Whole-Body Movements Increase Arm Use Outcomes of Wrist-Worn Accelerometers in Stroke Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
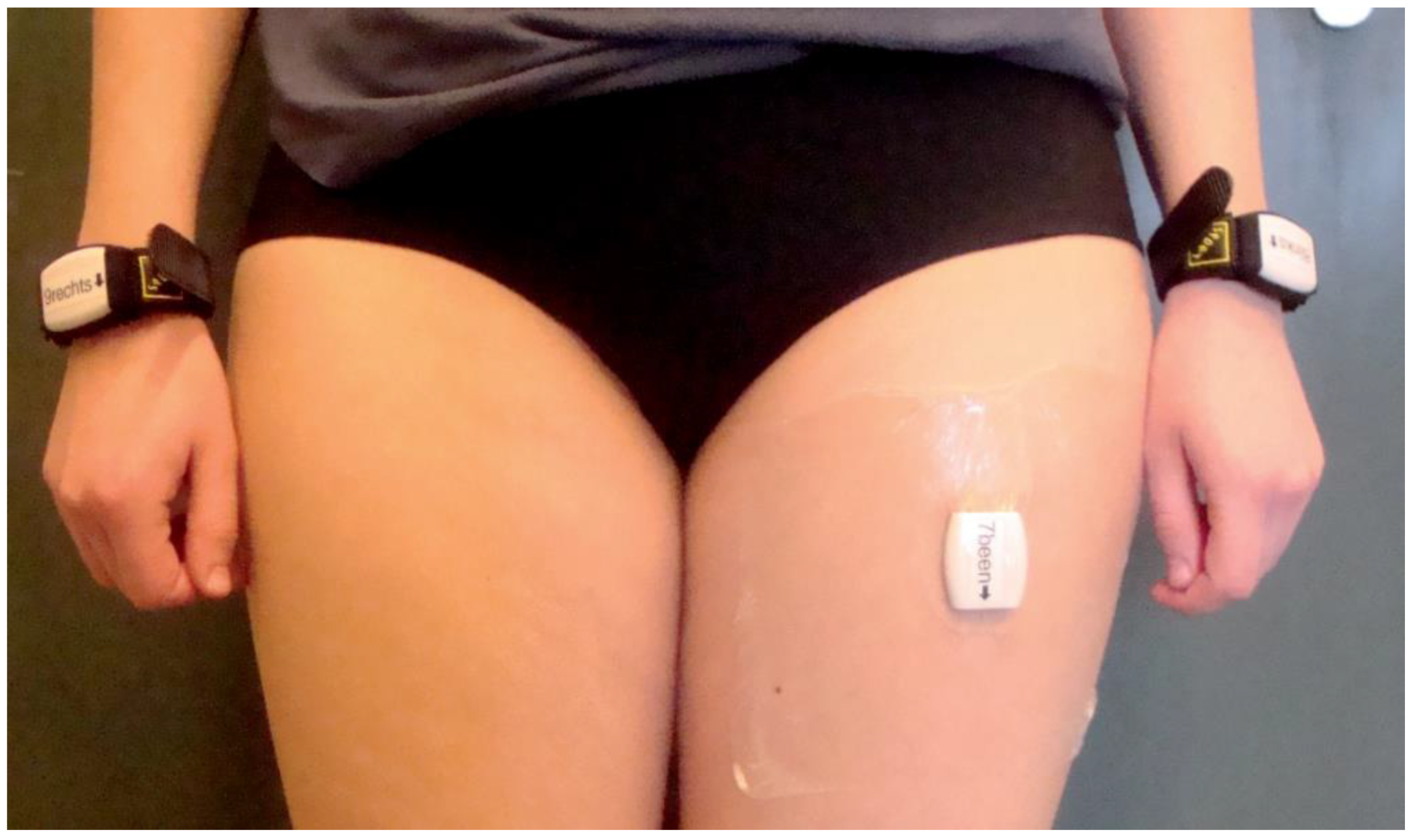
2.3. Arm Use Measurements
2.4. Analysis of Sensor Data
- Paretic arm use: Calculated by summing the movement counts of the sensor on the paretic arm over all 30 s epochs.
- Ratio between arms: Calculated as the paretic arm use during all whole-body postures and movements divided by the nonparetic arm use during all whole-body postures and movements.
- Nonparetic arm use: Calculated by summing the movement counts of the sensor on the nonparetic arm over all 30 s epochs.
- Paretic arm use: Calculated by summing the movement counts of the sensor on the paretic arm over all 30 s epochs of which the posture was sitting or standing. An epoch was classified as sitting or standing when at least 90% of the 48 samples of the leg sensor were classified as sitting or standing.
- Ratio between arms: Calculated as the paretic arm use during sitting and standing divided by the nonparetic arm use during sitting and standing.
- Nonparetic arm use: Calculated by summing the movement counts of the sensor on the nonparetic arm over all 30 s epochs classified as sitting or standing.
2.5. Statistical Analysis
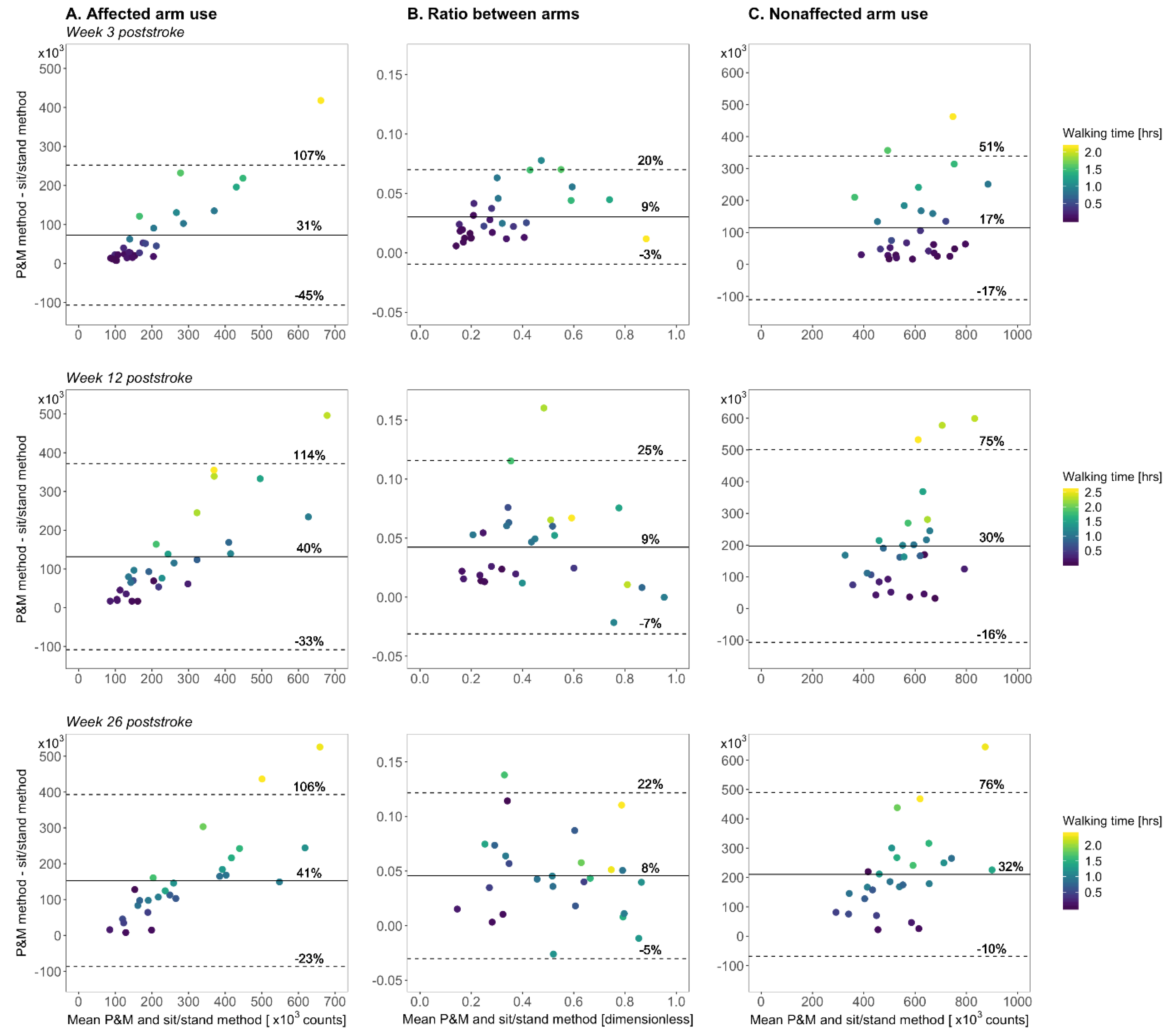
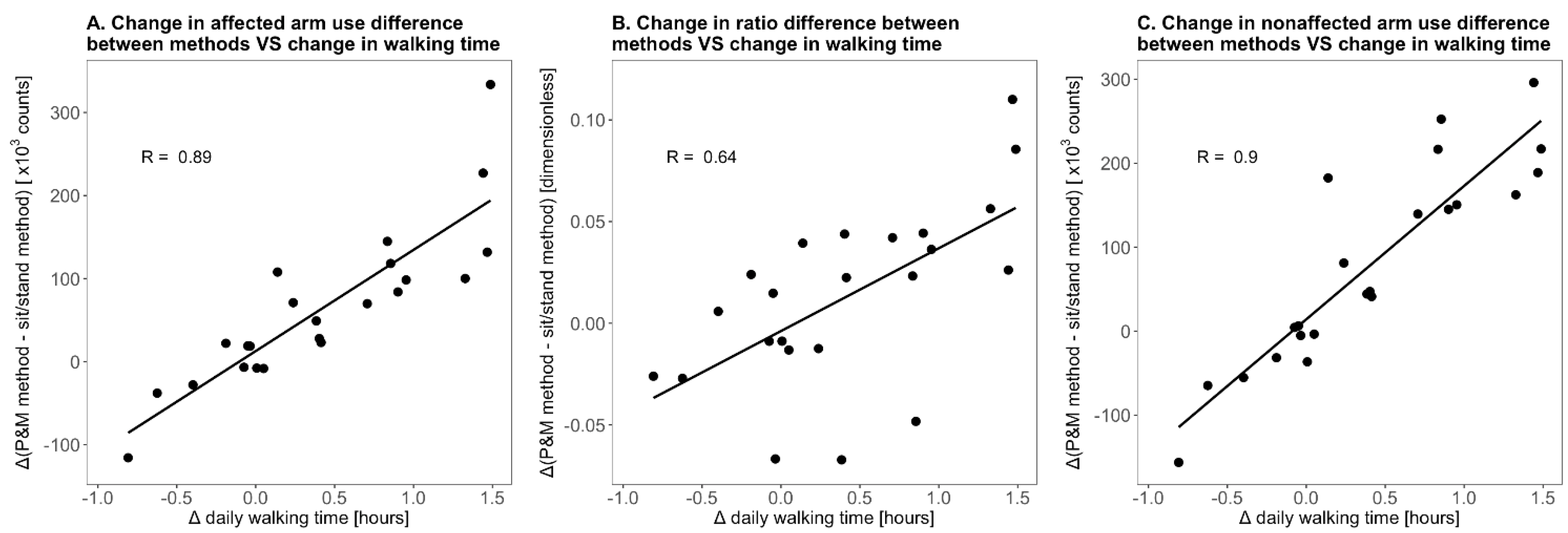
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwakkel, G.; Veerbeek, J.M.; Wegen, E.E.H.V.; Wolf, S.L. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015, 14, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Michielsen, M.E.; De Niet, M.; Ribbers, G.; Stam, H.J.; Bussmann, J.B. Evidence of a logarithmic relationship between motor capacity and actual performance in daily life of the paretic arm following stroke. J. Rehabil. Med. 2009, 41, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doman, C.A.; Waddell, K.J.; Bailey, R.R.; Moore, J.L.; Lang, C.E. Changes in Upper-Extremity Functional Capacity and Daily Performance During Outpatient Occupational Therapy for People with Stroke. Am. J. Occup. Ther. 2016, 70, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Waddell, K.J.; Strube, M.J.; Tabak, R.G.; Haire-Joshu, D.; Lang, C.E. Upper Limb Performance in Daily Life Improves Over the First 12 Weeks Poststroke. Neurorehabilit. Neural Repair 2019, 33, 836–847. [Google Scholar] [CrossRef]
- Lang, C.E.; Wagner, J.M.; Edwards, D.F.; Dromerick, A.W. Upper Extremity Use in People with Hemiparesis in the First Few Weeks After Stroke. J. Neurol. Phys. Ther. 2007, 31, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, M.E.; Selles, R.W.; Stam, H.J.; Ribbers, G.; Bussmann, J.B. Quantifying Nonuse in Chronic Stroke Patients: A Study Into Paretic, Nonparetic, and Bimanual Upper-Limb Use in Daily Life. Arch. Phys. Med. Rehabil. 2012, 93, 1975–1981. [Google Scholar] [CrossRef]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. Quantifying Real-World Upper-Limb Activity in Nondisabled Adults and Adults with Chronic Stroke. Neurorehabilit. Neural Repair 2015, 29, 969–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Niet, M.; Bussmann, J.B.; Ribbers, G.; Stam, H.J. The Stroke Upper-Limb Activity Monitor: Its Sensitivity to Measure Hemiplegic Upper-Limb Activity During Daily Life. Arch. Phys. Med. Rehabil. 2007, 88, 1121–1126. [Google Scholar] [CrossRef]
- Chin, L.F.; Hayward, K.; Soh, A.J.A.; Tan, C.M.; Wong, C.J.R.; Loh, J.W.; Loke, G.J.H.; Brauer, S. An accelerometry and observational study to quantify upper limb use after stroke during inpatient rehabilitation. Physiother. Res. Int. 2019, 24, e1784. [Google Scholar] [CrossRef]
- Bailey, R.R.; Lang, C.E. Pt Upper-limb activity in adults: Referent values using accelerometry. J. Rehabil. Res. Dev. 2013, 50, 1213–1222. [Google Scholar] [CrossRef]
- Moore, S.A.; Da Silva, R.; Balaam, M.; Brkic, L.; Jackson, D.; Jamieson, D.; Ploetz, T.; Rodgers, H.; Shaw, L.; Van Wijck, F.; et al. Wristband Accelerometers to motiVate arm Exercise after Stroke (WAVES): Study protocol for a pilot randomized controlled trial. Trials 2016, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Held, J.P.O.; Luft, A.R.; Veerbeek, J.M. Encouragement-Induced Real-World Upper Limb Use after Stroke by a Tracking and Feedback Device: A Study Protocol for a Multi-Center, Assessor-Blinded, Randomized Controlled Trial. Front. Neurol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fanchamps, M.; Selles, R.; Stam, H.; Bussmann, J. Development and validation of a clinically applicable arm use monitor for people after stroke. J. Rehabil. Med. 2018, 50, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Uswatte, G.; Foo, W.L.; Olmstead, H.; Lopez, K.; Holand, A.; Simms, L.B. Ambulatory Monitoring of Arm Movement Using Accelerometry: An Objective Measure of Upper-Extremity Rehabilitation in Persons with Chronic Stroke. Arch. Phys. Med. Rehabilitation 2005, 86, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Regterschot, G.R.H.; Bussmann, J.B.J.; Fanchamps, M.H.J.; Meskers, C.G.M.; Ribbers, G.M.; Selles, R.W. Objectively measured arm use in daily life improves during the first six months poststroke: A longitudinal observational cohort study. J. NeuroEng. Rehabil. 2021, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [Green Version]
- Franck, J.A.; Smeets, R.J.E.M.; Seelen, H.A.M. Changes in arm-hand function and arm-hand skill performance in patients after stroke during and after rehabilitation. PLoS ONE 2017, 12, e0179453. [Google Scholar] [CrossRef] [Green Version]
- Franck, J.A.; Halfens, J.; Smeets, R.; Seelen, H. Concise Arm and Hand Rehabilitation Approach in Stroke (CARAS): A practical and evidence-based framework for clinical rehabilitation management. Open J. Occup. Ther. 2015, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Fanchamps, M.H.J.; Horemans, H.L.D.; Ribbers, G.M.; Stam, H.J.; Bussmann, J.B.J. The Accuracy of the Detection of Body Postures and Movements Using a Physical Activity Monitor in People after a Stroke. Sensors 2018, 18, 2167. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 4 November 2020).
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Salazar, A.; Ojeda, B.; Dueñas, M.; Fernández, F.; Failde, I. Simple generalized estimating equations (GEEs) and weighted generalized estimating equations (WGEEs) in longitudinal studies with dropouts: Guidelines and implementation in R. Stat. Med. 2016, 35, 3424–3448. [Google Scholar] [CrossRef]
- Højsgaard, S.; Halekoh, U.; Yan, J.; Ekstrøm, C. Generalized Estimating Equation Package. CRAN Repository, 2019. Available online: https://cran.r-project.org (accessed on 4 November 2020).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Estimated Marginal Means, Aka Least-Squares Means. CRAN Repository, 2020. Available online: https://cran.r-project.org (accessed on 4 November 2020).
- Domholdt, E. Physical Therapy Research: Principles and Applications; Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Desrosiers, J.; Malouin, F.; Bourbonnais, D.; Richards, C.L.; Rochette, A.; Bravo, G. Arm and leg impairments and disabilities after stroke rehabilitation: Relation to handicap. Clin. Rehabil. 2003, 17, 666–673. [Google Scholar] [CrossRef]



| Age in Years | 55.9 ± 9.2 [37–75] |
| Gender | 26 males, 7 females |
| Affected body side | 12 left side, 21 right side |
| Dominant side affected | 11 (33%) |
| Admitted to rehabilitation clinic in weeks poststroke | 1.6 ± 0.7 [0.4–3.0] |
| Discharge from a rehabilitation clinic in weeks poststroke | 10.5 ± 4.7 [3.7–20.3] |
| NIHSS a values week 12 poststroke | 2.1 ± 2.7 [0–11] |
| Fugl-Meyer upper extremity assessment: | |
| week 3 poststroke | 25.5 ± 20.6 [4–64] |
| week 12 poststroke | 41.2 ± 21.8 [4–64] |
| week 26 poststroke | 51.2 ± 16.8 [9–64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regterschot, G.R.H.; Selles, R.W.; Ribbers, G.M.; Bussmann, J.B.J. Whole-Body Movements Increase Arm Use Outcomes of Wrist-Worn Accelerometers in Stroke Patients. Sensors 2021, 21, 4353. https://doi.org/10.3390/s21134353
Regterschot GRH, Selles RW, Ribbers GM, Bussmann JBJ. Whole-Body Movements Increase Arm Use Outcomes of Wrist-Worn Accelerometers in Stroke Patients. Sensors. 2021; 21(13):4353. https://doi.org/10.3390/s21134353
Chicago/Turabian StyleRegterschot, Gerrit Ruben Hendrik, Ruud W. Selles, Gerard M. Ribbers, and Johannes B. J. Bussmann. 2021. "Whole-Body Movements Increase Arm Use Outcomes of Wrist-Worn Accelerometers in Stroke Patients" Sensors 21, no. 13: 4353. https://doi.org/10.3390/s21134353






