Hydrogen-Terminated Diamond Surface as a Gas Sensor: A Comparative Study of Its Sensitivities
Abstract
:1. Introduction
2. Experimental
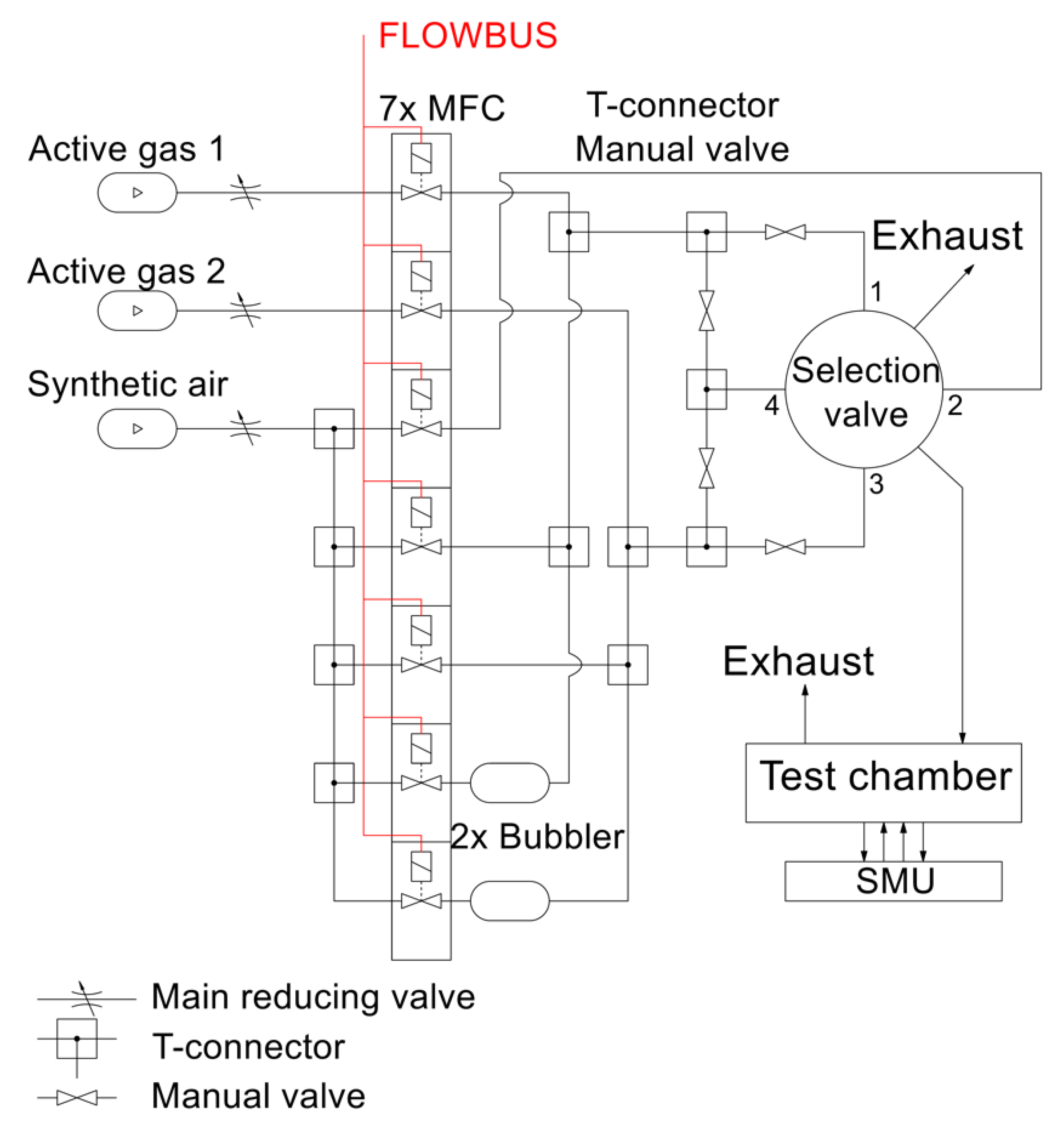
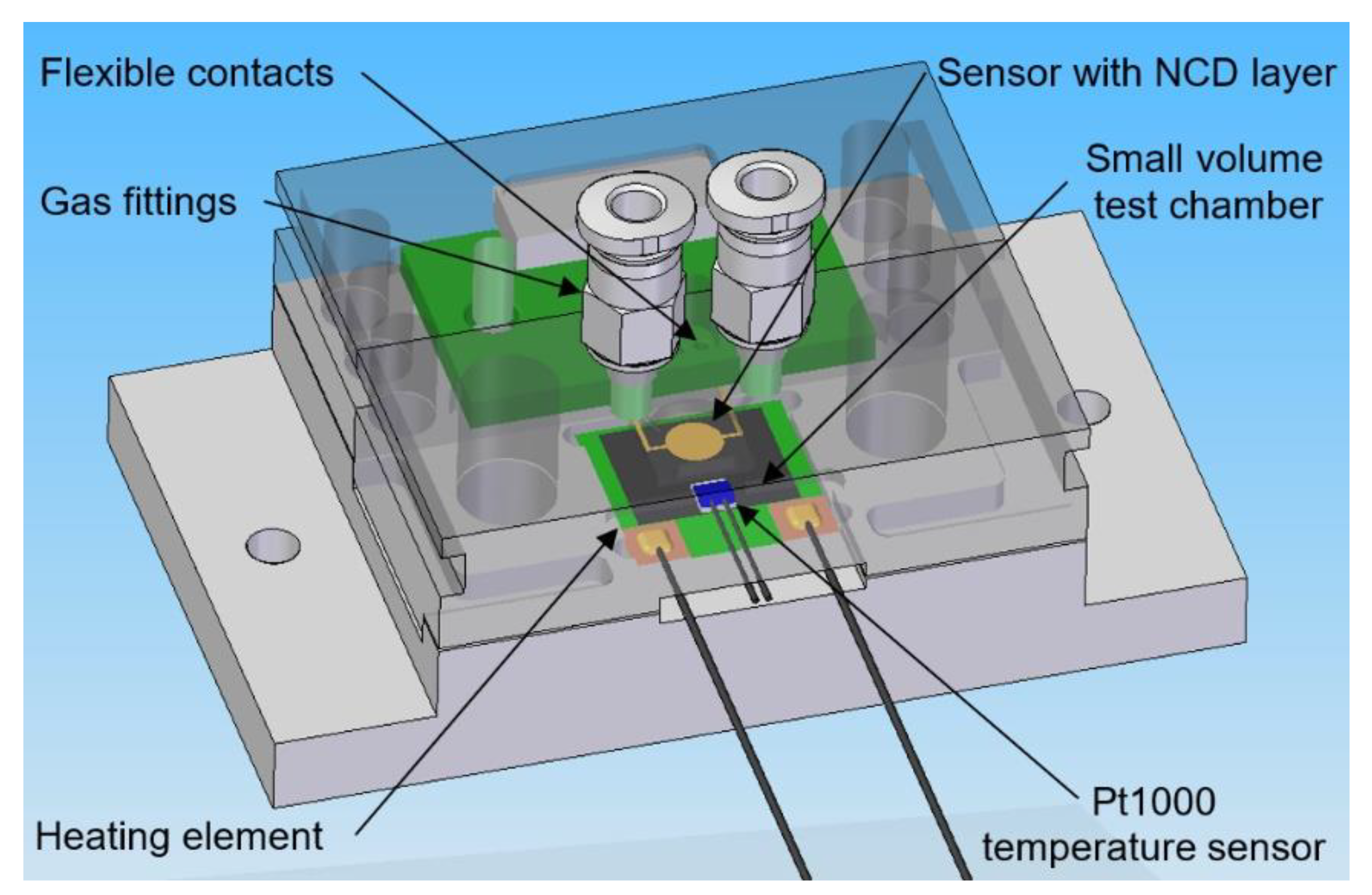
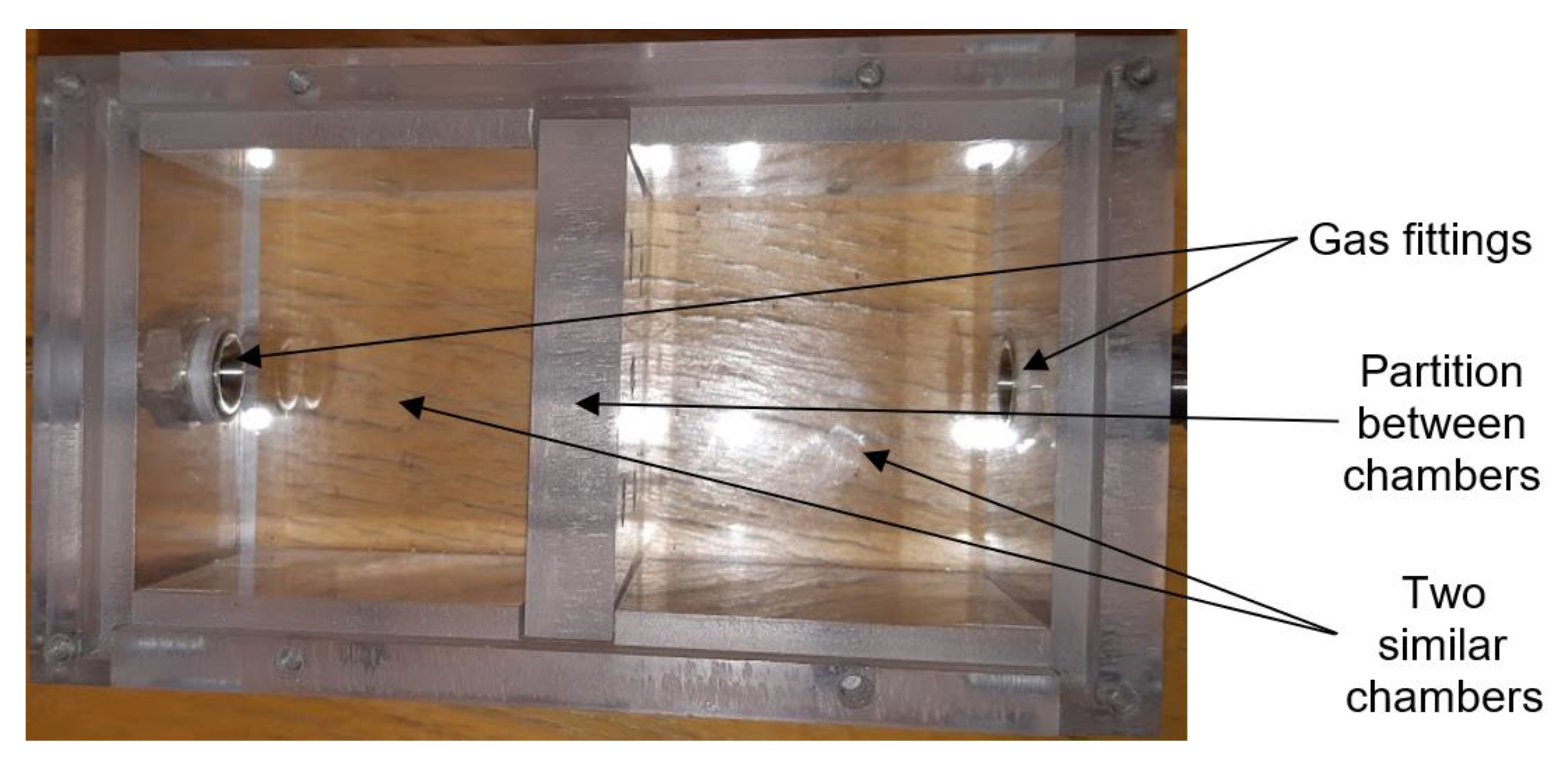
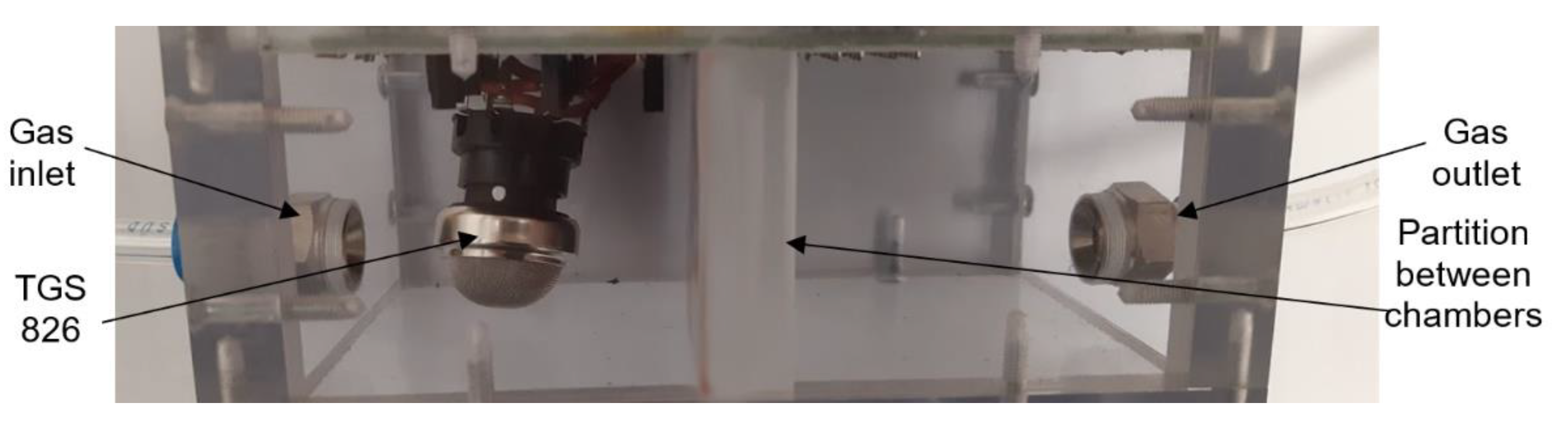
2.1. Experimental Setup for Gas Sensor Testing
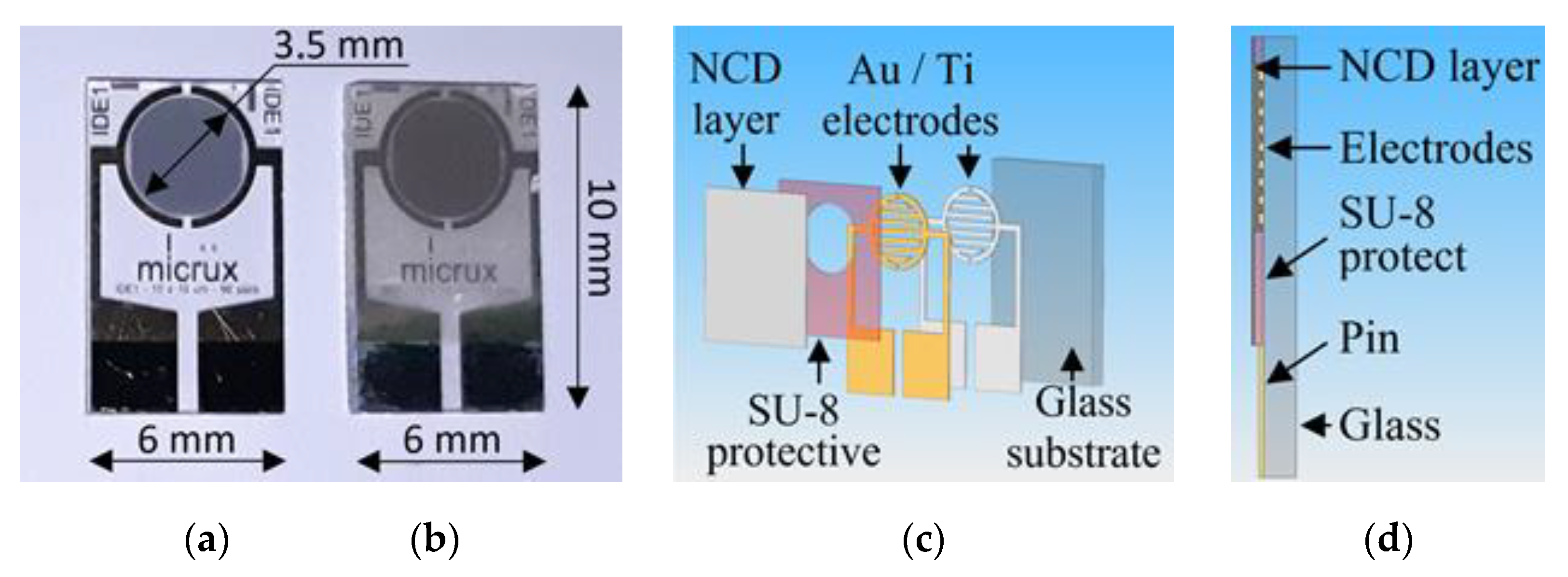
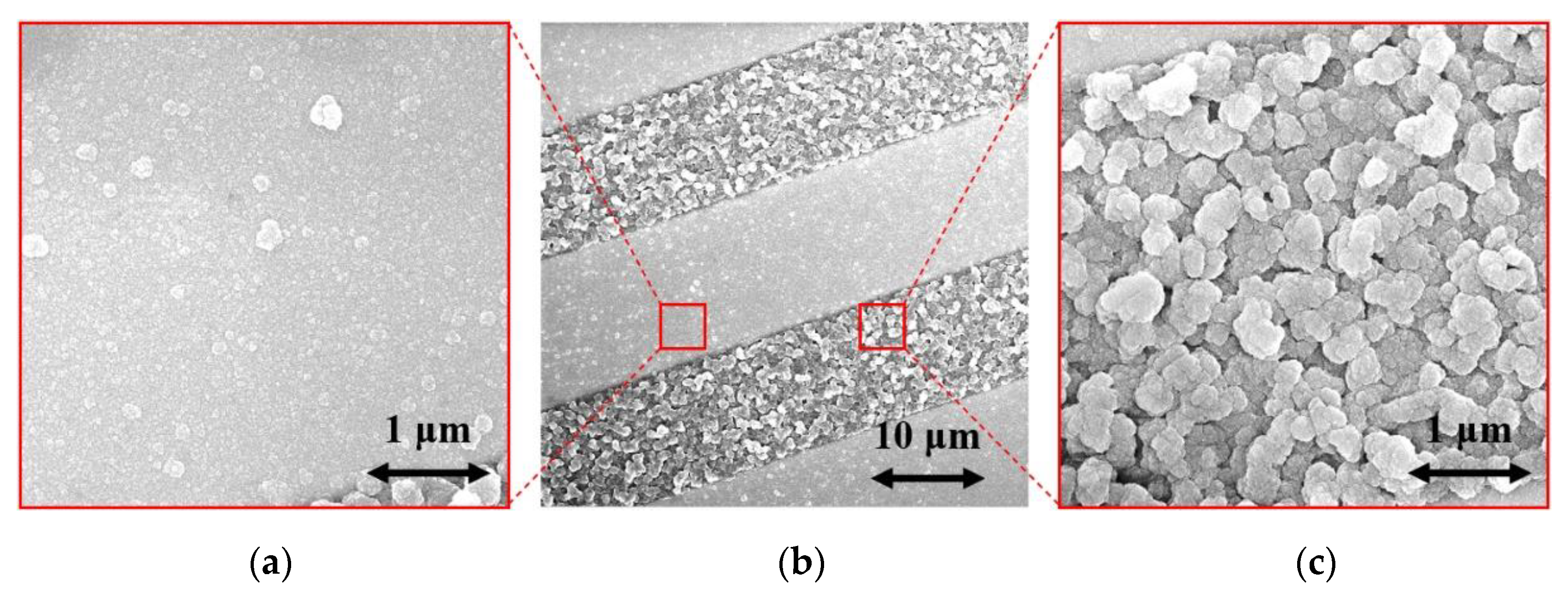
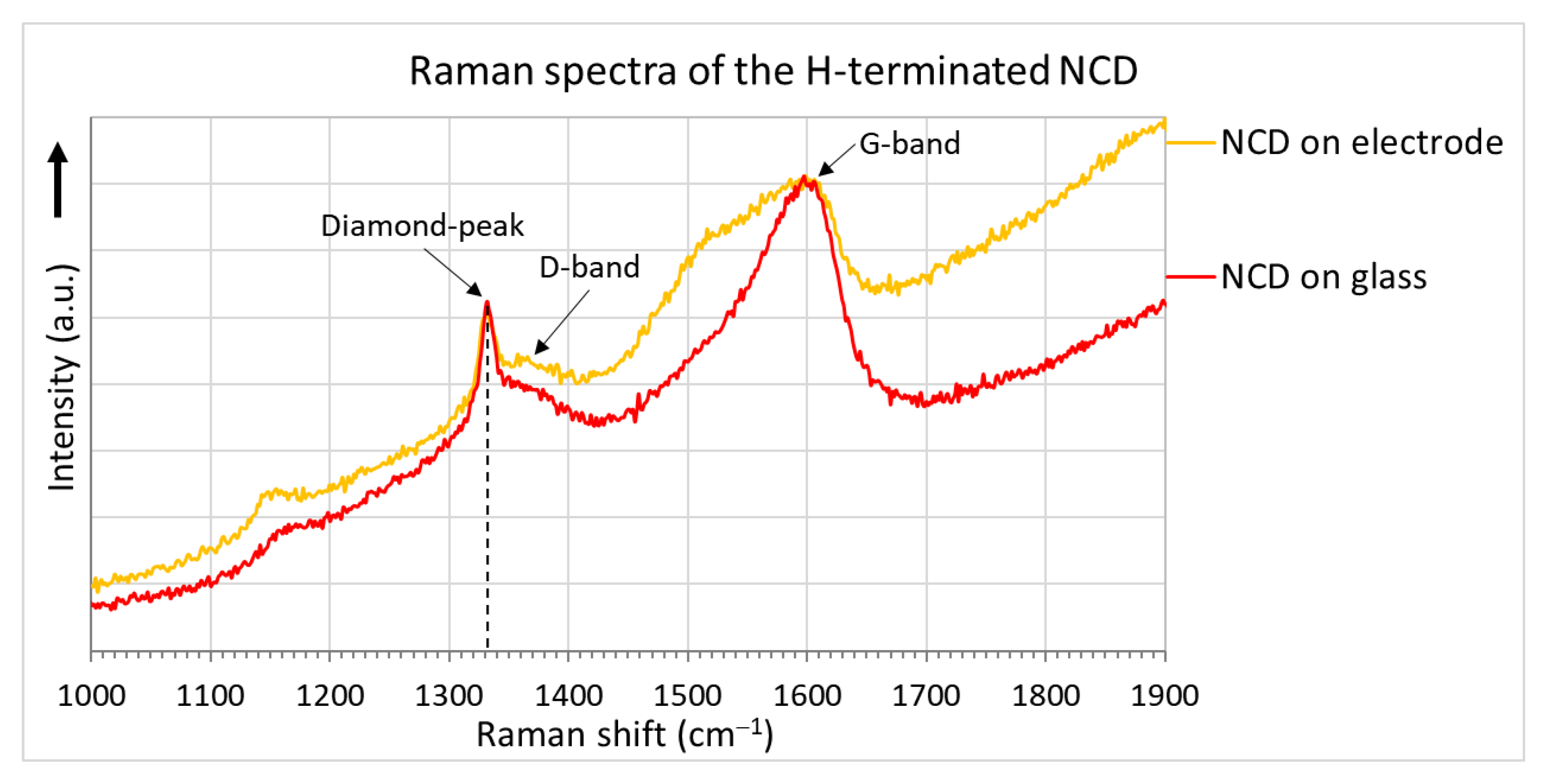
2.2. Sensor Elements with H-Terminated NCD
2.3. Figaro TGS 826 Commercial Sensor
2.4. Pyreos PY2055 Commercial Sensor
3. Results
3.1. H-Terminated NCD Conductivity Sensors
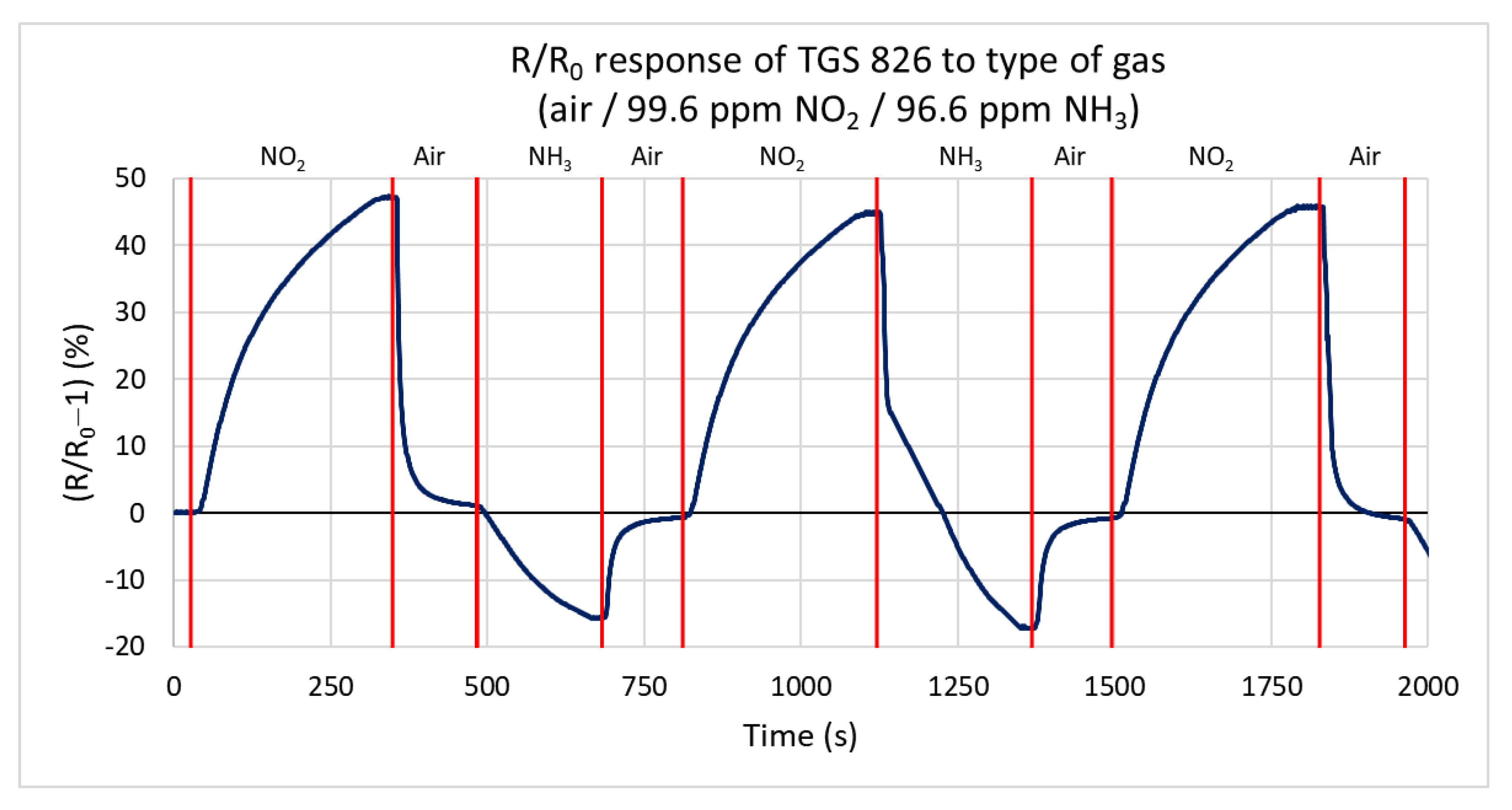
3.2. TGS 826 SnO2 Conductivity Sensor
3.3. PY2055 Infrared Sensor
3.4. Comparison of Sensors
4. Discussion
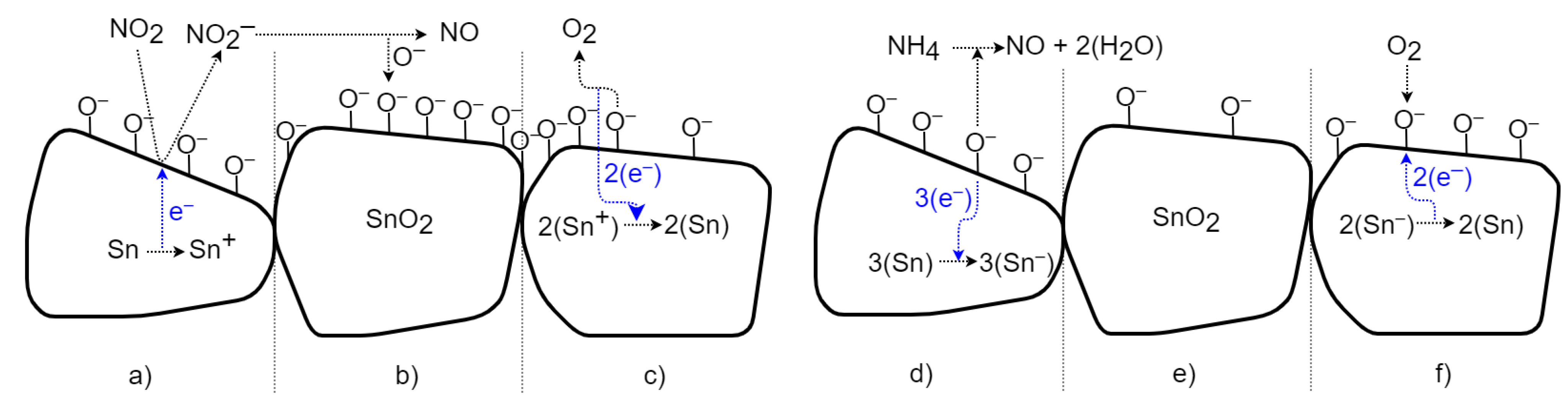
4.1. SnO2 Surface Gas Interaction Model
4.2. H-Terminated NCD Surface Gas Interaction Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helwig, A.; Mueller, G.; Garrido, J.A.; Eickhoff, M. Gas Sensing Properties of Hydrogen-Terminated Diamond. Sens. Actuators B Chem. 2008, 133, 156–165. [Google Scholar] [CrossRef]
- Liao, M.; Shen, B.; Wang, Z. Ultra-Wide Bandgap Semiconductor Materials; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128172568. [Google Scholar]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd, ed.; Wiley: Hoboken, NJ, USA, 2007; ISBN 0471143235. [Google Scholar]
- Adachi, S. Properties of Group-IV, III-V and II-VI Semiconductors; Wiley: Hoboken, NJ, USA, 2005; ISBN 9780470090343. [Google Scholar]
- Joshi, R.K.; Weber, J.E.; Hu, Q.; Johnson, B.; Zimmer, J.W.; Kumar, A. Carbon Monoxide Sensing at Room Temperature via Electron Donation in Boron Doped Diamond Films. Sens. Actuators B Chem. 2010, 145, 527–532. [Google Scholar] [CrossRef]
- Gurbuz, Y.; Kang, W.P.; Davidson, J.L.; Kinser, D.L.; Kerns, D.V. Diamond Microelectronic Gas Sensors. Sens. Actuators B Chem. 1996, 33, 100–104. [Google Scholar] [CrossRef]
- Sato, H.; Kasu, M. Electronic Properties of H-terminated Diamond during NO2 and O3 Adsorption and Desorption. Diam. Relat. Mater. 2012, 24, 99–103. [Google Scholar] [CrossRef]
- Laposa, A.; Kroutil, J.; Davydova, M.; Taylor, A.; Voves, J.; Klimsa, L.; Kopecek, J.; Husak, M. Inkjet Seeded CVD-Grown Hydrogenated Diamond Gas Sensor Under UV-LED Illumination. IEEE Sens. J. 2020, 20, 1158–1165. [Google Scholar] [CrossRef]
- Davydova, M.; Kulha, P.; Laposa, A.; Hruska, K.; Demo, P.; Kromka, A. Gas Sensing Properties of Nanocrystalline Diamond at Room Temperature. Beilstein J. Nanotechnol. 2014, 5, 2339–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davydova, M.; Stuchlik, M.; Rezek, B.; Larsson, K.; Kromka, A. Sensing of Phosgene by a Porous-Like Nanocrystalline Diamond Layer with Buried Metallic Electrodes. Sens. Actuators B Chem. 2013, 188, 675–680. [Google Scholar] [CrossRef]
- Mueller, G.; Krstev, I.; Maier, K.; Helwig, A.; Stutzmann, M.; Garrido, J. Resettable, Low-Temperature Accumulation Gas Sensors Based on Hydrogenated Diamond Transducers. Eurosensors 2015, 120, 590–593. [Google Scholar] [CrossRef] [Green Version]
- Kromka, A.; Davydova, M.; Rezek, B.; Vanecek, M.; Stuchlik, M.; Exnar, P.; Kalbac, M. Gas Sensing Properties of Nanocrystalline Diamond Films. Diam. Relat. Mater. 2010, 19, 196–200. [Google Scholar] [CrossRef]
- FIGARO USA, INC. TGS 826—For the Detection of Ammonia: Product Information. Available online: https://www.figarosensor.com/product/docs/TGS%20826%20%2805_04%29.pdf (accessed on 22 December 2020).
- Štulík, K.; Barek, J.; Janata, J.; Král, V.; Kronďák, M.; Šťastný, M. Sensors: General Aspects of Chemical Sensing; VŠCHT: Prague, Czech Republic, 2007; ISBN 9788086238203. [Google Scholar]
- Pyreos Limited, Thin Film Pyroelectric Dual Channel Sensor: Product Information. Available online: https://pyreos.com/wp-content/uploads/2020/11/Pyreos-Analog-TO-Two-Channels.pdf (accessed on 22 December 2020).
- FIGARO USA, INC. FIGARO GAS SENSORS: 1-Series and 8-Series: Product Catalogue. Available online: https://www.figarosensor.com/product/docs/figaro_tgs_serien.pdf (accessed on 1 February 2020).
- Nahlik, J.; Laposa, A.; Voves, J.; Kroutil, J.; Drahokoupil, J.; Davydova, M. A High Sensitivity UV Photodetector with Inkjet Printed ZnO/Nanodiamond Active Layers. IEEE Sens. J. 2019, 19, 5587–5593. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. SnO2 Thin Film Sensor with Enhanced Response for NO2 Gas at Lower Temperatures. Sens. Actuators B Chem. 2011, 156, 743–752. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Sun, Y.; Li, P.; Ji, J.; Chen, Y.; Zhang, W.; Hu, J. Fabrication and Gas Sensing Properties of Au-loaded SnO2 Composite Nanoparticles for Highly Sensitive Hydrogen Detection. Sens. Actuators B Chem. 2017, 240, 664–673. [Google Scholar] [CrossRef]






| IDT Sensors with H-Terminated Diamond | Commercial Sensors | ||||
|---|---|---|---|---|---|
| Temp. (°C) | Sensor No. 1 | Sensor No. 2 | Figaro TGS 826 (SnO2) | Pyreos PY2055 (IR Sensor) | |
| R0 (kΩ) | 125 | 216 | 111 | 52.9 | 0.12 (V) |
| 75 | 223 | 107 | |||
| 40 | 219 | 113 | |||
| (R-R0)/R0 response to 96.6 ppm NH3 (%) | 125 | 39 | 13 | −16.9 | N.A. ((U-U0)/U0) |
| 75 | 4 | 5 | |||
| 40 | N.A. | 2.5 | |||
| (R-R0)/R0 response to 99.6 ppm NO2 (%) | 125 | −41 | −11 | 47.8 | −46 ((U-U0)/U0) |
| 75 | −4.5 | −7 | |||
| 40 | N.A. | −5 | |||
| Time response to 96.6 ppm NH3 (Ω/s) | 125 | 908 | 183 | −2238 | N.A. |
| 75 | 84 | 123 | |||
| 40 | N.A. | 83 | |||
| Time response to 99.6 ppm NO2 (Ω/s) | 125 | −998 | −375 | 297 | 1.7 (%/s) |
| 75 | −75 | −214 | |||
| 40 | N.A. | −102 | |||
| Sensitivity to NH3 (%/ppm) | 125 | 0.259 | 0.092 | −0.135 | N.A. |
| Sensitivity to NO2 (%/ppm) | 125 | −0.161 | −0.058 | 0.482 | −0.315 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kočí, M.; Kromka, A.; Bouřa, A.; Szabó, O.; Husák, M. Hydrogen-Terminated Diamond Surface as a Gas Sensor: A Comparative Study of Its Sensitivities. Sensors 2021, 21, 5390. https://doi.org/10.3390/s21165390
Kočí M, Kromka A, Bouřa A, Szabó O, Husák M. Hydrogen-Terminated Diamond Surface as a Gas Sensor: A Comparative Study of Its Sensitivities. Sensors. 2021; 21(16):5390. https://doi.org/10.3390/s21165390
Chicago/Turabian StyleKočí, Michal, Alexander Kromka, Adam Bouřa, Ondrej Szabó, and Miroslav Husák. 2021. "Hydrogen-Terminated Diamond Surface as a Gas Sensor: A Comparative Study of Its Sensitivities" Sensors 21, no. 16: 5390. https://doi.org/10.3390/s21165390
APA StyleKočí, M., Kromka, A., Bouřa, A., Szabó, O., & Husák, M. (2021). Hydrogen-Terminated Diamond Surface as a Gas Sensor: A Comparative Study of Its Sensitivities. Sensors, 21(16), 5390. https://doi.org/10.3390/s21165390






