The Effects of Temperature on the Growth of a Lead-Free Perovskite-Like (CH3NH3)3Sb2Br9 Single Crystal for An MSM Photodetector Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
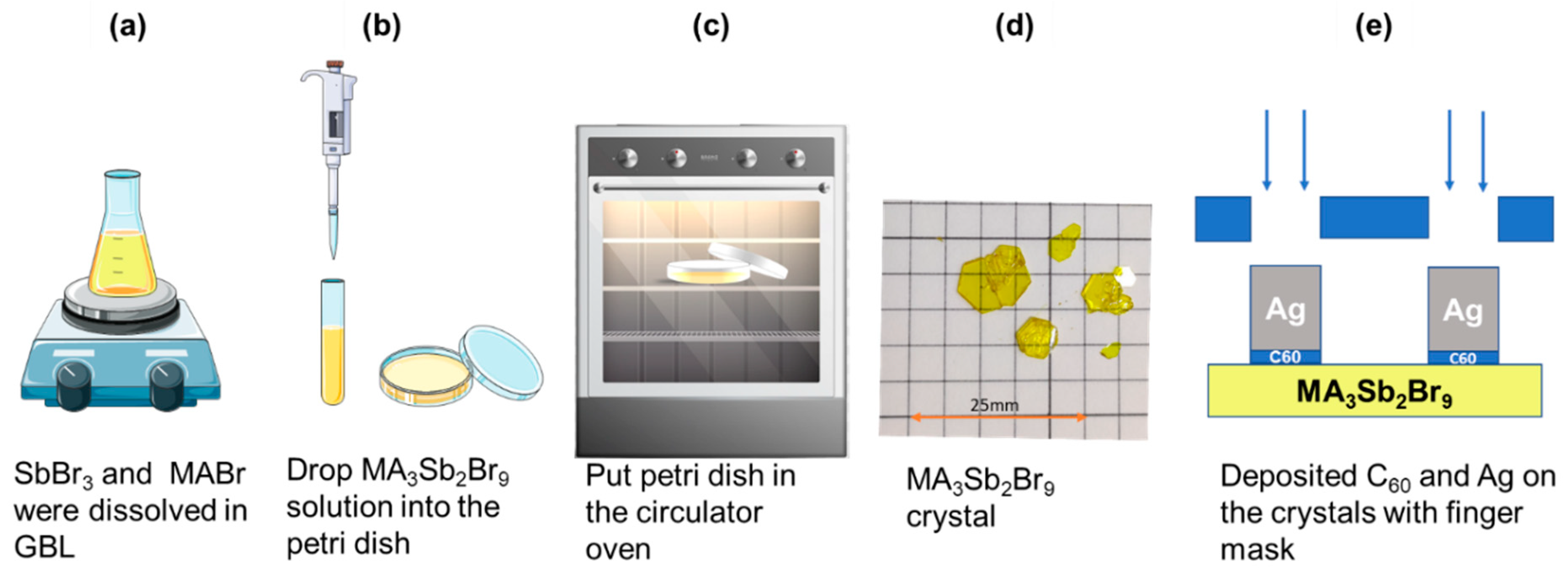
2.2. Preparation of MA3Sb2Br9 Single Crystals and Photodetector Device
2.3. Characterization
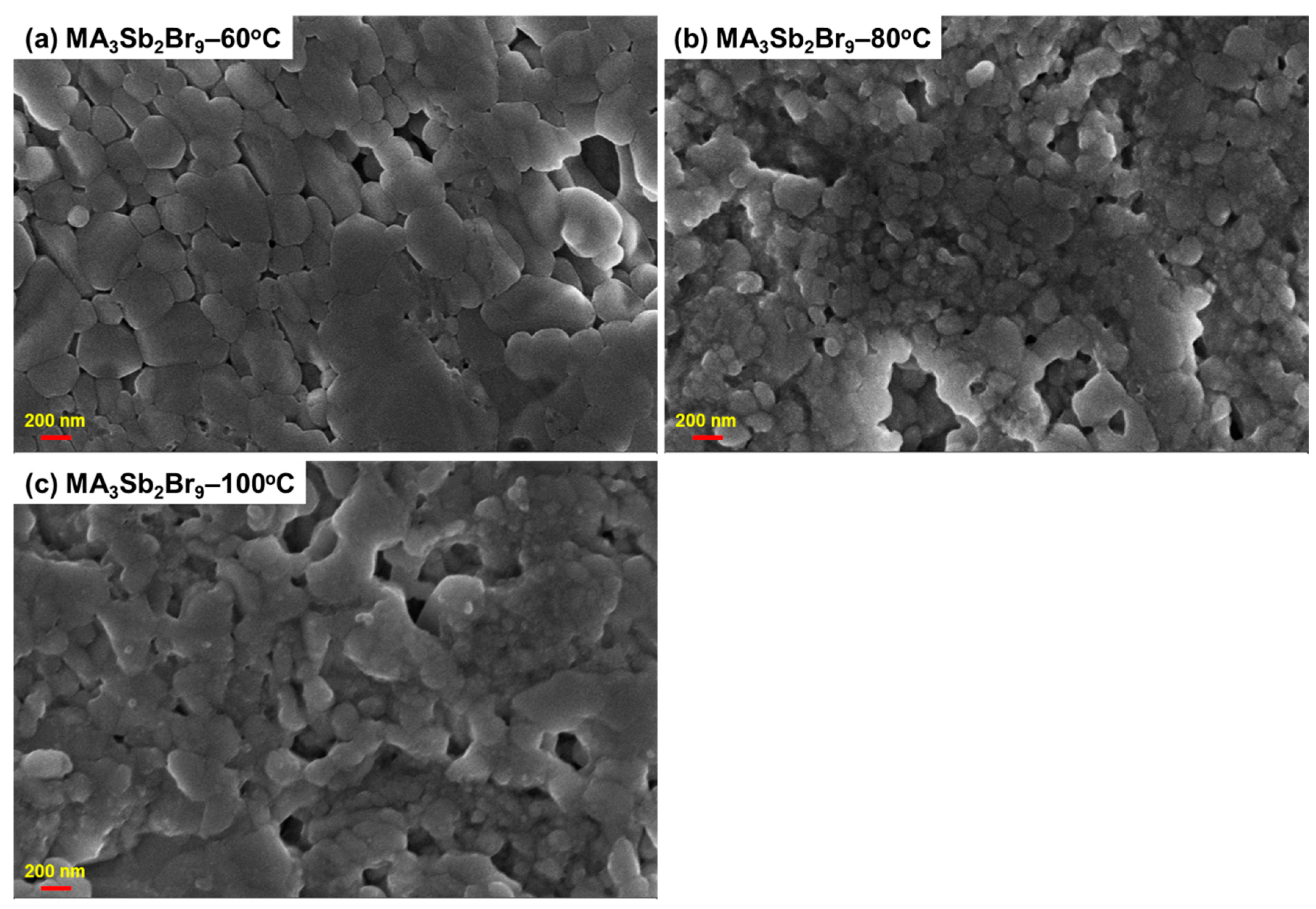
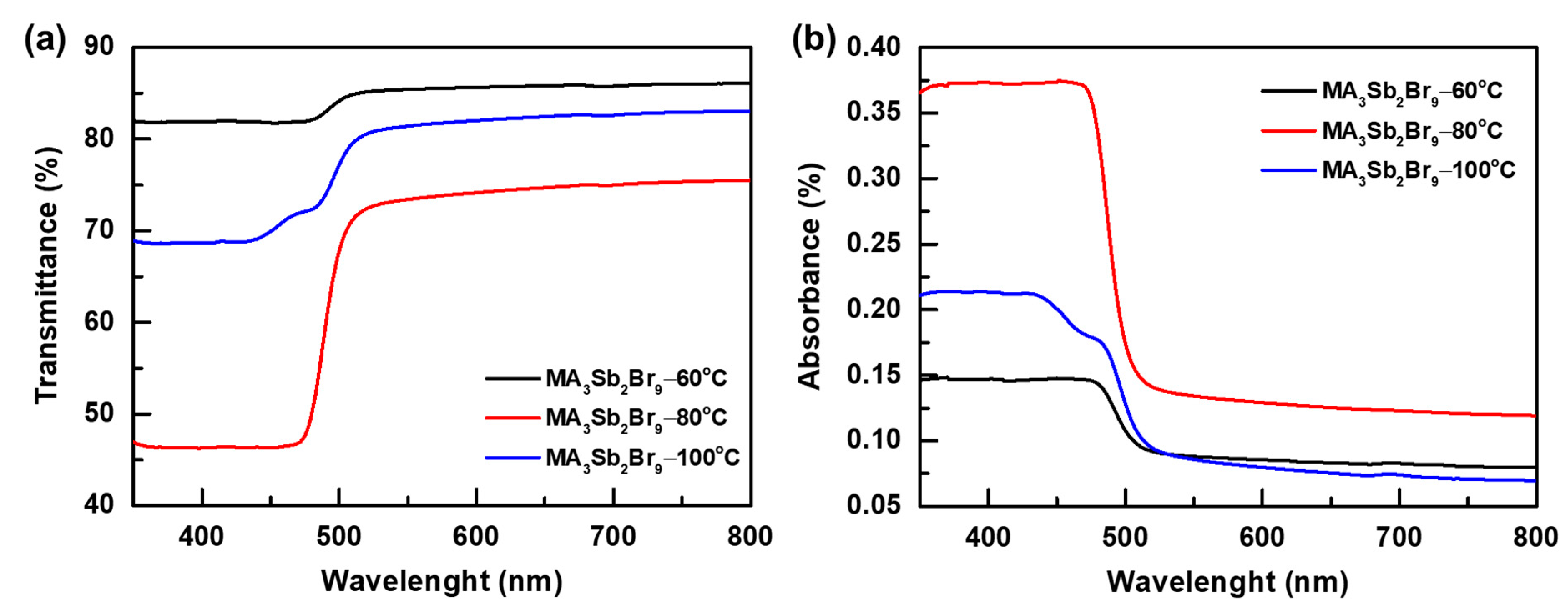
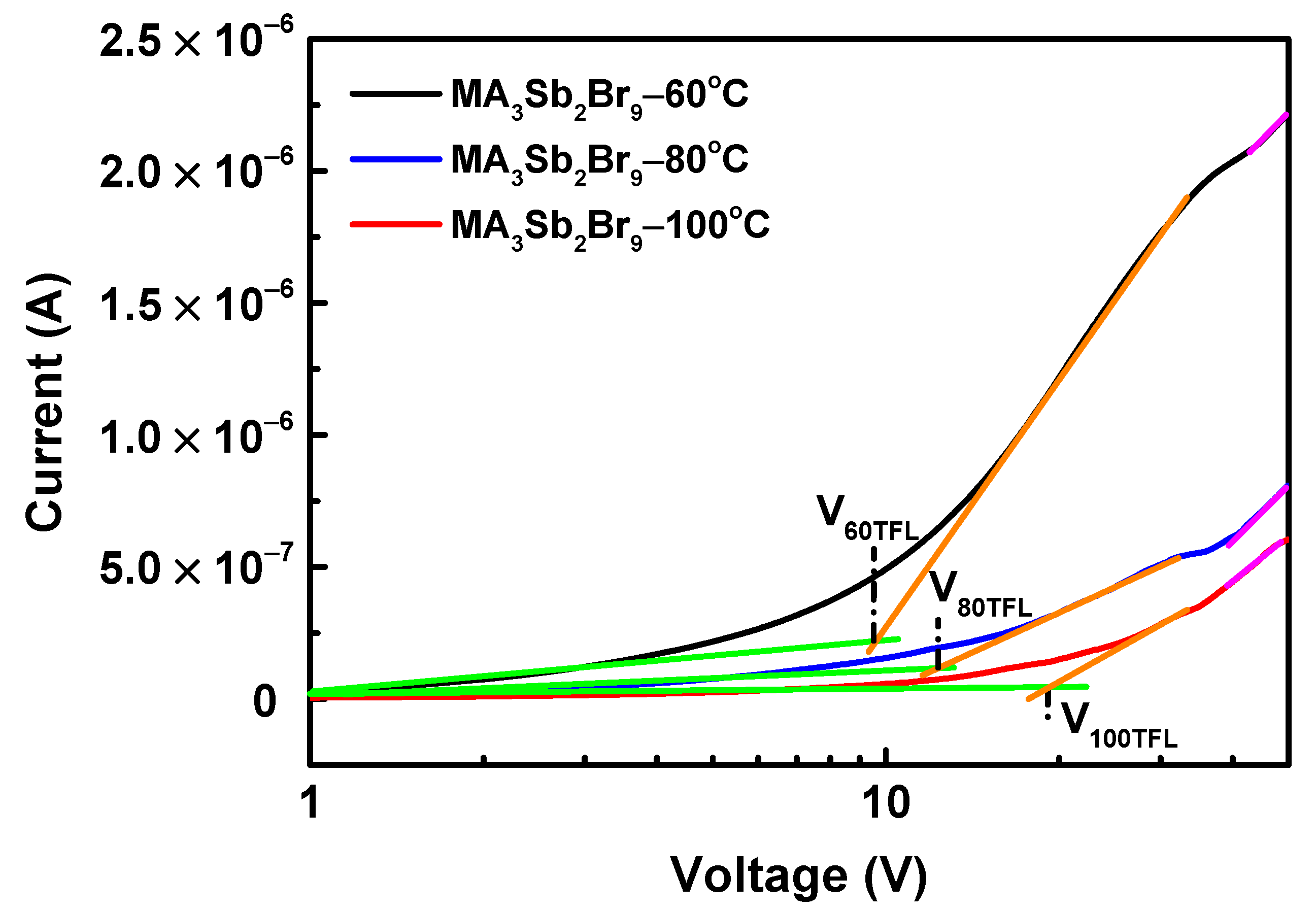
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.W.; Seol, D.J.; Cho, A.N.; Park, N.G. High-Efficiency Perovskite Solar Cells Based on the Black Polymorph of HC(NH2)2PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Correa-Baena, J.P.; Graetzel, M.; Hagfeldt, A.; Abate, A. Perovskite Solar Cells: From the Atomic Level to Film Quality and Device Performance. Angew. Chem. Int. Ed. 2018, 57, 2554–2569. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhang, C.; Huang, J.; Fan, H.; Wang, H.; Wang, P.; Zhan, C.; Liu, C.M.; Li, X.; Yang, L.; et al. A Cation-Exchange Approach for the Fabrication of Efficient Methylammonium Tin Iodide Perovskite Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 6688–6692. [Google Scholar] [CrossRef]
- Yao, F.; Peng, J.; Li, R.; Li, W.; Gui, P.; Li, B.; Liu, C.; Tao, C.; Lin, Q.; Fang, G. Room-temperature liquid diffused separation induced crystallization for high-quality perovskite single crystals. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Yao, J.; Qiu, B.; Zhang, Z.G.; Xue, L.; Wang, R.; Zhang, C.; Chen, S.; Zhou, Q.; Sun, C.; Yang, C.; et al. Cathode engineering with perylene-diimide interlayer enabling over 17% efficiency single-junction organic solar cells. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Chen, L.C.; Tien, C.-H.; Lee, K.L.; Kao, Y.T. Efficiency Improvement of MAPbI3 Perovskite Solar Cells Based on a CsPbBr3 Quantum Dot/Au Nanoparticle Composite Plasmonic Light-Harvesting Layer. Energies 2020, 13, 1471. [Google Scholar] [CrossRef] [Green Version]
- National Renewable Energy Laboratory. Best Research-Cell Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200104.pdf (accessed on 4 January 2021).
- Ahmadi, M.; Wu, T.; Hu, B. A Review on Organic–Inorganic Halide Perovskite Photodetectors: Device Engineering and Fundamental Physics. Adv. Mater. 2017, 29, 1605242. [Google Scholar] [CrossRef]
- Chen, L.C.; Lee, K.L.; Lee, K.Y.; Huang, Y.W.; Lin, R.M. Study of Metal–Semiconductor–Metal CH3NH3PbBr3 Perovskite Photodetectors Prepared by Inverse Temperature Crystallization Method. Sensors 2020, 20, 297. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Zhang, F.; Luo, X.; Zhang, D.; Duan, L. Mixed 2D/3D perovskite with fine phase control modulated by a novel cyclopentanamine hydrobromide for better stability in light-emitting diodes. Chem. Eng. J. 2020, 393, 124787. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, S.-G.; Kim, Y.C.; Han, I.T.; Jang, H.J.; Lee, J.Y.; Park, N.-G. CsPbBr3/CH3NH3PbCl3 Double Layer Enhances Efficiency and Lifetime of Perovskite Light-Emitting Diodes. ACS Energy Lett. 2020, 5, 2191–2199. [Google Scholar] [CrossRef]
- Maculan, G.; Sheikh, A.D.; Abdelhady, A.L.; Saidaminov, M.; Haque, A.; Murali, B.; Alarousu, E.; Mohammed, O.F.; Wu, T.; Bakr, O.M. CH3NH3PbCl3 Single Crystals: Inverse Temperature Crystallization and Visible-Blind UV-Photodetector. J. Phys. Chem. Lett. 2015, 6, 3781–3786. [Google Scholar] [CrossRef] [Green Version]
- Ju, D.; Jiang, X.; Xiao, H.; Chen, X.; Hu, X.; Tao, X. Narrow band gap and high mobility of lead-free perovskite single crystal Sn-doped MA3Sb2I9. J. Mater. Chem. A 2018, 6, 20753–20759. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, J.; Zhao, C.; Zou, Y.; Kong, W.; Yu, Z.; Shan, Y.; Dong, Q.; Zhou, D.; Yu, W.; Guo, C. Modulating the optical and electrical properties of MAPbBr3 single crystals via voltage regulation engineering and application in memristors. Light. Sci. Appl. 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Bi, W.; Wang, A.; Liu, X.; Kang, Y.; Dong, Q. Efficient lateral-structure perovskite single crystal solar cells with high operational stability. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-C.; Tien, C.-H.; Jhou, Y.-C.; Lin, W.-C. Co-Solvent Controllable Engineering of MA0.5FA0.5Pb0.8Sn0.2I3 Lead–Tin Mixed Perovskites for Inverted Perovskite Solar Cells with Improved Stability. Energies 2020, 13, 2438. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, F.; Wei, Q.; Li, H.; Shang, Y.; Zhou, W.; Wang, C.; Cheng, P.; Chen, Q.; Chen, L.; et al. Ultra-high open-circuit voltage of tin perovskite solar cells via an electron transporting layer design. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hima, A.; Lakhdar, N. Enhancement of efficiency and stability of CH3NH3GeI3 solar cells with CuSbS2. Opt. Mater. 2020, 99, 109607. [Google Scholar] [CrossRef]
- Kanoun, A.-A.; Kanoun, M.B.; Merad, A.E.; Goumri-Said, S. Toward development of high-performance perovskite solar cells based on CH3NH3GeI3 using computational approach. Sol. Energy 2019, 182, 237–244. [Google Scholar] [CrossRef]
- Li, X.; Zhong, X.; Hu, Y.; Li, B.; Sheng, Y.; Zhang, Y.; Weng, C.; Feng, M.; Han, H.; Wang, J. Organic–Inorganic Copper(II)-Based Material: A Low-Toxic, Highly Stable Light Absorber for Photovoltaic Application. J. Phys. Chem. Lett. 2017, 8, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Guo, Y.; Hu, W.; Tan, Y.; Zhang, X.; Feng, J.; Tang, X. Opportunity of the Lead-Free All-Inorganic Cs3Cu2I5 Perovskite Film for Memristor and Neuromorphic Computing Applications. ACS Appl. Mater. Interfaces 2020, 12, 23094–23101. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.M.; Phuyal, D.; Davies, M.; Li, M.; Philippe, B.; de Castro, C.S.; Qiu, Z.; Kim, J.; Watson, T.; Tsoi, W.C.; et al. An effective approach of vapour assisted morphological tailoring for reducing metal defect sites in lead-free, (CH3NH3)3Bi2I9 bismuth-based perovskite solar cells for improved performance and long-term stability. Nano Energy 2018, 49, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Fu, X.; Yang, T.; Li, D.; Fan, P.; Li, H.; Jiang, F.; Li, L.; Luo, Z.; Zhuang, X.; et al. Highly stable lead-free Cs3Bi2I9 perovskite nanoplates for photodetection applications. Nano Res. 2019, 12, 1894–1899. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Nienhaus, L.; Kurchin, R.C.; Shin, S.S.; Wieghold, S.; Hartono, N.T.; Layurova, M.; Klein, N.D.; Poindexter, J.R.; Polizzotti, A.; et al. A-Site Cation in Inorganic A3Sb2I9 Perovskite Influences Structural Dimensionality, Exciton Binding Energy, and Solar Cell Performance. Chem. Mater. 2018, 30, 3734–3742. [Google Scholar] [CrossRef]
- Singh, A.; Najman, S.; Mohapatra, A.; Lu, Y.-J.; Hanmandlu, C.; Pao, C.-W.; Chen, Y.-F.; Lai, C.-S.; Chu, C.W. Modulating Performance and Stability of Inorganic Lead-Free Perovskite Solar Cells via Lewis-Pair Mediation. ACS Appl. Mater. Interfaces 2020, 12, 32649–32657. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N.; et al. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 2015, 3, 23829–23832. [Google Scholar] [CrossRef]
- Waleed, A.; Tavakoli, M.M.; Gu, L.; Wang, Z.; Zhang, D.; Manikandan, A.; Zhang, Q.; Zhang, R.; Chueh, Y.-L.; Fan, Z. Lead-Free Perovskite Nanowire Array Photodetectors with Drastically Improved Stability in Nanoengineering Templates. Nano Lett. 2016, 17, 523–530. [Google Scholar] [CrossRef]
- Liu, J.; Ozaki, M.; Yakumaru, S.; Handa, T.; Nishikubo, R.; Kanemitsu, Y.; Saeki, A.; Murata, Y.; Murdey, R.; Wakamiya, A. Lead-Free Solar Cells based on Tin Halide Perovskite Films with High Coverage and Improved Aggregation. Angew. Chem. Int. Ed. 2018, 57, 13221–13225. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tu, J.; Hu, X.; Huang, Z.; Meng, X.; Yang, J.; Duan, X.; Tan, L.; Li, Z.; Chen, Y. Enhanced Hole Transportation for Inverted Tin-Based Perovskite Solar Cells with High Performance and Stability. Adv. Funct. Mater. 2019, 29, 1808059. [Google Scholar] [CrossRef]
- Wolanyk, J.; Xiao, X.; Fralaide, M.; Lauersdorf, N.J.; Kaudal, R.; Dykstra, E.; Huang, J.; Shinar, J.; Shinar, R. Tunable perovskite-based photodetectors in optical sensing. Sen. Actuators B Chem. 2020, 321, 128462. [Google Scholar] [CrossRef]
- Park, B.-W.; Philippe, B.; Zhang, X.; Rensmo, H.; Boschloo, G.; Johansson, E.M.J. Bismuth Based Hybrid Perovskites A3Bi2I9(A: Methylammonium or Cesium) for Solar Cell Application. Adv. Mater. 2015, 27, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Mobin, S.M. Recent Progress and Challenges in A3Sb2X9-Based Perovskite Solar Cells. ACS Omega 2020, 5, 28404–28412. [Google Scholar] [CrossRef]
- Yang, B.; Li, Y.J.; Tang, Y.X.; Mao, X.; Luo, C.; Wang, M.S.; Deng, W.Q.; Han, K.L. Constructing Sensitive and Fast Lead-Free Single-Crystalline Perovskite Photodetectors. J. Phys. Chem. Lett. 2018, 9, 3087–3092. [Google Scholar] [CrossRef]
- Zheng, Z.; Hu, Q.; Zhou, H.; Luo, P.; Nie, A.; Zhu, H.; Gan, L.; Zhuge, F.; Ma, Y.; Song, H.; et al. Submillimeter and lead-free Cs3Sb2Br9 perovskite nanoflakes: Inverse temperature crystallization growth and application for ultrasensitive photodetectors. Nanoscale Horizons 2019, 4, 1372–1379. [Google Scholar] [CrossRef]
- Yang, J.M.; Choi, E.S.; Kim, S.Y.; Kim, J.H.; Park, J.H.; Park, N.G. Perovskite-related (CH3NH3)3Sb2Br9 for forming-free memristor and low-energy-consuming neuromorphic computing. Nanoscale 2019, 11, 6453–6461. [Google Scholar] [CrossRef]
- Li, L.; Deng, Y.; Bao, C.; Fang, Y.; Wei, H.; Tang, S.; Zhang, F.; Huang, J. Self-Filtered Narrowband Perovskite Photodetectors with Ultrafast and Tuned Spectral Response. Adv. Opt. Mater. 2017, 5, 1700672. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Zhang, S.; Li, J.; Wang, C.; Zhao, C.; Nie, P.; Dong, Y.; Zhang, X.; Zhao, S.; et al. Lead-Free Cs 3 Sb 2 Br 9 Single Crystals for High Performance Narrowband Photodetector. Adv. Opt. Mater. 2020, 8, 2001072. [Google Scholar] [CrossRef]



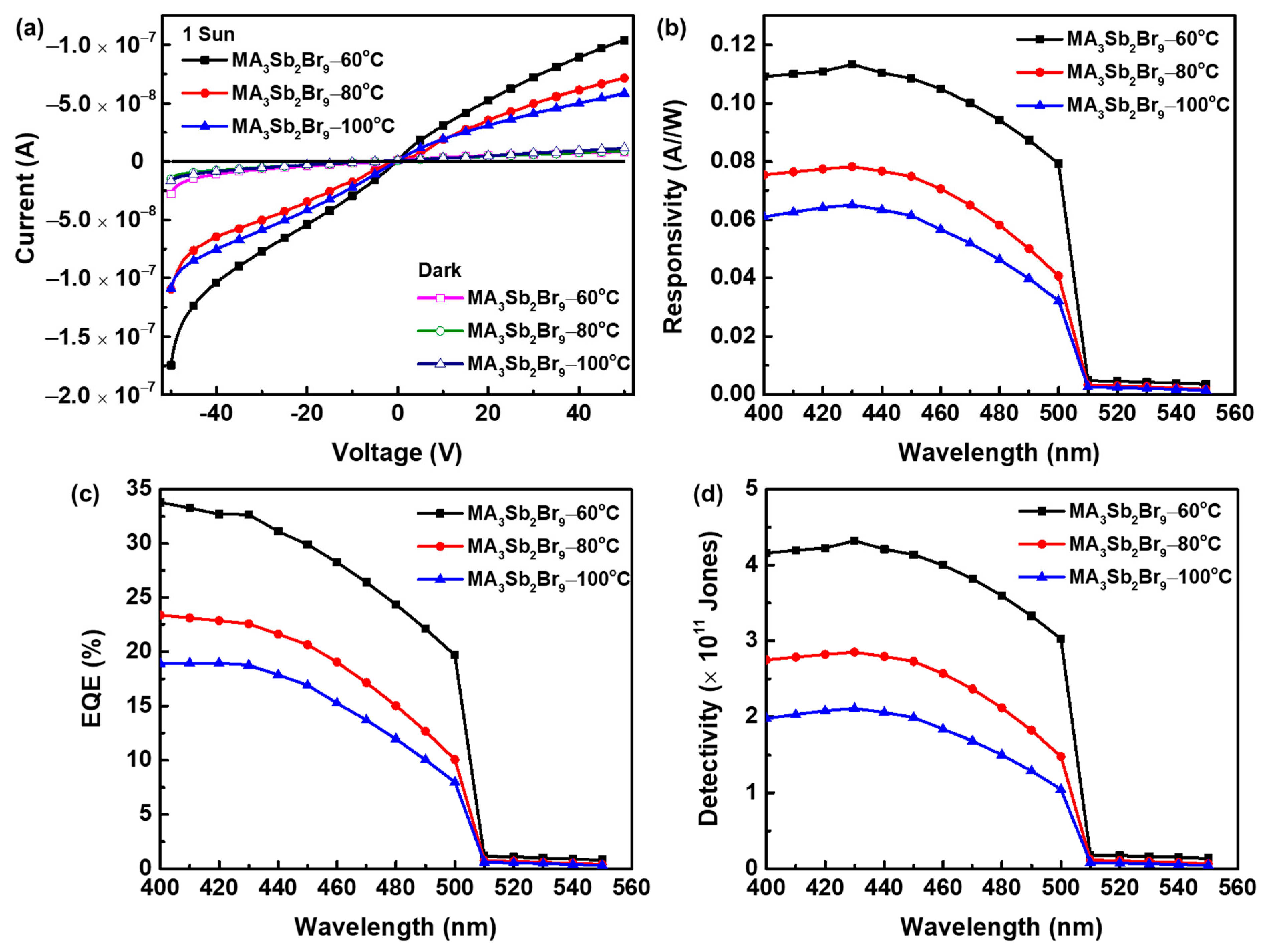
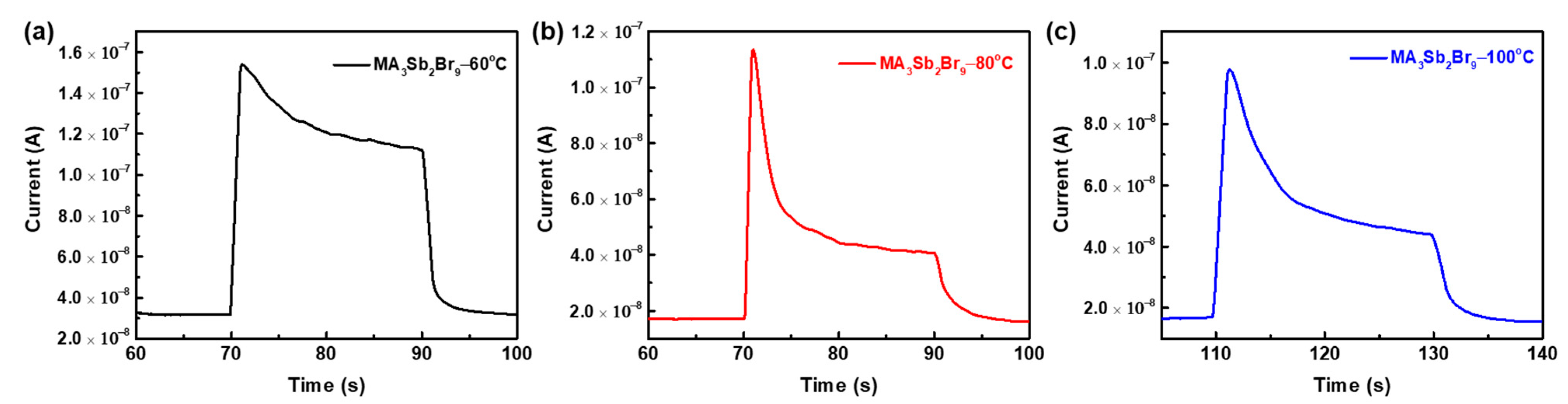

| Device Structure | Preparation Method | Rise /Decay Times (ms) | Responsivity (A/W) | EQE (%) | Detectivity (Jones) | Ref. |
|---|---|---|---|---|---|---|
| ITO/MA3Sb2I9 single crystals/ITO | Slowly cooling process | 0.4/0.9 | 40 | - | 1012 | [39] |
| ITO/MA3Sb2Br9 single crystals/ITO | Slowly cooling process | 1000 | 0.03 | - | 5 × 108 | [39] |
| Au/Ti/Si/SiO2/Cs3Sb2Br9 nanoflakes/Ti/Au | ITC process | 24/48 | 3.8 | - | 2.6 × 1012 | [40] |
| Au/Si/SiO2/Cs3Sb2Br9 single crystals/Au | Solvothermal process | 0.2/3.0 | 2.29 | 18 | 3.77 × 1012 | [42] |
| Ag/C60/MA3Sb2Br9 single crystals/C60/Ag | ITC process (oven) | 47.1/1162 | 0.113 | 32.7 | 4.32 × 1011 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hun, C.-M.; Tien, C.-H.; Lee, K.-L.; Lai, H.-Y.; Chen, L.-C. The Effects of Temperature on the Growth of a Lead-Free Perovskite-Like (CH3NH3)3Sb2Br9 Single Crystal for An MSM Photodetector Application. Sensors 2021, 21, 4475. https://doi.org/10.3390/s21134475
Hun C-M, Tien C-H, Lee K-L, Lai H-Y, Chen L-C. The Effects of Temperature on the Growth of a Lead-Free Perovskite-Like (CH3NH3)3Sb2Br9 Single Crystal for An MSM Photodetector Application. Sensors. 2021; 21(13):4475. https://doi.org/10.3390/s21134475
Chicago/Turabian StyleHun, Chien-Min, Ching-Ho Tien, Kuan-Lin Lee, Hong-Ye Lai, and Lung-Chien Chen. 2021. "The Effects of Temperature on the Growth of a Lead-Free Perovskite-Like (CH3NH3)3Sb2Br9 Single Crystal for An MSM Photodetector Application" Sensors 21, no. 13: 4475. https://doi.org/10.3390/s21134475
APA StyleHun, C.-M., Tien, C.-H., Lee, K.-L., Lai, H.-Y., & Chen, L.-C. (2021). The Effects of Temperature on the Growth of a Lead-Free Perovskite-Like (CH3NH3)3Sb2Br9 Single Crystal for An MSM Photodetector Application. Sensors, 21(13), 4475. https://doi.org/10.3390/s21134475







