AChE | 0–100.000 µM | 1.800 µM | 2014 | [143] |
| Paraoxon | Phosphorescence | Mn-ZnSQDs/AChE | - | 0.100 pM | 2014 | [125] |
| Monocrotophos | Fluorescence | NaYF4:Yb,Er/UCNPs-AChE | 0.009–89.606 nM | 10.305 nM | 2015 | [148] |
| Paraoxon | Fluorescence | CdTeQDs | - | 4.300 pM | 2016 | [131] |
| Methyl-paraoxon | Colorimetric | PAA-CeO2/AChE | - | 0.108 µM | 2016 | [141] |
| Dichlorvos | 0.035 M | |||||
| Paraoxon | Fluorescence | TPyP-CdTeQDs | 9.090 pM–1.090 µM | 3.150 pM | 2016 | [132] |
| Parathion | Fluorescence | QDs-AChE | - | 3.433 nM | 2016 | [127] |
| Paraoxon | Fluorescence | QDs-AChE/ChOx | 0.001–0.01 µM | 0.050 µM | 2017 | [136] |
| Dibrom | ||||||
| Diclorvos | ||||||
| Paraoxon-ethyl | Fluorescence | carbon dots | - | 0.220 ± 0.020 µM | 2017 | [145] |
| Paraoxon | Fluorescence | carbon quantum dots-AChE/CHOx | 0.182–181.688 nM | 0.182 nM | 2017 | [128] |
| Paraoxon | Fluorometric | gold nanocrystal-TYR | - | 0.363 nM | 2017 | [133] |
| Diclorvos | Photoluminescence | graphene quantum dots-AChE/ChOx | 0.453–45.253 µM | 0.778 µM | 2018 | [142] |
| Methyl-paraoxon | 0.340 µM | |||||
| Paraoxon-ethyl | Colorimetric and fluorometric | monoclonal antibody-BChE | 0–7.170 nM | 0.100 nM | 2018 | [144] |
| Paraoxon | Fluorescence | carbon dots-AChE | 0–1.817 nM | 0.472 nM | 2018 | [129] |
| Diclorvos | Fluorescence | QDs-nanoporphyrin | 45.253–90.506 nM | 45.253 nM | 2019 | [139] |
| Paraoxon | Fluorescence | BSPOTPE-SiO2-MnO2-AChE | 3.634–362.358 nM | 3.634 nM | 2019 | [130] |
| Paraoxon | LC–MS/MS | AChE | - | 5.087 nM | 2020 | [138] |
| Diclorvos | 23.021 pM |
| Type of Phosphonates | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Trichlorfon | Fluorescence | Mn-doped ZnSNCs/AChE | 0–100.000 µM | 1.800 µM | 2014 | [143] |
| Dipterex | Chemiluminescence | Lum-AgNP | 0–80.134 µM | 80.134 µM | 2015 | [151] |
| Trichlorfon | Fluorescence | 1, 8-naphthalimide/AChE-ChOx | - | 0.018 nM | 2016 | [152] |
| Trichlorfon | SERS | AuNPs | 0.388–27.978 µM | 3.885 µM | 2019 | [153] |
| Type of Phosphorothiotes (s =) | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Chlorpyrifos | SPR | monoclonal antibody | 0.051–0.154 nM | 0.143 nM | 2007 | [185] |
| Chlorpyrifos | cFLISA | CdTe/QDs-streptavidin | - | 10.839 nM | 2010 | [183] |
| Chlorpyrifos | Fluorescence | QDs/TCP | 0–2.852 nM | 2.852 nM | 2010 | [181] |
| Chlorpyrifos | Fluorescence | CdTeQDs | 0.100–10.000 nM | 0.100 nM | 2010 | [182] |
| Chlorpyrifos | cFLISA | QDs/antibody | 43.355–586.155 nM | 143.187 nM | 2010 | [184] |
| Diazinon | Fluorescence | (CdTe/CdS) QDs | 0.329–98.571 µM | 0.394 µM | 2010 | [146] |
| Methyl parathion | SERS | AuNPs/mono-6-thio-b-cyclodextrin with mono-6-thio-b-cyclodextrin | - | 0.300 µM | 2010 | [177] |
| Parathion | Fluorescence | PAH/CdTeQDs-AChE | 0.100–1.000 µM | 0.011 nM | 2011 | [122] |
| Parathion | Fluorescence | CdTeQDs-ChOx/AChE | - | 0.001 nM | 2011 | [134] |
| Diazinon | Fluorescence | QDs-MIP | 0.164–1.971 µM | 0.164 µM | 2012 | [167] |
| Methyl parathion | Fluorescence | CdSe/CdTeQDs-AChE | 0.190–37.992 µM | 0.190 µM | 2012 | [166] |
| Methyl parathion | Photoluminescence | CdSe/ZnSe 2MI/ZnS 8MI-QDs-AChE | 0.190–30.394 nM | 0.190 µM | 2012 | [137] |
| Parathion Diazinon | Photoluminescence | SiQDs-AChE/ChOx | 25.712 pM–2.571 µM 24.610 pM–2.461 µM | 0.112 nM 0.222 nM | 2013 | [195] |
| Methyl parathion | Electrochemiluminescence | GNs-CdTe/QDs-AChE | 0–0.570 µM | 0.228 nM | 2013 | [168] |
| Chlorpyrifos | SPR | Fe3O4@PDA NPs-AChE | 0.001–10.000 µM | 0.760 nM | 2013 | [186] |
| Chlorpyrifos | Fluorescence | Mn-ZnS NCs | 0–100.000 µM | 1.800 µM | 2014 | [143] |
| Methyl parathion | Fluorescence | CdTeQDs/ CTAB-OPH | - | 68.386 nM | 2014 | [170] |
| Chlorpyrifos-methyl | LFIA | monoclonal antibody | 0.310–18.605 µM | 0.310 µM | 2014 | [201] |
| Methyl parathion | Fluorescence | NaYF4:Yb,Er/UCNPs-AChE | - | 2.545 pM | 2015 | [148] |
| Methyl parathion | Fluorescence | tyrosinase-carbon dots | 0.100 nM–0.100 mM | 0.048 nM | 2015 | [174] |
| Methyl parathion | Fluorescence | CdTeQDs- Trypsin | - | 68.386 pM | 2015 | [171] |
| Chlorpyrifos | Chemiluminescent | Lum-AgNP | 0–68.456 µM | 68.456 µM | 2015 | [151] |
| Methyl parathion | Fluorescence | CuInS2QDs-Pb2+ | 0.100–38.000 µM | 0.060 µM | 2015 | [175] |
| Methyl parathion | Chemiluminescence | bispecific monoclonal antibody-HRP/ALP | - | 1.254 nM | 2015 | [172] |
| Methyl parathion | Colorimetric | gold nanorods-AChE | 0.120–40.000 pM | 0.039 pM | 2015 | [169] |
| Chlorpyrifos Primophos-methyl | Colorimetric | AuNPs | - | 0.685 µM 0.786 µM | 2016 | [202] |
| Parathion | Fluorescence | CdTeQDs | - | 4.300 pM | 2016 | [131] |
| Methyl parathion Chlorpyrifos | SERS | AuNPs | 0–9.878 nM 0–7.416 nM | 9.878 nM 7.416 nM | 2016 | [189] |
| Diazinon | Fluorescence | AIE/TPE-maleimide-AChE | 0.986–1.643 nM | 1.643 nM | 2016 | [196] |
| Methyl parathion | Fluorescence | 1, 8-naphthalimide-AChE/ChOx | - | 1.277 pM | 2016 | [152] |
| Trizophos Methyl parathion | Colorimetric | Antibody/AuNPs | - | 0.064 nM 3.115 nM | 2016 | [198] |
| Parathion | Fluorescence | QDs-AChE | - | 3.433 µM | 2016 | [127] |
| Parathion | Fluorescence | [Cd(atc)(H2O)2]n (NMOF1)-anti parathion | 3.433 nM–3.433 µM | 3.433 nM | 2016 | [193] |
| Trizophos | Colorimetric | GAA-MPA-AuNPs | 0.500–500.000 µM | 0.080 µM | 2016 | [199] |
| Chlorpyrifos | SERS | popcorn like AuNPs | 1.000–6.250 µM | 1.000 µM | 2017 | [190] |
| Methyl parathion | Fluorescence | MOS2-QDs | - | 0.3229 µM | 2017 | [176] |
| Methyl parathion | Chemiluminescent | bifunctional antibody- HRP/ALP | - | 0.220 nM | 2017 | [173] |
| Parathion | Fluorescence | CdSe/ZnS QDs-AChE/ChOx | - | 0.050 µM | 2017 | [136] |
| Trizophos | Colorimetric | AuNPs /mcAbs | - | 0.045 nM | 2017 | [200] |
| Chlorpyrifos | Fluorescence | g-C3N4/AuNPs-AChE | - | 6.900 pM | 2018 | [180] |
| Chlorpyrifos | Colorimetric and chemiluminescent | dual-g-C3N4/BiFeO3 | - | 0.930 nM | 2018 | [192] |
| Chlorpyrifos | SPR | TiO2/P3HT/AuNPs | 0.010–16.000 µM | 7.500 nM | 2018 | [187] |
| Chlorpyrifos | SPR | staphylococcal protein A | 0.713–142.617 nM | 15.973 nM | 2019 | [188] |
| Chlorpyrifos Coumaphos | SERS | AuNPs | 0.003–28.523 µM 0.002–27.566 µM | 28.523 µM 0.002 µM | 2019 | [153] |
| Diazinon | Fluorescence | UCNPs/Cu2+- AChE | 0.033–164.285 nM | 0.164 nM | 2019 | [197] |
| Demeton | Fluorescence | QDs-nanoporphyrin | 38.709–77.418 nM | 38.709 nM | 2019 | [139] |
| Chlorpyrifos | LC–MS/MS | AChE | - | 37.080 nM | 2020 | [138] |
| Methyl parathion | SERS | silver nanoparticles-Al2O3 | - | 1.000 fM | 2020 | [178] |
| Chlorpyrifos | SERS | silver nitrate | 1.000 mM–1.000 nM | 1.000 nM | 2020 | [191] |
| Methyl parathion | SERS | AuNPs | - | 0.004 µM | 2020 | [179] |
| Fenirothion | SPR | tantalum(V) oxide nanoparticles | 0.250–4.000 μM | 0.038 µM | 2020 | [203] |
| Type of Phosphorothioates (S-Substituted) | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Omethoate | Colorimetric | indoxyl acetate-R-DmAChE | 4.691–46.907 µM | 29.551 µM | 2012 | [137] |
| Profenofos | SPR | SAM/ 2,2-azobis (2-amidinopropane) hydrochloride | 0.003–0.268 nM | 0.964 pM | 2012 | [213] |
| Omethoate | Fluorescence | gold-based nanobeacon | 0.268–26.800 µM | 2.350 µM | 2015 | [210] |
| Profenofos | SPR | MIPs-Ag | - | 0.007 nM | 2016 | [212] |
| Profenofos | Fluorescence | CdTe/CdS-QDs (AMO-aptamer) | - | 0.100 µM 0.230 µM | 2016 | [214] |
| Omethoate | ||||||
| Malaoxon | Fluorescence | QDs-AChE/ChOx | - | 0.050 µM | 2017 | [136] |
| Omethoate | Colorimetric | silver metallization of AuNRs-ALP | - | 0.390 nM | 2020 | [211] |
| Profenofos | Luminescent | Eu-IRMOF-3-EBA | - | 0.002 nM | 2020 | [215] |
| Azamethiphos | Fluorescence | 8-((E)-((thiophen-2-yl)methylimino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one (L) to copper (II) ion | 0–50.000 µM | 50.000 µM | 2020 | [216] |
| Type of Phosphorodithioates | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Malathion | Colorimetric | IPA/sol-gel derived silica inks-AChE | 0–10.000 µM | 0.001 µM | 2009 | [121] |
| Malathion | SERS | MCR-WALS | 0.372–37.232 µM | 0.372 µM | 2015 | [231] |
| Malathion | Colorimetric | dithiobis-nitrobenzoic acid-AChE | 0–24.216 µM | 0.303 µM | 2015 | [229] |
| Dimethoate | Fluorescence | NaYF4:Yb,Er/UCNPs-AChE | 0.009–87.237 nM | 0.292 nM | 2015 | [148] |
| Malathion | Colorimetric | gold nanorods- HRP | 0.003–1.816 mM | 0.005 mM | 2016 | [230] |
| Malathion | Colorimetric | palladium-gold | 0.003–605.404 nM | 181.621 nM | 2017 | [232] |
| Malathion | Fluorescence | QDs-AChE/ChOx | 0.001–0.100 µM | 0.05 µM | 2017 | [137] |
| Phosmet | Fluorescence | PDs/Ab based poly [2-methoxy-5-(2-ethylhexyloxy)-1, 4-(1-cyanovinylene-1, 4-phenylene)] | 0.007–0.126 nM | 0.126 nM | 2017 | [233] |
| Carbophenothion | SERS | AuNPs | 0.003–29.167 µM | 0.292 µM | 2019 | [153] |
| Malathion | 0.003–30.270 µM | 0.327 µM | ||||
| Phosalone | 0.003–27.188 µM | 2.717 µM | ||||
| Phosmet | 0.003–31.515 µM | 0.315 µM | ||||
| Dimethoate | Fluorescence | QDs-nanoporphyrin | 0.044–0.630 µM | 0.044 µM | 2019 | [139] |
| Dimethoate | SPR | P(EGDMA-MATrp) | 0.040–4.360 nM | 0.033 nM | 2019 | [234] |
| Ethion | Luminescent | Eu-IRMOF-3-ethylbenzoylacetate | - | 0.003 nM | 2020 | [215] |
| Type of Phosphoramidates | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Fenamithion | Luminescence | CdSeQDs -p-sulfonatocalix[4]arene | 0–100.000 µM | 0.012 µM | 2009 | [237] |
| Fenamithion | Fluorescence and colorimetric | RB-AgNPs | - | 10.000 nM | 2011 | [238] |
| Fenamiphos | Colorimetric | AuNPs | - | 0.247 µM | 2016 | [202] |
| Type of Phosphoramidothioates | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Methamidophos | Colorimetric | indoxyl acetate-R-DmAChE | - | 223.955 µM | 2012 | [136] |
| Acephate | Colorimetric | Fe3O4(MNP)-AChE/ChOx | - | 0.005 mM | 2013 | [140] |
| Isocarbophos | LFIA | monoclonal antibody | 0.346–20.740 µM | 0.346 µM | 2014 | [201] |
| Isocarbophos | Fluorescence | gold-based nanobeacon | - | 0.035 µM | 2015 | [210] |
| Acephate | Fluorescence | 1, 8-naphthalimide-AChE/ChOx | - | 0.006 nM | 2016 | [152] |
| Isocarbophos | Fluorescence | CdTe/CdS-QDs(aptamer) | - | 0.170 µM | 2016 | [214] |
| Type of Phosphonofluoridates | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Sarin | Colorimetric | LA-AUNPs-AChE | 28.200–225.000 pM | 28.200 pM | 2011 | [246] |
| Sarin | Colorimetric | Fe2O3-MNPs-AChE | - | 1.000 nM | 2013 | [140] |
| Type of Carbamates | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Carbaryl | SPR | SAM/mAbs | - | 6.858 mM | 2005 | [258] |
| Carbaryl | SPR | mAbs | 0.089–0.268 nM | 0.248 nM | 2007 | [185] |
| Methomyl | Fluorescence | C[4]/SiO2/CdTe | - | 0.080 µM | 2007 | [256] |
| Carbofuran | LFIA | anti-carbofuran | 0–128.000 µM | 32.000 µM | 2009 | [257] |
| Trizophos | anti-trizophos | 0–32.000 µM | 4.000 µM | |||
| Bendiocarb | Colorimetric | idophenyl acetate-AChE | 0–10.000 nM | 0.001 µM | 2009 | [121] |
| Carbaryl | 10.000 nM | |||||
| Carbofuran | Colorimetric | indoxyl acetate-R-DmAChE | 0.005–45.197 µM0.006–61.648 µM | 27.118 µM | 2012 | [137] |
| Methomyl | 1.726 µM | |||||
| Carbaryl | Photoluminescence | SiQDs-AChE/ChOx | 0.037–3722.29 nM | 0.037 nM | 2013 | [195] |
| Carbaryl | Colorimetric | PABSA-AuNPs | 0.100 nM–1 mM | 0.250 µM | 2013 | [259] |
| Carbaryl | Fluorescence | CdSe/ZnSQDs | - | 0.147 µM | 2015 | [260] |
| Metolcarb | Fluorescence | NOC4 | 0.100 nM–1.000 mM | 0.100 µM | 2015 | [265] |
| Carbaryl | Chemiluminescent | Lum-AgNP | 0–4.970 µM | 4.970 µM | 2015 | [151] |
| 0–108.472 µM | 108.472 µM | |||||
| Carbofuran | ||||||
| Carbaryl | Colorimetric and chemiluminescent | dual-g-C3N4/BiFeO3 | 0.005–0.298 µM | 0.164 nM | 2018 | [192] |
| Carbofuran | SERS | AuNPs | 0.005–45.197 µM | 0.904 µM | 2019 | [153] |
| 0.006–61.648 µM | 0.123 µM | |||||
| Methomyl | ||||||
| Carbendazim | SERS | AuNPs | 0–52.305 µM | 0.523 µM | 2019 | [266] |
| Carbofuran | SPR | P(EGDMA-MATrp) | - | 0.032 nM | 2019 | [234] |
| Carbendazim | SPR | AuNPs- Fe3O4/mAbs | 0.262–784.572 nM | 2.301 nM | 2019 | [267] |
| Aldicarb Carbofuran Carbofuran-3 hydroxy Carbaryl | Liquid Chromatography Tandem Mass Spectrometry | AChE | - | 0.039 µM | 2020 | [138] |
| - | 0.007 µM | |||||
| - | 5.901 pM | |||||
| - | 0.007 µM | |||||
| Carbaryl | HFF-QCM | mAbs | 0.497 pM–4.970 nM | 0.248 nM | 2020 | [261] |
| Carbaryl | Fluorescence | CdTeQDs | - | 0.596 nM | 2020 | [262] |
| Carbaryl | Fluorescence | CQDs-AuNPs-AChE | 0.994–745.453 nM | 0.298 nM | 2020 | [263] |
| Carbaryl | Colorimetric | silver reduced-graphene oxide | 0.100–50.000 μM | 42.000 nM | 2020 | [264] |
| Type of Neonicotinoids | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Acetamiprid | Luminescence | CdTeQDs and p sulfonatocalix[4]arene | 0–1000.000 µM | 0.034 µM | 2009 | [237] |
| Acetamiprid | Colorimetric | AuNPs | 0.660–6.600 µM | 0.044 µM | 2011 | [277] |
| Acetamiprid | Fluorescence | CdSe/ZnSe/ZnS QDs-AChE | 0.225–44.910 nM | 4.491 nM | 2013 | [283] |
| Acetamiprid | Colorimetric | AuNPs-ABA | 0.075–7.500 µM | 0.005 µM | 2013 | [278] |
| Acetamiprid | Colorimetric | AuNPs | 0.449–44.910 µM | 0.449 µM | 2014 | [273] |
| Thiacloprid | ELISA | Anti-thiacloprid | 0–0.723 µM | 0.017 µM | 2014 | [285] |
| Imidacloprid | Anti-imidacloprid | 0–0.228 µM | 0.008 µM | |||
| Acetamiprid | Fluorescence | AuNPs | 0.112–22.455 µM | 75.448 µM | 2014 | [279] |
| Imidacloprid | Chemiluminescence | bispecific monoclonal antibody-HRP/ALP | - | 0.001 µM | 2015 | [172] |
| Acetamiprid | Colorimetric | hemin-reduced graphene oxide | 0.100–10.000 µM | 40.000 nM | 2015 | [274] |
| Acetamiprid | Fluorescence | NH2-NaYF4: Yb, Ho@SiO2/UCNPs/GNPs | - | 0.003 µM | 2016 | [275] |
| Acetamiprid | Fluorescence | ZnS:Mn-aptamer and MWCNTs | 0–150.000 nM | 0.700 nM | 2016 | [276] |
| Acetamiprid | Fluorescence | AuNPs/SWNTs/SiNPs-streptavidin | 0–1000.000 nM | 127.000 pM | 2016 | [284] |
| Imidacloprid | Colorimetric | AuNPs-AChE | 0.156–1.565 µM | 0.939 µM | 2016 | [202] |
| Acetamiprid | Chemiluminescence | AuNPs | 0.800 nM–0.630 µM | 62.000 pM | 2016 | [280] |
| Acetamiprid | Colorimetric | AuNPs | 0–50.000 µM | 0.400 µM | 2016 | [281] |
| Imidacloprid | Chemiluminescent | bispecific antibody-HRP/ALP | - | 0.227 nM | 2017 | [173] |
| Acetamiprid | Colorimetric | AuNPs | - | 0.560 nM | 2020 | [282] |
| Imidacloprid | LFIA | monoclonal antibody | 0–78.229 pM | 78.229 pM | 2020 | [286] |
| Imidacloprid | SERS | AgNPs | 0–0.110 nM | 0.110 nM | 2020 | [287] |
| Type of Pyrethroids/Pyrethrins | Method | Material | Range of Detection | LOD | Year | References |
|---|---|---|---|---|---|---|
| Cyhalothrin | Fluorescence | MIPs-CdSe/SiO2 | 0.100–1000.000 µM | 0.101 µM | 2010 | [294] |
| Cyphenothrin | Fluorescence | MIPs-Mn doped ZnS QDs | 0.100–80.000 µM | 9.000 µM | 2014 | [297] |
| Cypermethrin | ELISA | MIPs-QDs | - | 0.003 µM | 2015 | [298] |
| Cyhalothrin | Fluorescence | SiO2-MIPs | 0–1.500 µM | 10.260 nM | 2016 | [295] |
| Cyhalothrin | Fluorescence | OVDAC/CdTeQDs | 0.100–16.000 µM | 0.030 µM | 2016 | [296] |
| Permethrin | SERS | AuNPs | 0.002–25.557 µM | 0.002 µM | 2019 | [153] |
| Transfluthrin | 0.002–26.943 µM | 2.694 µM |
| Type of Organochlorines | Methods | Materials | LOD | Year | References |
|---|---|---|---|---|---|
| DDT | SPR | anti-DDT monoclonal antibody (LIB-DDT5.25) | 0.141 nM | 2007 | [185] |
| Dieldrin | SERS | alkyl dithiol-functionalized metal nanoparticles-induced plasmonic hot spots | 0.123 µM | 2015 | [306] |
| Aldrin | 0.418 µM | ||||
| Endosulfan | 3.534 µM | ||||
| Lindane | 0.830 µM | ||||
| DDT | HFF-QCM | anti-DDT monoclonal antibody | 0.068 nM | 2020 | [261] |
| Optical Method | Insecticides | Materials | LOD | References |
|---|---|---|---|---|
| Fluorescence | Dichlorvos | CdTeQDs-AChE | 0.0021 nM | [134] |
| Colorimetric | Sarin | lipoic acid-AuNPs-AChE | 0.0282 nM | [246] |
| SERS | Imidacloprid | AgNPs | 0.1100 nM | [287] |
| SPR | Carbofuran | P(EGDMA-MATrp) | 0.0320 nM | [234] |
| Chemiluminescence | Acetamiprid | AuNPs | 0.0620 nM | [280] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Hashim, H.S. Recent Advances on Detection of Insecticides Using Optical Sensors. Sensors 2021, 21, 3856. https://doi.org/10.3390/s21113856
Fauzi NIM, Fen YW, Omar NAS, Hashim HS. Recent Advances on Detection of Insecticides Using Optical Sensors. Sensors. 2021; 21(11):3856. https://doi.org/10.3390/s21113856
Chicago/Turabian StyleFauzi, Nurul Illya Muhamad, Yap Wing Fen, Nur Alia Sheh Omar, and Hazwani Suhaila Hashim. 2021. "Recent Advances on Detection of Insecticides Using Optical Sensors" Sensors 21, no. 11: 3856. https://doi.org/10.3390/s21113856





