Modular Data Acquisition System for Recording Activity and Electrical Stimulation of Brain Tissue Using Dedicated Electronics
Abstract
:1. Introduction
- Recover samples of data recorded by each hardware channel from the multiplexed input.
- Provide experiment control mechanisms.
- Efficiently store sampled data as well as any other information that could be useful from any further data analysis perspective (e.g., markers of an experimental paradigm).
- Visualise data according to the experimental conditions and user’s preferences.
- Present hardware architecture of the custom-designed electronics that can be used in electrophysiological experiments with a living brain.
- Show in-depth organisation of the DAQ software accentuating challenges and applied solutions.
- Discuss system performance regarding the quality of signal processing of the ASIC, software stability and resource management.
- Render preliminary results of data acquisition with living rat brains.
- Provide an outline of possible future experiments and software enhancements enabling more sophisticated paradigms and real-time data processing.
- Hardware architecture of the system and electric signal processing pipeline logic are presented in Section 2.1 and Section 2.2.
- DAQ software design including real-time data visualisation is described in Section 2.3.
- DAQ tests in terms of signal processing performance and biological experiment methodologies are outlined in Section 2.4 and Section 2.5 respectively.
- Section 3 is devoted to the presentation and discussion of the obtained results regarding software (Section 3.1) and Neurostim-3 ASIC basic signal processing capabilities (e.g., gain, noise, filter bandwidth; Section 3.2). Additionally, Section 3.3 provides examples from ongoing electrophysiological experiments performed using the presented system.
- Our findings and plans for further system enhancements are summarised in Section 4.
2. Materials and Methods
2.1. Architecture of the System
2.2. System Hardware
- Custom-designed elements:
- −
- ASIC Boards (ABs) housing Neurostim-3 ASICs and MEAs’ connectors.
- −
- Interface Board (IB) which distributes output signals and power among up to eight Neurostim-3 ASICs (currently available IB can handle two ASICs, but extension to the full system requires replication of a few IB elements only) and allows simple debugging procedures.
- Commercially available hardware supporting operation of IB and ABs:
- −
- −
2.2.1. Neurostim-3
- Set global ASIC parameter e.g., output current range, analogue multiplexer timing.
- Set per-channel parameter e.g., enable/disable electrical stimulation.
- Prepare each channel to drive current through an electrode and record signals based on the contents of the Real-Time Data Command.
2.2.2. ASIC Board
- Neurostim-3 ASIC under copper shield.
- Connector allowing usage of MEAs. Since there are many vendors of MEAs using different sockets [24,25], there are several versions of ABs to maintain compatibility with them. MEA becomes an interface between animal brain and Neurostim-3 ASIC. When dipped into the neural tissue, MEAs sample surrounding electric potential distribution changes due to the nearby cell population activity. They can also be used to output current driven by Neurostim-3 ASIC to artificially stimulate neurons.
- Analogue-Digital Converter (ADC) [26] converting multiplexed Neurostim-3 output data to its digital 12bit representation.
- Low-Voltage Differential Signalling (LVDS) [27] chip providing communication with the IB.
2.2.3. Interface Board
2.3. Software Design
- Removes risks of data acquisition process failure due to any Data Consumer application error.
- Allows remote experiment control from many sites; moreover, one might want to run computationally extensive data analysis in real-time that is not possible to be performed using a single computer but might be split into smaller tasks operating on subsets of data on many machines, thus creating a computing cluster.
- GPIB [23] provides remote control of the PSU.
- Logger gathers all information about the current state of each process and saves it for further investigation in simple text files.
- Data Player is meant to replay past experiment dataset files without the need for external tools. This way, one can pass the name of the dataset file and specific time range and then examine the data using Visualisation Client.
- System Monitor keeps track of all processes created by the system, monitors their resource utilisation and provides means of changing process priority and affinity for better performance.
- ASIC control Finite State Machine Monitor is a utility that in real-time tries to evaluate Output Bitstream contents to keep track of internal state registers of the ASICs. This tool comes in handy because no hardware mechanism would allow polling Neurostim-3 ASIC about its current state (Input Bitstream represents values sampled by each ASIC channel only).
- Setup Manager is a simple database-like file, keeping values of all critical parameters. Its instance is shared by all system processes, including Data Consumers.
2.3.1. In-Depth Data Processing Pipeline Overview
- No protocol—the device would operate in the read-only mode, which means it would only sample incoming signals with output current circuitry disabled.
- Pulse Libraries—user provides two MATLAB (MathWorks, Natick, USA) files [35], one of them keeps definitions of current pulse shape and amplitude, the second is a table telling when each channel should output given pulse.
- Square Wave—for testing purposes, it is beneficial to be able to output a continuous standard signal of arbitrary frequency to be applied on a subset of channels. Currently, both time and amplitude symmetrical square continuous wave pattern is supported.
- Prebuilt Experiment—this is a single file holding description of stimulation protocol and pulses used in JavaScript Object Notation (JSON) [36] format. Such a file is always created when the new experiment has been scheduled successfully and saved alongside the output dataset file.
- Archiver—using Hierarchical Data Format (HDF5) file format [40] stores all Simulation & Data chunks with the system Setup Data on the permanent storage device as dataset file. These files can be used for offline data analysis by external tools or become an input for the Data Player. It is possible to enable gzip [41] data compression, which is especially useful when only a few Neurostim-3 ASICs are enabled to reduce the amount of data produced during the experiment.
- TCP/UDP Data Servers—allow external Data Consumers to connect to the stream of data. In the case of the TCP connection, Data Consumer may specify that it does not require data from all channels, thus optimising network connection load.
2.3.2. Visualisation Client
- When using TCP protocol Visualisation Client must provide the Server Application a list of channels that user is interested in. After a connection is established, the TCP Data Server sends this client only a part of the Simulation & Data corresponding to the requested channels, thus reducing the network load.
- UDP multicast transmission does not require any direct communication with the Server Application. UDP Data Client tries to collect as many packets as possible to build whole Simulation & Data chunk. All packets are indexed so that the client knows which packets were possibly dropped (if any). In the case of undelivered packets output, the data chunk would have holes. Only then data from the user required channels are extracted.
2.3.3. Graphical User Interface
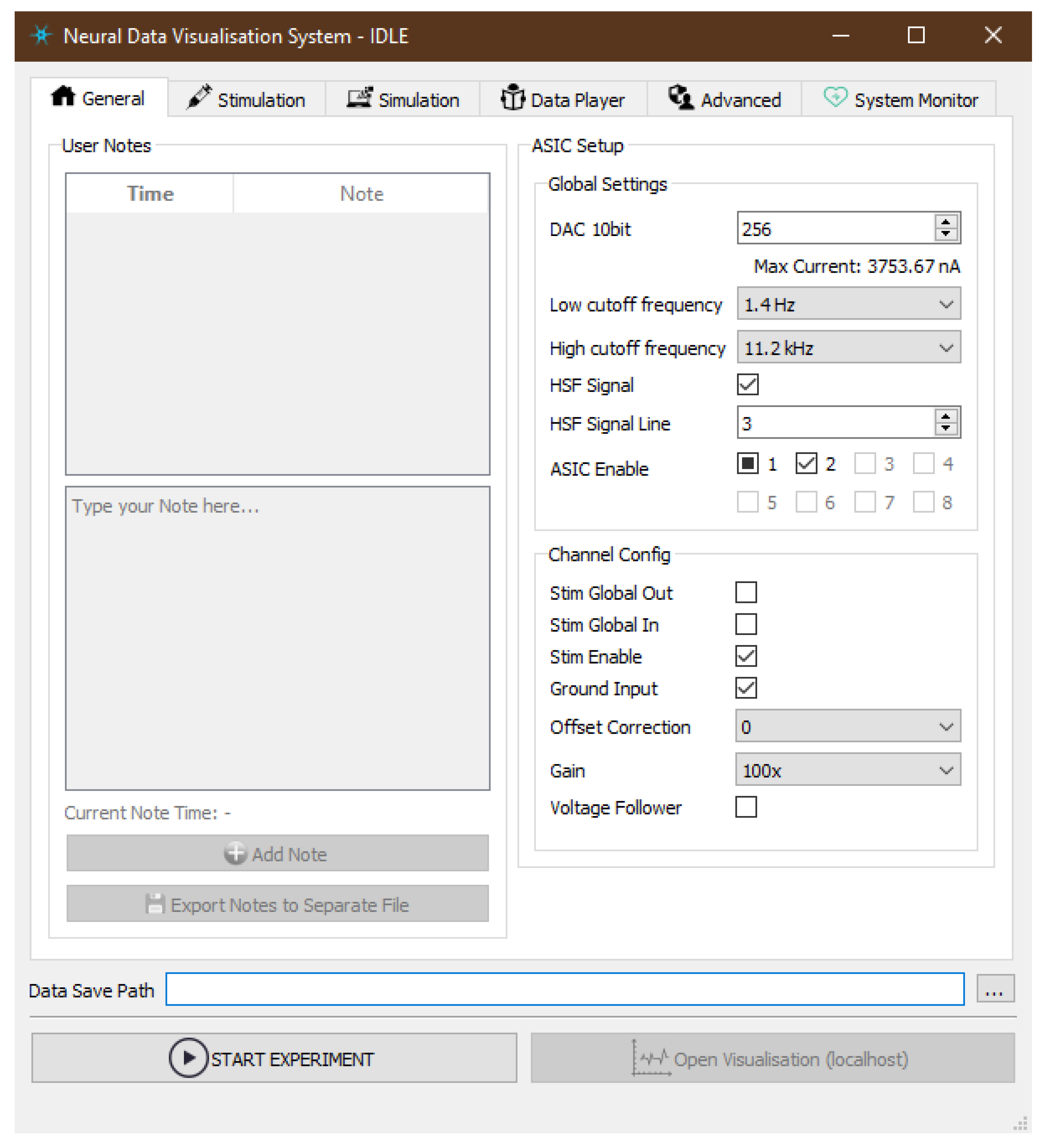
- General—exposes the ASIC parameters that would be applied in hardware at the beginning of the measurement. Global Settings define maximum output current value (DAC 10bit), hardware band-pass filter options and allow enabling individual ASICs. Channel Config is additional set of states applied at per-channel level in order to enable specific parts of its electronic circuitry like output current source (Stim Enable) or signal gain which can be set to seven discreet levels between 100 / and 500 /. Other visible parameters are useful when it comes to Neurostim-3 ASIC testing and debugging procedures. Although each ASIC can be configured independently of each other, currently we do not explore such a possibility.
- Stimulation—allows loading files describing output current stimulation protocol of an experiment.
- Simulation—when any of ASIC Simulators is enabled, one could examine here internal state of Finite State Machine (FSM) and choose how accurate in terms of the number of samples data simulation is.
- Data Player—configures dataset replay schedule.
- Advanced—enables power cycle procedure of the Neurostim-3 hardware right before the new experiment is scheduled. Moreover, here one can decide upon whether apply GZIP (GZIP) compression to saved data or not.
- System Monitor—presents summary as well as detailed information about hardware resources being used by DAQ.
2.3.4. Software Dependencies
2.4. Neurostim-3 Testing Basis
2.4.1. Software
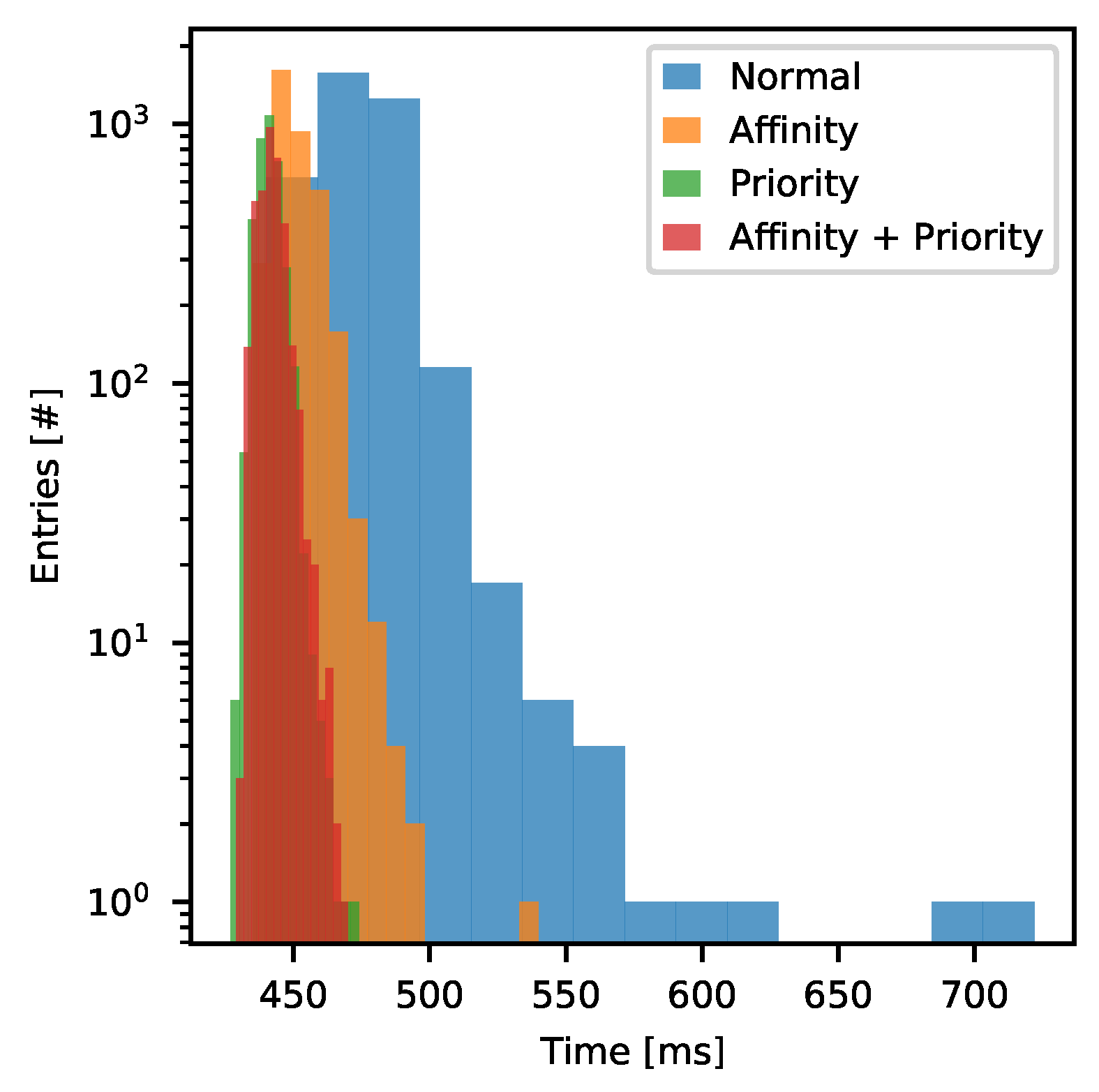
- Normal—the process was running without process scheduling hints.
- Affinity—the process was assigned to the 0th CPU core while the other processes of the DAQ could run freely on any but 0th CPU core.
- Priority—priority of the Stimulation Stream Builder process was changed to high, while the rest of DAQ processes were running with below normal priority.
- Affinity + Priority—two of the above combined.
2.4.2. Inband Analogue Signal Response
2.4.3. Gain
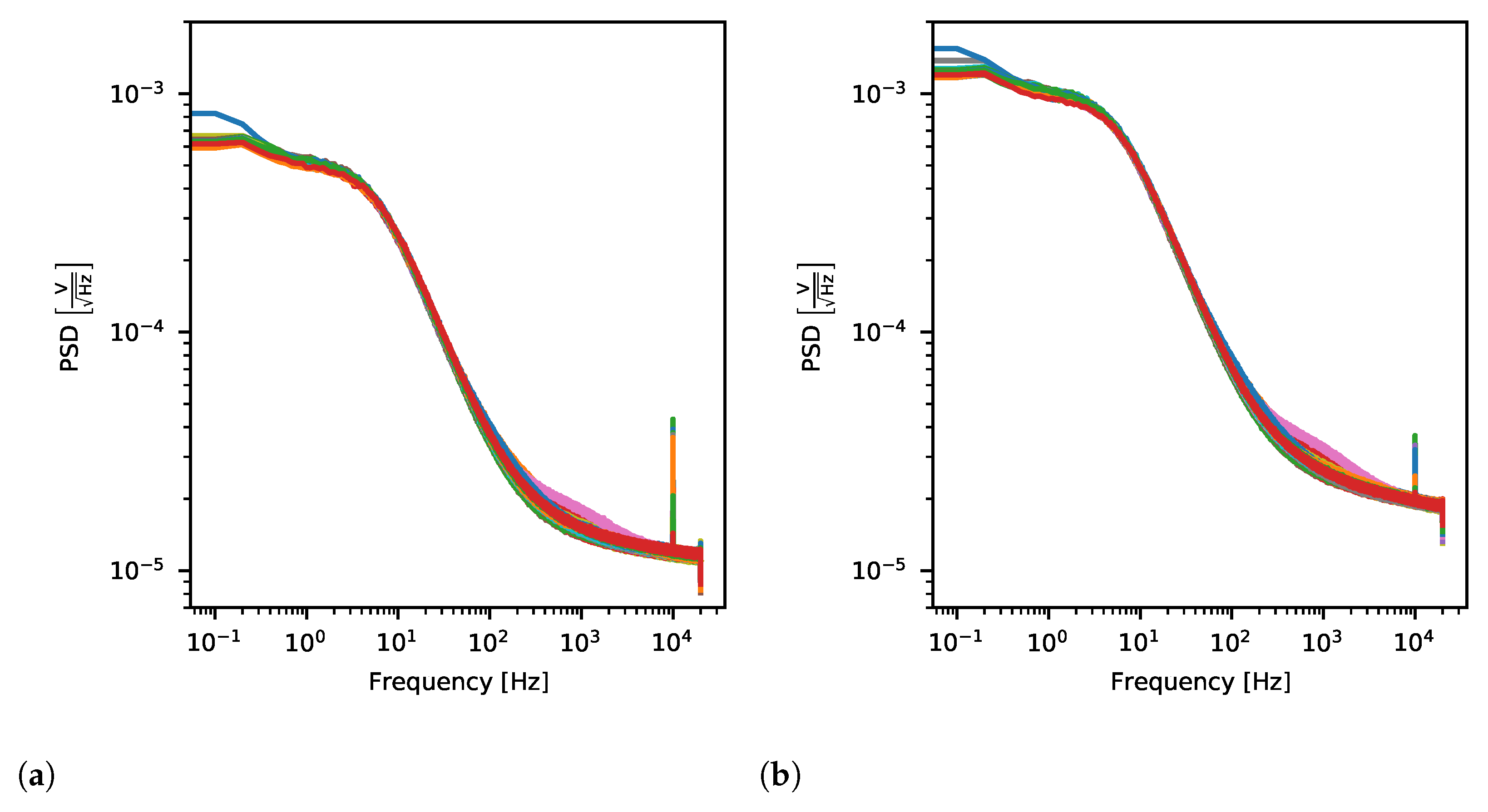
2.4.4. Noise
2.4.5. Filtering
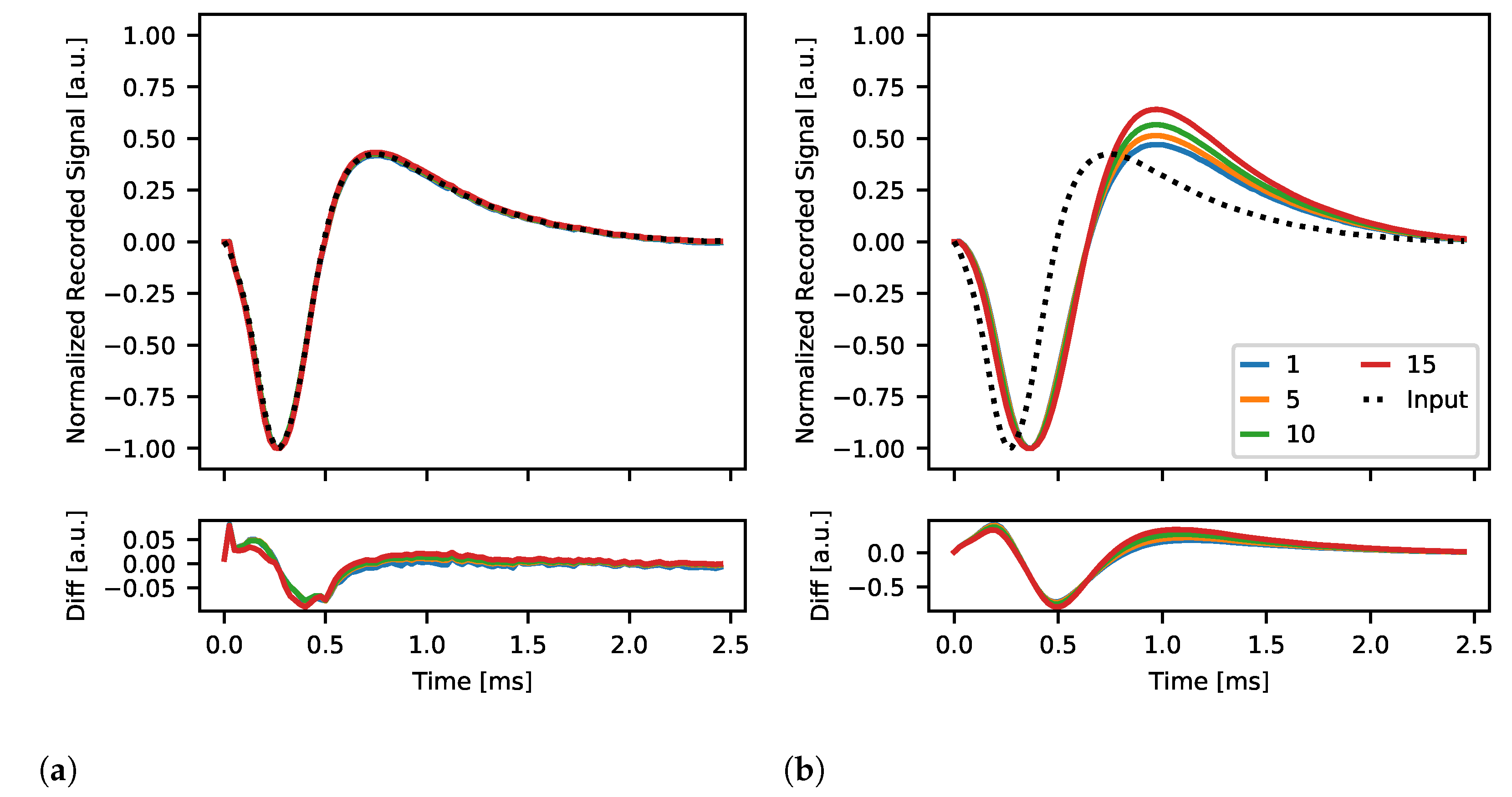
2.4.6. Spike Pulse Stimulation
2.5. Biological Experiments
3. Results and Discussion
3.1. Software Metrics
3.2. Neurostim-3 Basic Characteristics
3.2.1. Inband Analogue Signal Response
3.2.2. Gain
3.2.3. Noise
3.2.4. Filtering
3.2.5. Spike Pulse Stimulation
3.3. Biological Experiments
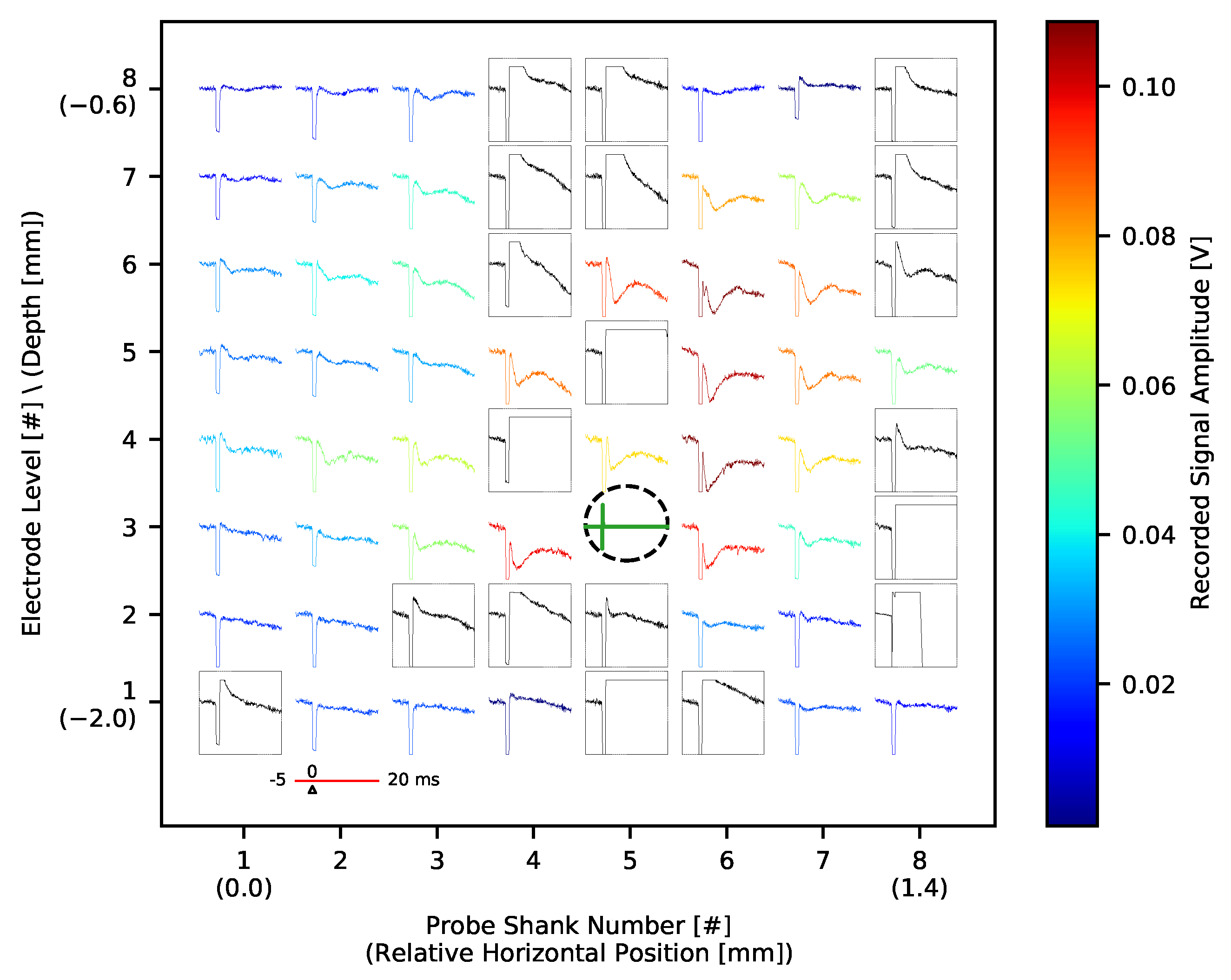
3.3.1. Spontaneous and Whisker Evoked Activity
3.3.2. Electrical Stimulation
4. Conclusions and Plans for the Future
- Multiplication of internal circuitry on the IB responsible for the management of additional ASICs (up to eight).
- Building ABs with two Neurostim-3 ASICs on them.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| µLED | micro Light Emitting Diode. 2 |
| 2-D | two-dimensional. 11, 15 |
| 4K | UHD 4K Ultra High Definition. 15 |
| AB | ASIC Board. 4–7, 19, 23, 35 |
| ADC | Analogue-Digital Converter. 56, 11 |
| ASIC | Application Specific Integrated Circuit. 1–13, 15–24, 26, 27, 29, 30, 32–35 |
| CMOS | Complementary Metal–Oxide–Semiconductor. 5–7 |
| CPU | Central Processing Unit. 8, 15, 19, 20, 24–26 |
| DAC | Digital-Analogue Converter. 18,20 |
| DAQ | Data Acquisition. 1–3, 15, 16, 19, 20, 22, 25, 26, 31, 34–36 |
| DLL | Dynamic-Link Library. 11 |
| DSP | Digital Signal Processing. 15 |
| EEG | Electroencephalography. 1 |
| EEP | Electric Evoked Potential. 33, 34 |
| EP | Evoked Potential. 23, 31, 35 |
| FFT | Fast Fourier Transform. 15 |
| FSM | Finite State Machine. 9, 16 |
| GIL | Global Interpreter Lock. 8 |
| GPIB | General Purpose Interface Bus. 5, 9 |
| GUI | Graphical User Interface. 15, 17, 18 |
| GZIP | GZIP. 13, 16 |
| HDF5 | Hierarchical Data Format. 13 |
| HT | Hyper-Threading. 20 |
| i.p. | Intraperitoneal Injection. 23 |
| IB | Interface Board. 4–7, 11, 17, 23, 35 |
| JSON | JavaScript Object Notation. 10 |
| LDO | Low-Dropout Regulator. 7 |
| LFP | Local Field Potential. 1, 2, 15, 21, 23, 29, 31–35 |
| LVDS | Low-Voltage Differential Signalling. 5, 6 |
| MEA | Multi-Electrode Array. 1–6, 17, 19, 23, 24, 31–35 |
| MEG | Magnetoencephalography. 1 |
| NIC | Network Interface Card. 20, 26 |
| NS3 | Neurostim-3. 4 |
| PBS | Phosphate Buffered Saline. 23 |
| PC | Personal Computer. 1, 3, 26 |
| PEG | Polyethylene Glycol. 23 |
| PSD | Power Spectral Density. 21, 29 |
| PSU | Power Supply Unit. 5, 7, 9 |
| RAM | Random Access Memory. 20, 24 |
| SNR | Signal-To-Noise Ratio. 28, 31 |
| TCP | Transmission Control Protocol. 7, 8, 11, 13, 14, 26, 34 |
| UDP | User Datagram Protocol. 7, 8, 14, 34 |
References
- Jackson, A.F.; Bolger, D.J. The Neurophysiological Bases of EEG and EEG Measurement: A Review for the Rest of Us. Psychophysiology 2014, 51, 1061–1071. [Google Scholar] [CrossRef]
- Ono, Y.; Ishiyama, A. Magnetoencephalography: Basic Theory and Estimation Techniques of Working Brain Activity. In Novel Trends in Brain Science; Onozuka, M., Yen, C.T., Eds.; Springer: Tokyo, Japan, 2008. [Google Scholar] [CrossRef]
- Obien, M.E.J.; Frey, U. Large-Scale, High-Resolution Microelectrode Arrays for Interrogation of Neurons and Networks. Adv. Neurobiol. 2019, 22, 83–123. [Google Scholar] [CrossRef]
- Liu, Q.; Farahibozorg, S.; Porcaro, C.; Wenderoth, N.; Mantini, D. Detecting Large-Scale Networks in the Human Brain Using High-Density Electroencephalography. Hum. Brain Mapp. 2017, 38, 4631–4643. [Google Scholar] [CrossRef] [Green Version]
- Rattay, F. The Basic Mechanism for the Electrical Stimulation of the Nervous System. Neuroscience 1999, 89, 335–346. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-Timescale, Genetically Targeted Optical Control of Neural Activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.; Davidson, T.; Mogri, M.; Deisseroth, K. Optogenetics in Neural Systems. Neuron 2011, 71, 9–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häusser, M. Optogenetics: the Age of Light. Nat. Methods 2014, 11, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, A.V.; Bonci, A. Optogenetics, Physiology, and Emotions. Front. Behav. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Egert, D.; Pettibone, J.R.; Lemke, S.; Patel, P.R.; Caldwell, C.M.; Cai, D.; Ganguly, K.; Chestek, C.A.; Berke, J.D. Cellular-Scale Silicon Probes for High-Density, Precisely Localized Neurophysiology. J. Neurophysiol. 2020, 124, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lee, K.; Villagracia, J.; Masmanidis, S.C. Open Source Silicon Microprobes for High Throughput Neural Recording. J. Neural Eng. 2020, 17, 016036. [Google Scholar] [CrossRef]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydın, Ç.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Hashemi Noshahr, F.; Nabavi, M.; Sawan, M. Multi-Channel Neural Recording Implants: A Review. Sensors 2020, 20. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Zhang, M.; Wang, Z. A Low-Noise Chopper Amplifier Designed for Multi-Channel Neural Signal Acquisition. IEEE J. -Solid-State Circuits 2019, 54, 2255–2265. [Google Scholar] [CrossRef]
- Kumar, A.; Rotter, S.; Aertsen, A. Spiking Activity Propagation in Neuronal Networks: Reconciling Different Perspectives on Neural Coding. Nat. Rev. Neurosci. 2010, 11, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, Y.; Schroeder, C. How Local Is the Local Field Potential? Neuron 2011, 72, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Hottowy, P.; Skoczeń, A.; Gunning, D.E.; Kachiguine, S.; Mathieson, K.; Sher, A.; Wiącek, P.; Litke, A.M.; Dąbrowski, W. Properties and Application of a Multichannel Integrated Circuit for Low-Artifact, Patterned Electrical Stimulation of Neural Tissue. J. Neural Eng. 2012, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szypulska, M.; Dwużnik, M.; Wiącek, P.; Skoczeń, A.; Fiutowski, T.; Jędraczka, M.; Dusik, J.; Ahmed, M.I.; Dąbrowski, W.; Hottowy, P.; et al. Modular ASIC-Based System for Large-Scale Electrical Stimulation and Recording of Brain Activity in Behaving Animals. In Proceedings of the 2016 MIXDES—23rd International Conference Mixed Design of Integrated Circuits and Systems, Łodź, Poland, 23–25 June 2016; pp. 217–222. [Google Scholar] [CrossRef]
- National Instruments 6537 DIO Manual. Available online: https://www.ni.com/pdf/manuals/374373g.pdf (accessed on 29 April 2021).
- NI-DAQmx. Available online: https://knowledge.ni.com/KnowledgeArticleDetails?id=kA00Z000000P8baSAC&l=pl-PL (accessed on 29 April 2021).
- NI-DAQmx Driver Download Page. Available online: https://www.ni.com/pl-pl/support/downloads/drivers/download.ni-daqmx.html#348669 (accessed on 29 April 2021).
- User’s Guide DP1308A Programmable Linear DC Power Supply. Available online: https://rigol.com.pl/pl/p/file/54fedfbce114fd2ed236c415e51e29c8/DP1308A_UserGuide_EN.pdf (accessed on 29 April 2021).
- IEEE Standard For Higher Performance Protocol for the Standard Digital Interface for Programmable Instrumentation; IEEE Std 488.1-2003 (Revision of IEEE Std 488.1-1987). 2003, pp. 1–140. Available online: https://standards.ieee.org/standard/488_1-2003.html (accessed on 29 April 2021). [CrossRef]
- Masmanidis Lab Probe Mapping. Available online: https://masmanidislab.neurobio.ucla.edu/images/microprobe_info.pdf (accessed on 29 April 2021).
- NeuroNexus Probe Mapping. Available online: https://neuronexus.com/support/product-documentation/mapping/probe-mapping (accessed on 29 April 2021).
- Maxim Integrated MAX11190 Manual. Available online: https://datasheets.maximintegrated.com/en/ds/MAX11190.pdf (accessed on 29 April 2021).
- LVDS Application and Data Handbook. Available online: https://www.ti.com/lit/pdf/SLLD009 (accessed on 29 April 2021).
- Intan Technologies: RHD SPI Interface Cables and Cable Adapters. Available online: http://intantech.com/RHD_SPI_cables.html?tabSelect=RHDSPIcables (accessed on 29 April 2021).
- Transmission Control Protocol. Available online: https://tools.ietf.org/html/rfc761 (accessed on 29 April 2021).
- User Datagram Protocol Multicast Transmission. Available online: https://tools.ietf.org/html/rfc8085#page-28 (accessed on 29 April 2021).
- Python Documentation. Available online: https://docs.python.org/3/ (accessed on 29 April 2021).
- Python Package Index. Available online: https://pypi.org/ (accessed on 29 April 2021).
- Python Global Interpreter Lock. Available online: https://wiki.python.org/moin/GlobalInterpreterLock (accessed on 29 April 2021).
- Python Synchronisation Manager. Available online: https://docs.python.org/3/library/multiprocessing.html#multiprocessing-managers (accessed on 29 April 2021).
- MATLAB Documentation of Saving Workspace Variables. Available online: https://www.mathworks.com/help/matlab/ref/save.html (accessed on 29 April 2021).
- JSON Data Format Documentation. Available online: https://www.json.org/json-en.html (accessed on 29 April 2021).
- NI-DAQmx C Reference Help. Available online: https://www.ni.com/pdf/manuals/370471am.zip (accessed on 29 April 2021).
- Numba Documentation. Available online: https://numba.readthedocs.io/en/stable/user/index.html (accessed on 29 April 2021).
- NumPy Documentation. Available online: https://numpy.org/doc/stable/release.html (accessed on 29 April 2021).
- Hierarchical Data Format 5 Documentation. Available online: https://portal.hdfgroup.org/display/HDF5/HDF5 (accessed on 29 April 2021).
- GZIP File Format Specification Version 4.3. Available online: https://tools.ietf.org/html/rfc1952 (accessed on 29 April 2021).
- Butterworth, S. On the Theory of Filter Amplifiers. Wirel. Eng. 1930, 7, 536–541. [Google Scholar]
- Cooley, J.W.; Tukey, J.W. An Algorithm for the Machine Calculation of Complex Fourier Series. Math. Comput. 1965, 19, 297–301. [Google Scholar] [CrossRef]
- PyQtGraph. Available online: http://www.pyqtgraph.org/ (accessed on 29 April 2021).
- PyQt5 Documentation. Available online: https://www.riverbankcomputing.com/static/Docs/PyQt5/ (accessed on 29 April 2021).
- Python Data Classes: PEP 557. Available online: https://www.python.org/dev/peps/pep-0557/ (accessed on 29 April 2021).
- H5PY Documentation. Available online: https://docs.h5py.org/en/stable/ (accessed on 29 April 2021).
- NI-VISA. Available online: https://www.ni.com/visa/default.htm (accessed on 29 April 2021).
- NI-VISA Driver Download Page. Available online: https://www.ni.com/pl-pl/support/downloads/drivers/download.ni-visa.html#346210 (accessed on 29 April 2021).
- PyVISA Documentation. Available online: https://pyvisa.readthedocs.io/en/latest/ (accessed on 29 April 2021).
- Arrayqueues Documentation and Source Code. Available online: https://github.com/portugueslab/arrayqueues (accessed on 29 April 2021).
- SciPy Documentation. Available online: https://docs.scipy.org/doc/scipy/reference/ (accessed on 29 April 2021).
- Psutil Documentation. Available online: https://psutil.readthedocs.io/en/latest/ (accessed on 29 April 2021).
- Marr, D.T.; Binns, F.; Hill, D.L.; Hinton, G.; Koufaty, D.A.; Miller, J.A.; Upton, M. Hyper-Threading Technology Architecture and Microarchitecture. Intel Technol. J. 2002, 6, 4–15. [Google Scholar]
- Intel Ethernet Connection I217-LM Product Page. Available online: https://ark.intel.com/content/www/us/en/ark/products/60019/intel-ethernet-connection-i217-lm.html (accessed on 29 April 2021).
- Intel 82579LM Gigabit Ethernet PHY Product Page. Available online: https://ark.intel.com/content/www/us/en/ark/products/47620/intel-82579lm-gigabit-ethernet-phy.html (accessed on 29 April 2021).
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef] [Green Version]
- SciPy Welch() Function. Available online: https://docs.scipy.org/doc/scipy/reference/generated/scipy.signal.welch.html (accessed on 29 April 2021).
- Kołodziej, K.; Szypulska, M.; Dąbrowski, W.; Hottowy, P. Modelling and Cancellation of the Stimulation Artifact for ASIC-based Bidirectional Neural Interface. In Proceedings of the 2018 25th International Conference “Mixed Design of Integrated Circuits and System” (MIXDES), Gdynia, Poland, 21–23 June 2018; pp. 449–453. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02010L0063-20190626 (accessed on 29 April 2021).
- NeuroNexus mapping A64 Package. Available online: https://neuronexus.com/files/probemapping/64-channel/A64-Maps.pdf (accessed on 29 April 2021).
- NanoZ Electroplating Device. Available online: https://white-matter.com/products/nanoz/ (accessed on 29 April 2021).
- Ferguson, J.E.; Boldt, C.; Redish, D.A. Creating Low-Impedance Tetrodes by Electroplating with Additives. Sens. Actuators. Phys. 2009, 156, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laura M., J. Fernandez, A.L. Sleep Spindles: Mechanisms and Functions. Physiol. Rev. 2020, 100, 805–868. [Google Scholar] [CrossRef]
- Kublik, E.; Swiejkowski, D.A.; Wróbel, A. Cortical Contribution to Sensory Volleys Recorded at Thalamic Nuclei of Lemniscal and Paralemniscal Pathways. Acta Neurobiol. Exp. 2003, 63, 377–382. [Google Scholar]
- Łęski, S.; Kublik, E.; Świejkowski, D.A.; Wróbel, A.; Wójcik, D.K. Extracting Functional Components of Neural Dynamics with Independent Component Analysis and Inverse Current Source Density. J. Comput. Neurosci. 2010, 29, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.; Di, S. Laminar Excitability Cycles in Neocortex. J. Neurophysiol. 1991, 65, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Arieli, A.; Sterkin, A.; Grinvald, A.; Aertsen, A. Dynamics of Ongoing Activity: Explanation of the Large Variability in Evoked Cortical Responses. Science 1996, 273, 1868–1871. [Google Scholar] [CrossRef] [Green Version]
- Musiał, P.; Kublik, E.; Wróbel, A. Spontaneous Variability Reveals Principal Components in Cortical Evoked Potentials. Neuroreport 1998, 9, 2627–2631. [Google Scholar] [CrossRef]























| Purpose | Tool Name | Version | References |
|---|---|---|---|
| Programming language | CPython | 3.8.3 64bit | [31] |
| Graphical User Interface (GUI) | PyQt5 | 5.14 | [45] |
| Plotting tool | PyQtGraph | 0.11 | [44] |
| Data storage | HDF5 | 1.8.14 64bit | [40] |
| h5py | 1.18 | [47] | |
| External device communication | NI-DAQmx | 18.1f1 | [20,21] |
| NI-VISA | 18.5 | [48,49] | |
| PyVISA | 1.10 | [50] | |
| Shared memory implementation | arrayqueues | 1.2.0b0 | [51] |
| Numerical data processing | NumPy | 1.18 | [39] |
| SciPy | 1.4.1 | [52] | |
| Python to machine code compiler | Numba | 0.49.1 | [38] |
| Process monitor | psutil | 5.7 | [53] |
| Gain | 100 | 150 | 200 | 250 | 300 | 400 | 500 |
| DAC 10bit | 120 | 80 | 60 | 48 | 40 | 30 | 24 |
| Scheduling Configuration | ||||
|---|---|---|---|---|
| Normal | Affinity | Priority | Affinity + Priority | |
| Mean time per Output Bitstream chunk [ms] | 474 | 450 | 441 | 442 |
| [ms] | 15.3 | 7.5 | 4.2 | 4.7 |
| Mean Central Processing Unit (CPU) | 97 | 100 | 92 | 100 |
| core utilization [%] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurgielewicz, P.; Fiutowski, T.; Kublik, E.; Skoczeń, A.; Szypulska, M.; Wiącek, P.; Hottowy, P.; Mindur, B. Modular Data Acquisition System for Recording Activity and Electrical Stimulation of Brain Tissue Using Dedicated Electronics. Sensors 2021, 21, 4423. https://doi.org/10.3390/s21134423
Jurgielewicz P, Fiutowski T, Kublik E, Skoczeń A, Szypulska M, Wiącek P, Hottowy P, Mindur B. Modular Data Acquisition System for Recording Activity and Electrical Stimulation of Brain Tissue Using Dedicated Electronics. Sensors. 2021; 21(13):4423. https://doi.org/10.3390/s21134423
Chicago/Turabian StyleJurgielewicz, Paweł, Tomasz Fiutowski, Ewa Kublik, Andrzej Skoczeń, Małgorzata Szypulska, Piotr Wiącek, Paweł Hottowy, and Bartosz Mindur. 2021. "Modular Data Acquisition System for Recording Activity and Electrical Stimulation of Brain Tissue Using Dedicated Electronics" Sensors 21, no. 13: 4423. https://doi.org/10.3390/s21134423
APA StyleJurgielewicz, P., Fiutowski, T., Kublik, E., Skoczeń, A., Szypulska, M., Wiącek, P., Hottowy, P., & Mindur, B. (2021). Modular Data Acquisition System for Recording Activity and Electrical Stimulation of Brain Tissue Using Dedicated Electronics. Sensors, 21(13), 4423. https://doi.org/10.3390/s21134423






