Internal Consistency of Sway Measures via Embedded Head-Mounted Accelerometers: Implications for Neuromotor Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
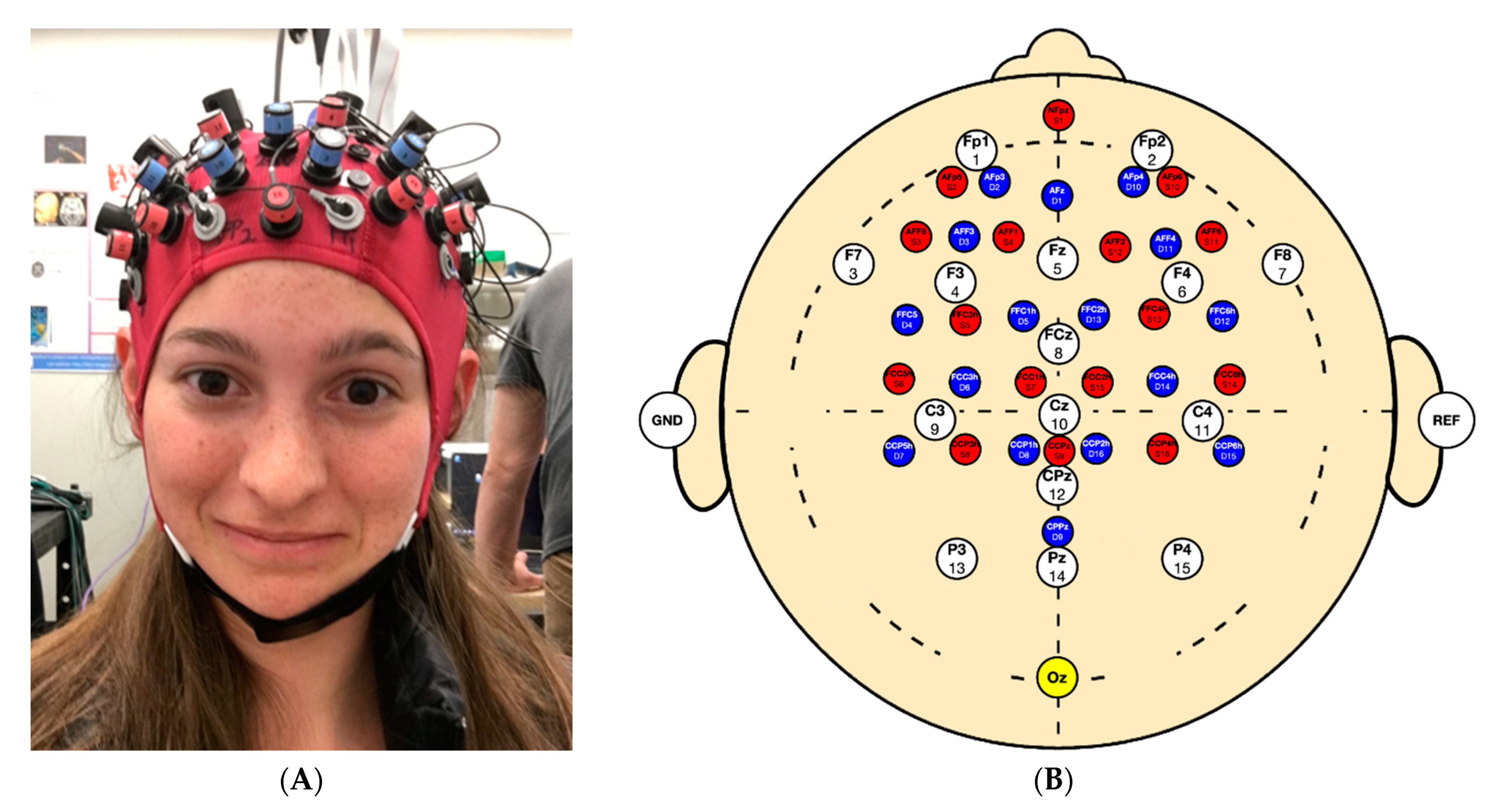
2.2. Testing Protocol
2.3. Data Processing
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Tilt-Correction Method for Linear Acceleration of a Single Recording
Appendix B. Mean Reliability of Each Sway Measure

References
- Maki, B.E.; Holliday, P.J.; Fernie, G.R. Aging and Postural Control: A Comparison of Spontaneous- and Induced-Sway Balance Tests. J. Am. Geriatr. Soc. 1990, 38, 1–9. [Google Scholar] [CrossRef]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Chiari, L. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Park. Relat. Disord. 2011, 17, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Moon, Y.; McGinnis, R.S.; Seagers, K.; Motl, R.W.; Sheth, N.; Wright, J.A.; Ghaffari, R.; Patel, S.; Sosnoff, J.J. Assessment of Postural Sway in Individuals with Multiple Sclerosis Using a Novel Wearable Inertial Sensor. Digit. Biomark. 2018, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.J.; Bruetsch, A.P.; Lynch, S.G.; Horak, F.B.; Huisinga, J.M. Instrumented balance and walking assessments in persons with multiple sclerosis show strong test-retest reliability. J. Neuroeng. Rehabil. 2017, 14, 43. [Google Scholar] [CrossRef]
- Grafton, S.T.; Ralston, A.B.; Ralston, J.D. Monitoring of postural sway with a head-mounted wearable device: Effects of gender, participant state, and concussion. Med. Devices Evid. Res. 2019, 12, 151–164. [Google Scholar] [CrossRef] [Green Version]
- King, L.A.; Mancini, M.; Fino, P.C.; Chesnutt, J.; Swanson, C.W.; Markwardt, S.; Chapman, J.C. Sensor-Based Balance Measures Outperform Modified Balance Error Scoring System in Identifying Acute Concussion. Ann. Biomed. Eng. 2017, 45, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Hubble, R.P.; Silburn, P.A.; Naughton, G.A.; Cole, M.H. Assessing stability in mild and moderate Parkinson’s disease: Can clinical measures provide insight? Gait Posture 2016, 49, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Whitney, S.L.; Roche, J.L.; Marchetti, G.F.; Lin, C.-C.; Steed, D.P.; Furman, G.R.; Musolino, M.C.; Redfern, M.S. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: A measure of balance. Gait Posture 2011, 33, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.; Chiari, L.; Horak, F.B. ISway: A sensitive, valid and reliable measure of postural control. J. Neuroeng. Rehabil. 2012, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsubaie, S.F.; Whitney, S.L.; Furman, J.M.; Marchetti, G.F.; Sienko, K.H.; Sparto, P.J. Reliability of Postural Sway Measures of Standing Balance Tasks. J. Appl. Biomech. 2018, 35, 11–18. [Google Scholar] [CrossRef]
- Saunders, N.W.; Koutakis, P.; Kloos, A.D.; Kegelmeyer, D.A.; Dicke, J.D.; Devor, S.T. Reliability and Validity of a Wireless Accelerometer for the Assessment of Postural Sway. J. Appl. Biomech. 2015, 31, 159–163. [Google Scholar] [CrossRef]
- Browne, J.; O’Hare, N. Review of the Different Methods for Assessing Standing Balance. Physiotherapy 2001, 87, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Noamani, A.; Nazarahari, M.; Lewicke, J.; Vette, A.H.; Rouhani, H. Validity of using wearable inertial sensors for assessing the dynamics of standing balance. Med. Eng. Phys. 2020, 77, 53–59. [Google Scholar] [CrossRef]
- Winter, D. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 19, 4075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynard, F.; Christe, D.; Terrier, P. Postural control in healthy adults: Determinants of trunk sway assessed with a chest-worn accelerometer in 12 quiet standing tasks. PLoS ONE 2019, 14, e0211051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewan, B.M.; James, R.C.; Kumar, N.A.; Sawyer, S.F. Kinematic Validation of Postural Sway Measured by Biodex Biosway (Force Plate) and SWAY Balance (Accelerometer) Technology. BioMed. Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Salisbury, J.P.; Keshav, N.U.; Sossong, A.D.; Sahin, N.T.; Shah, N.; Cikajlo, I.; Rey-Martinez, J.; Stanmore, E. Concussion Assessment With Smartglasses: Validation Study of Balance Measurement Toward a Lightweight, Multimodal, Field-Ready Platform. JMIR Mhealth Uhealth 2018, 6, e15. [Google Scholar] [CrossRef]
- Lubetzky, A.V.; Wang, Z.; Krasovsky, T. Head mounted displays for capturing head kinematics in postural tasks. J. Biomech. 2019, 86, 175–182. [Google Scholar] [CrossRef]
- Baracks, J.; Casa, D.J.; Covassin, T.; Sacko, R.; Scarneo, S.E.; Schnyer, D.; Yeargin, S.W.; Neville, C. Acute Sport-Related Concussion Screening for Collegiate Athletes Using an Instrumented Balance Assessment. J. Athl. Train. 2018, 53, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Bonnette, S.; Diekfuss, J.A.; Grooms, D.; Myer, G.D.; Meehan, W.P.; Howell, D.R. Integrated linear and nonlinear trunk dynamics identify residual concussion deficits. Neurosci. Lett. 2020, 729, 134975. [Google Scholar] [CrossRef]
- Cohen, H.S.; Mulavara, A.P.; Peters, B.T.; Sangi-Haghpeykar, H.; Bloomberg, J.J. Standing balance tests for screening people with vestibular impairments. Laryngoscope 2014, 124, 545–550. [Google Scholar] [CrossRef] [Green Version]
- D’Silva, L.; Kluding, P.M.; Whitney, S.L.; Dai, H.; Santos, M. Postural sway in individuals with type 2 diabetes and concurrent benign paroxysmal positional vertigo. Int. J. Neurosci. 2017, 127, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Kosse, N.M.; Caljouw, S.; Vervoort, D.; Vuillerme, N.; Lamoth, C.J.C. Validity and Reliability of Gait and Postural Control Analysis Using the Tri-axial Accelerometer of the iPod Touch. Ann. Biomed. Eng. 2014, 43, 1935–1946. [Google Scholar] [CrossRef]
- Williams, J.M.; Dorey, C.; Clark, S.; Clark, C. The within-day and between-day reliability of using sacral accelerations to quantify balance performance. Phys. Ther. Sport 2016, 17, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Horst, F.; Müller, S.; Steinberg, F.; Doppelmayr, M. Current State and Future Prospects of EEG and fNIRS in Robot-Assisted Gait Rehabilitation: A Brief Review. Front. Hum. Neurosci. 2019, 13, 172. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Chen, Y.; Farrand, J.; Craft, M.A.; Carlson, B.W.; Yuan, H. Amplitude of fNIRS Resting-State Global Signal Is Related to EEG Vigilance Measures: A Simultaneous fNIRS and EEG Study. Front. Neurosci. 2020, 14, 560878. [Google Scholar] [CrossRef]
- Peterson, S.M.; Ferris, D.P. Differentiation in Theta and Beta Electrocortical Activity between Visual and Physical Perturbations to Walking and Standing Balance. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heebner, N.R.; Akins, J.S.; Lephart, S.M.; Sell, T.C. Reliability and validity of an accelerometry based measure of static and dynamic postural stability in healthy and active individuals. Gait Posture 2015, 41, 535–539. [Google Scholar] [CrossRef]
- Ozinga, S.J.; Alberts, J.L. Quantification of postural stability in older adults using mobile technology. Exp. Brain Res. 2014, 232, 3861–3872. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mendez, R.; Sekine, M.; Tamura, T. Postural sway parameters using a triaxial accelerometer: Comparing elderly and young healthy adults. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 899–910. [Google Scholar] [CrossRef]
- Johnston, W.; Heiderscheit, B.; Sanfilippo, J.; Brooks, M.A.; Caulfield, B. Athletes with a concussion history in the last two years have impairments in dynamic balance performance. Scand. J. Med. Sci. Sports 2020. [Google Scholar] [CrossRef] [PubMed]
- Diop, S.; Grizzle, J.W.; Moraal, P.E.; Stefanopoulou, A. Interpolation and Numerical Differentiation for Observer Design. In Proceedings of the 1994 American Control Conference-ACC ’94, Baltimore, MD, USA, 29 June–1 July 1994. [Google Scholar]
- Alessandrini, M.; Micarelli, A.; Viziano, A.; Pavone, I.; Costantini, G.; Casali, D.; Paolizzo, F.; Saggio, G. Body-worn triaxial accelerometer coherence and reliability related to static posturography in unilateral vestibular failure. Acta Otorhinolaryngol. Ital. 2017, 37, 231–236. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Use R! Springer New York: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Flash, T.; Hogan, N. The coordination of arm movements: An experimentally confirmed mathematical model. J. Neurosci. 1985, 5, 1688–1703. [Google Scholar] [CrossRef] [PubMed]

| Sex | Age | Weight (kg) | Height (m) | BMI (kg/cm2) | Nasion to Inion (cm) | Preauricular Point to Preauricular Point (cm) | Head Circumference (cm) | |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 23 | 48 | 1.60 | 18.75 | 39.0 | 37 | 56.0 |
| 2 | F | 30 | 61 | 1.55 | 25.39 | 35.0 | 34 | 55.0 |
| 3 | F | 24 | 50 | 1.58 | 20.03 | 39.0 | 36 | 57.0 |
| 4 | M | 27 | 96 | 1.88 | 27.16 | 42.0 | 36 | 60.0 |
| 5 | F | 20 | 50 | 1.64 | 18.59 | 37.0 | 34 | 52.5 |
| 6 | M | 21 | 64 | 1.63 | 24.09 | 37.0 | 33 | 55.0 |
| 7 | M | 24 | 77 | 1.83 | 22.99 | 38.0 | 38 | 58.5 |
| 8 | F | 21 | 58 | 1.78 | 18.31 | 37.5 | 32 | 56.0 |
| 9 | F | 26 | 58 | 1.63 | 21.83 | 35.0 | 34 | 52.5 |
| 10 | M | 25 | 90 | 1.88 | 25.46 | 36.0 | 32 | 57.0 |
| Metric | Description | Directions | Units |
|---|---|---|---|
| RMS | Sway magnitude | ML, AP, Transverse Plane | |
| P2P | Range | ML, AP | |
| Ellipse Area | Direction change | Transverse Plane | |
| Jerk | Smoothness of motion | Resultant Jerk from ML, AP and V data |
| Metric | ICC | Lower Bound | Upper Bound | F | df1 | df2 | p | Classification |
|---|---|---|---|---|---|---|---|---|
| Ellipse Area | 0.78 | 0.52 | 0.92 | 4.44 | 8 | 48 | >0.001 | Good |
| Anteroposterior Root Mean Square Acceleration | 0.76 | 0.48 | 0.92 | 4.10 | 8 | 48 | 0.001 | Good |
| Total Root Mean Square Acceleration | 0.84 | 0.66 | 0.95 | 6.28 | 8 | 48 | >0.001 | Good |
| Mediolateral Root Mean Square Acceleration | 0.79 | 0.55 | 0.93 | 4.71 | 8 | 48 | >0.001 | Good |
| Anteroposterior Peak-to-Peak | 0.67 | 0.30 | 0.89 | 3.05 | 8 | 48 | 0.007 | Moderate |
| Mediolateral Peak-to-Peak | 0.65 | 0.24 | 0.88 | 2.83 | 8 | 48 | 0.012 | Moderate |
| Jerk | 0.95 | 0.90 | 0.98 | 21.19 | 8 | 48 | >0.001 | Excellent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapointe, A.P.; Ritchie, J.N.; Vitali, R.V.; Burma, J.S.; Soroush, A.; Oni, I.; Dunn, J.F. Internal Consistency of Sway Measures via Embedded Head-Mounted Accelerometers: Implications for Neuromotor Investigations. Sensors 2021, 21, 4492. https://doi.org/10.3390/s21134492
Lapointe AP, Ritchie JN, Vitali RV, Burma JS, Soroush A, Oni I, Dunn JF. Internal Consistency of Sway Measures via Embedded Head-Mounted Accelerometers: Implications for Neuromotor Investigations. Sensors. 2021; 21(13):4492. https://doi.org/10.3390/s21134492
Chicago/Turabian StyleLapointe, Andrew P., Jessica N. Ritchie, Rachel V. Vitali, Joel S. Burma, Ateyeh Soroush, Ibukunoluwa Oni, and Jeff F. Dunn. 2021. "Internal Consistency of Sway Measures via Embedded Head-Mounted Accelerometers: Implications for Neuromotor Investigations" Sensors 21, no. 13: 4492. https://doi.org/10.3390/s21134492






