Evaluation of Periodontal Risk Factors with Quantitative Light-Induced Fluorescence Based Fluorescent Plaque Index, in Comparison to Radiographic and Oral Health Habit Scoring: A Retrospective Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Questionnaire
2.3. QLF Analysis
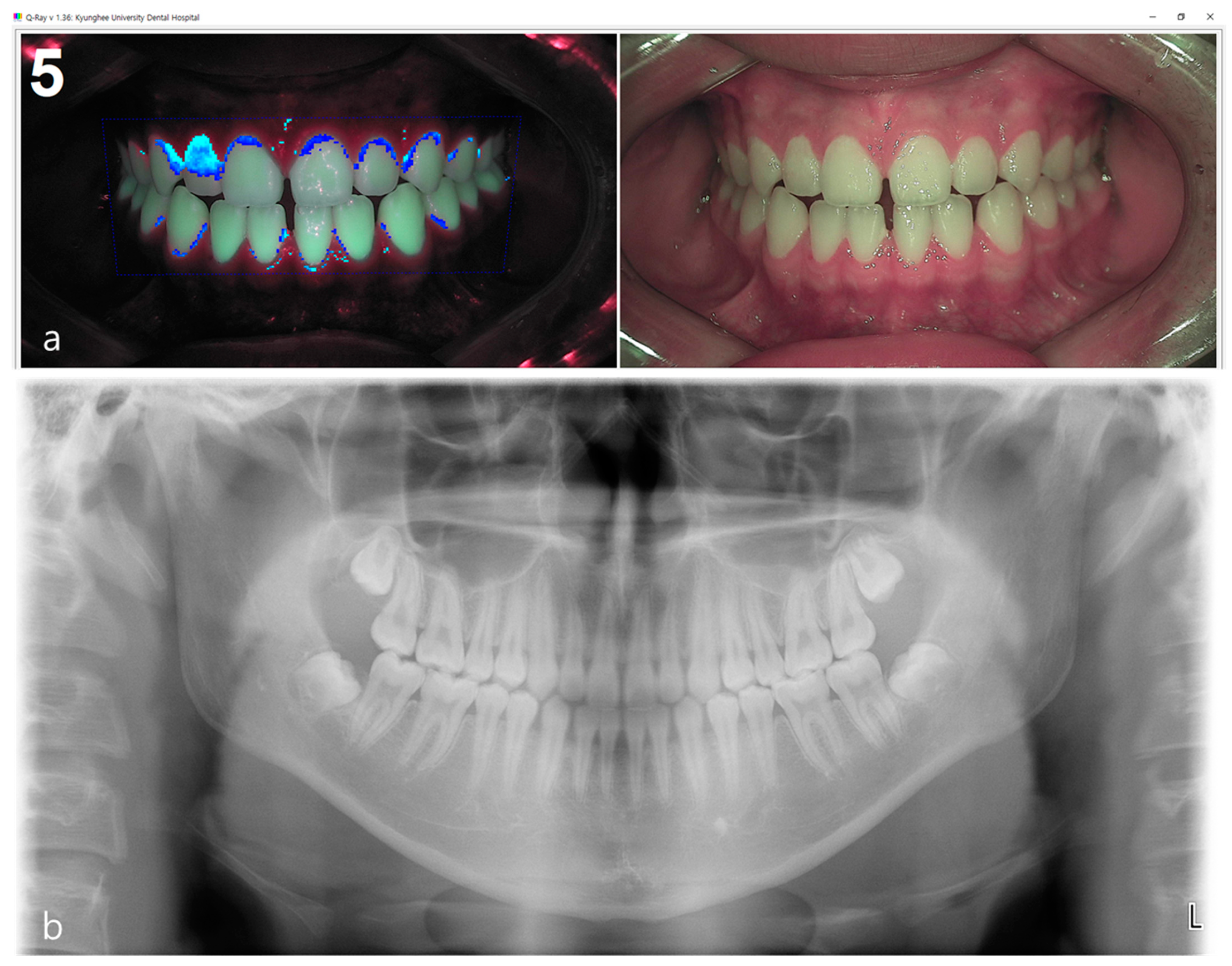
2.3.1. Acquisition of White-Light and Fluorescent Images
2.3.2. Fluorescent Plaque Index (FPI) Scoring
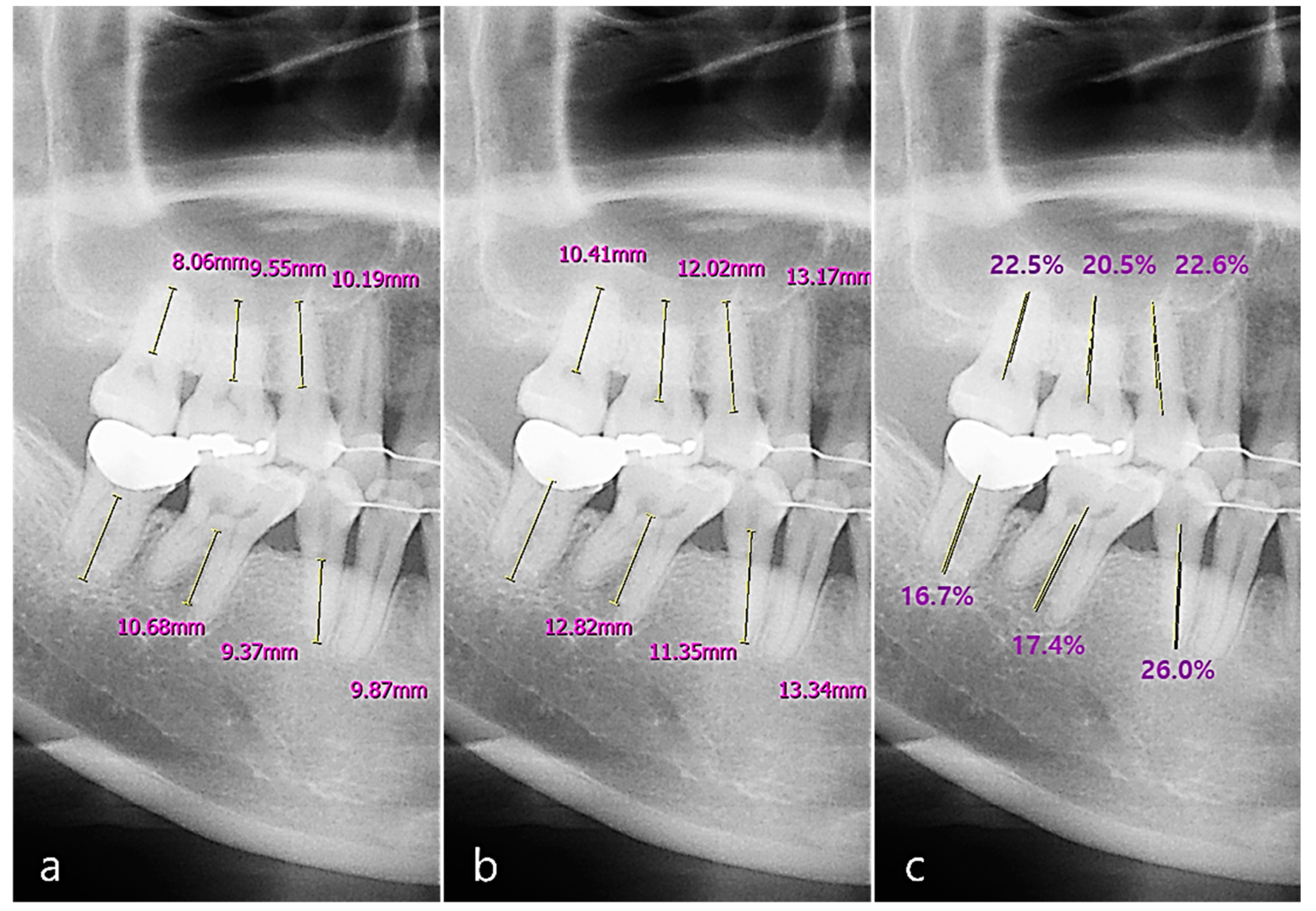
2.4. Radiographic Image Analysis
2.4.1. Acquisition of Panoramic and Periapical Images
2.4.2. Radiographic Bone Loss (RBL) Scoring
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Periodontol. 2018, 89, S9–S16. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Kwon, H.J.; Lee, S.G.; Choi, Y.H. The relationship between periodontal disease and the prevalence of cardiovascular diseases. J. Korean Acad. Oral Health 2007, 31, 387–395. [Google Scholar]
- Baek, H.J.; Choi, Y.H.; Lee, S.G.; Song, K.B.; Kwon, H.J. The association of metabolic syndrome and periodontitis in Korean adult population. J. Korean Acad. Oral Health 2010, 34, 338–345. [Google Scholar]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Ecological approaches to oral biofilms: Control without killing. Caries Res. 2015, 49 (Suppl. S1), 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef]
- Lang, N.P.; Ostergaard, E.; Löe, H. A fluorescent plaque disclosing agent. J. Periodontal Res. 1972, 7, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.D. Indices for measurement of soft accumulations in clinical studies of oral hygiene and periodontal disease. J. Periodontal Res. 1974, 9 (Suppl. S14), 7–30. [Google Scholar] [CrossRef]
- Park, S.W.; Kim, S.K.; Lee, H.S.; Lee, E.S.; de Jong, E.D.; Kim, B.I. Comparison of fluorescence parameters between three generations of QLF devices for detecting enamel caries in vitro and on smooth surfaces. Photodiagnosis Photodyn. Ther. 2019, 25, 142–147. [Google Scholar] [CrossRef]
- Kim, H.E.; Kwon, H.K.; Kim, B.I. Recovery percentage of remineralization according to severity of early caries. Am. J. Dent. 2013, 26, 132–136. [Google Scholar]
- Jun, M.K.; Park, S.W.; Lee, E.S.; Kim, B.R.; Kim, B.I. Diagnosis and management of cracked tooth by quantitative light-induced fluorescence technology. Photodiagnosis Photodyn. Ther. 2019, 26, 324–326. [Google Scholar]
- Han, S.Y.; Kim, B.R.; Ko, H.Y.; Kwon, H.K.; Kim, B.I. Validity and reliability of autofluorescence-based quantification method of dental plaque. Photodiagnosis Photodyn. Ther. 2015, 12, 587–591. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, B.R.; Ko, H.Y.; Kwon, H.K.; Kim, B.I. Assessing the use of Quantitative Light-induced Fluorescence-Digital as a clinical plaque assessment. Photodiagnosis Photodyn. Ther. 2016, 13, 34–39. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions-Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [Green Version]
- Garnick, J.J.; Silverstein, L. Periodontal probing: Probe tip diameter. J. Periodontol. 2000, 71, 96–103. [Google Scholar] [CrossRef]
- Keagle, J.G.; Garnick, J.J.; Searle, J.R.; King, G.E.; Morse, P.K. Gingival resistance to probing forces. I. Determination of optimal probe diameter. J. Periodontol. 1989, 60, 167–171. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Periodontol. 2018, 45, S46–S73. [Google Scholar] [CrossRef] [Green Version]
- Lange, D.E. Failure of periodontal treatment as a result of diagnostic mistakes. Dtsch. Zahnärztl. Z. 1982, 37, 604–609. (In German) [Google Scholar] [PubMed]
- Gröndahl, H.G.; Jönsson, E.; Lindahl, B. Diagnosis of marginal bone destruction with orthopantomography and intraoral full mouth radiography. Swed. Dent. J. 1971, 64, 439–446. [Google Scholar]
- Walsh, T.F.; Al-Hokail, O.S.; Fosam, E.B. The relationship of bone loss observed on panoramic radiographs with clinical periodontal screening. J. Clin. Periodontol. 1997, 24, 153–157. [Google Scholar] [CrossRef]
- Yoon, S.U.; Lee, E.S.; Park, Y.N. Subjective oral status perceptionand oral health impact profile (OHIP) according to the oral health management among international and domestic university students. J. Korean Soc. Dent. Hyg. 2021, 21, 203–209. [Google Scholar]
- Park, S.W.; Kahharova, D.; Lee, J.Y.; Lee, E.S.; de Josselin de Jong, E.; Khudanov, B.; Kim, B.I. Clinical assessment of an automated fluorescent plaque index scoring with quantitative light-induced fluorescence. Photodiagnosis Photodyn. Ther. 2020, 32, 102011. [Google Scholar] [CrossRef] [PubMed]
- Shaker, Z.M.H.; Parsa, A.; Moharamzadeh, K. Development of a Radiographic Index for Periodontitis. Dent. J. 2021, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Coulthwaite, L.; Pretty, I.A.; Smith, P.W.; Higham, S.M.; Verran, J. The microbiological origin of fluorescence observed in plaque on dentures during QLF analysis. Caries Res. 2006, 40, 112–116. [Google Scholar] [CrossRef]
- Thomas, R.Z.; van der Mei, H.C.; van der Veen, M.H.; de Soet, J.J.; Huysmans, M.C. Bacterial composition and red fluorescence of plaque in relation to primary and secondary caries next to composite: An in situ study. Oral Microbiol. Immunol. 2008, 23, 7–13. [Google Scholar] [CrossRef]
- van der Veen, M.H.; Volgenant, C.M.; Keijser, B.; Ten Cate, J.B.; Crielaard, W. Dynamics of red fluorescent dental plaque during experimental gingivitis--A cohort study. J. Dent. 2016, 48, 71–76. [Google Scholar] [CrossRef]
- Lee, E.S.; de Josselin de Jong, E.; Kim, B.I. Detection of dental plaque and its potential pathogenicity using quantitative light-induced fluorescence. J. Biophotonics 2019, 12, e201800414. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S. Intermicrobial Interactions as a Driver for Community Composition and Stratification of Oral Biofilms. J. Mol. Biol. 2015, 427, 3662–3675. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Abbas, D.K. Radiographic quantification of alveolar bone level changes. Comparison of 3 currently used methods. J. Clin. Periodontol. 1986, 13, 810–813. [Google Scholar] [CrossRef]
- Björn, H.; Holmberg, K. Radiographic determination of periodontal bone destruction in epidemiological research. Odontol. Revy 1966, 17, 232–250. [Google Scholar]
- Lang, N.P.; Hill, R.W. Radiographs in periodontics. J. Clin. Periodontol. 1977, 4, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Soikkonen, K.; Wolf, J.; Tenkanen, M. Clinical and panoramic assessment of marginal bone loss. A cadaver study. Proc. Finn. Dent. Soc. 1990, 86, 137–141. [Google Scholar]
- Akesson, L.; Häkansson, J.; Rohlin, M. Comparison of panoramic and intraoral radiography and pocket probing for the marginal bonelevel. J. Clin. Periodontol. 1992, 19, 326–332. [Google Scholar] [CrossRef]
- Pepelassi, E.A.; Tsiklakis, K.; Diamanti-Kipioti, A. Radiographic detection and assessment of the periodontal endosseous defects. J. Clin. Periodontol. 2000, 27, 224–230. [Google Scholar] [CrossRef]
- Ziebolz, D.; Szabadi, I.; Rinke, S.; Hornecker, E.; Mausberg, R.F. Initial periodontal screening and radiographic findings—A comparison of two methods to evaluate the periodontal situation. BMC Oral Health 2011, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Persson, R.E.; Tzannetou, S.; Feloutzis, A.G.; Brägger, U.; Persson, G.R.; Lang, N.P. Comparison between panoramic and intra-oral radiographs for the assessment of alveolar bone levels in a periodontal maintenance population. J. Clin. Periodontol. 2003, 30, 833–839. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.W.; Huang, C.T.; Lee, J.H.; Li, C.H.; Chang, S.W.; Siao, M.J.; Lai, T.M.; Ibragimov, B.; Vrtovec, T.; Ronneberger, O.; et al. A benchmark for comparison of dental radiography analysis algorithms. Med. Image Anal. 2016, 31, 63–76. [Google Scholar] [CrossRef]
- Chang, H.J.; Lee, S.J.; Yong, T.H.; Shin, N.Y.; Jang, B.G.; Kim, J.E.; Huh, K.H.; Lee, S.S.; Heo, M.S.; Choi, S.C.; et al. Deep Learning Hybrid Method to Automatically Diagnose Periodontal Bone Loss and Stage Periodontitis. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Sheiham, A.; Nicolau, B. Evaluation of social and psychological factors in periodontal disease. Periodontol. 2000 2005, 39, 118–131. [Google Scholar] [CrossRef]
- Sakki, T.K.; Knuuttila, M.L.; Vimpari, S.S.; Kivelä, S.L. Lifestyle, dental caries and number of teeth. Community Dent. Oral Epidemiol. 1994, 22, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Shizukuishi, S. [Smoking and periodontal disease]. Clin. Calcium. 2007, 17, 226–232. [Google Scholar]
- Chen, M.S.; Stone, D.B. Toothbrushing, flossing, and dental visits in relation to socioeconomic characteristics of white American families. Community Dent. Oral Epidemiol. 1983, 11, 325–332. [Google Scholar] [CrossRef]
- Davis, M.M.; Jones, D.W. The role of lifestyle management in the overall treatment plan for prevention and management of hypertension. Semin. Nephrol. 2002, 22, 35–43. [Google Scholar] [CrossRef]
- Nyman, S.; Rosling, B.; Lindhe, J. Effect of professional tooth cleaning on healing after periodontal surgery. J. Clin. Periodontol. 1975, 2, 80–86. [Google Scholar] [CrossRef]
- Rosling, B. Periodontally treated dentitions: Their maintenance and prognosis. Int. Dent. J. 1983, 33, 147–151. [Google Scholar]
- Westfelt, E.; Rylander, H.; Dahlén, G.; Lindhe, J. The effect of supragingival plaque control on the progression of advanced periodontal disease. J. Clin. Periodontol. 1998, 25, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, B.; Ohrn, K.; Oscarson, N.; Lindberg, P. The effectiveness of an individually tailored oral health educational programme on oral hygiene behaviour in patients with periodontal disease: A blinded randomized-controlled clinical trial (one-year follow-up). J. Clin. Periodontol. 2009, 36, 1025–1034. [Google Scholar] [CrossRef]




| Oral Health Habits | Score |
|---|---|
| Tooth brushing/day | |
| None | 0 |
| 1~2 | 1 |
| 3 or more | 2 |
| Tooth brushing before bed/week | |
| 0 | 0 |
| 1~3 | 1 |
| 4~6 | 2 |
| 7 | 3 |
| Use of oral hygiene products | |
| No | 0 |
| Yes | 1 |
| Use of interdental brush or dental floss | |
| No | 0 |
| Sometimes | 1 |
| Always use with toothbrush | 2 |
| Scaling experience | |
| None | 0 |
| >2 years | 1 |
| <2 years | 2 |
| <1 year | 3 |
| <6 months | 4 |
| The average of oral health habit score | Total | 7.52 ± 2.07 |
| Male | 6.98 ± 2.20 | |
| Female | 7.88 ± 1.91 | |
| The patient number of FPI | 0 | 125 (53.9%) |
| 1 | 57 (24.6%) | |
| 2 | 6 (2.6%) | |
| 3 | 16 (6.9%) | |
| 4 | 15 (6.5%) | |
| 5 | 13 (5.6%) | |
| The average of RBL score | Total | 1.50 ± 0.32 |
| Maxilla | 1.50 ± 0.33 | |
| Mandible | 1.50 ± 0.36 | |
| Anterior | 1.44 ± 0.36 | |
| Posterior | 1.56 ± 0.33 |
| Variable | Multiple Generalized Linear Model | ||||
|---|---|---|---|---|---|
| B Estimate | CI | p-Value | |||
| Oral health habit score | FPI | −0.241 | −0.332 | −0.150 | <0.0001 |
| A30 | −0.352 | −0.537 | −0.167 | 0.000 | |
| A120 | −0.122 | −0.208 | −0.035 | 0.006 | |
| Age | FPI | 0.018 | 0.004 | 0.032 | 0.010 |
| A30 | 0.027 | −0.001 | 0.055 | 0.051 | |
| A120 | 0.001 | −0.012 | 0.014 | 0.851 | |
| Sex (female) | FPI | −0.458 | −0.846 | −0.070 | 0.021 |
| A30 | −0.674 | −1.463 | 0.114 | 0.093 | |
| A120 | −0.187 | −0.555 | 0.181 | 0.318 | |
| Variable | Multiple Generalized Linear Model | |||||
|---|---|---|---|---|---|---|
| B Estimate | CI | p-Value | ||||
| Oral health habit score | RBL | Total | 0.002 | −0.017 | 0.021 | 0.8707 |
| Maxilla | −0.002 | −0.022 | 0.019 | 0.8640 | ||
| Mandible | 0.005 | −0.017 | 0.027 | 0.6385 | ||
| Anterior | 0.005 | −0.017 | 0.027 | 0.6590 | ||
| Posterior | −0.002 | −0.021 | 0.017 | 0.8245 | ||
| Age | RBL | Total | 0.009 | 0.006 | 0.012 | <0.0001 |
| Maxilla | 0.008 | 0.005 | 0.011 | <0.0001 | ||
| Mandible | 0.010 | 0.007 | 0.014 | <0.0001 | ||
| Anterior | 0.007 | 0.003 | 0.010 | 0.000 | ||
| Posterior | 0.011 | 0.008 | 0.014 | <0.0001 | ||
| Sex (female) | RBL | Total | −0.060 | −0.141 | 0.021 | 0.1477 |
| Maxilla | −0.058 | −0.146 | 0.029 | 0.1893 | ||
| Mandible | −0.077 | −0.170 | 0.015 | 0.1018 | ||
| Anterior | −0.039 | −0.135 | 0.057 | 0.4212 | ||
| Posterior | −0.084 | −0.167 | −0.002 | 0.044 | ||
| Coefficient of Correlation | p-Value | ||
|---|---|---|---|
| RBL_Total | FPI | −0.0008 | 0.9909 |
| A30 | −0.0347 | 0.5996 | |
| A120 | 0.0585 | 0.3752 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.H.; Choi, J.-Y.; Lee, S.R.; Kim, S.-H. Evaluation of Periodontal Risk Factors with Quantitative Light-Induced Fluorescence Based Fluorescent Plaque Index, in Comparison to Radiographic and Oral Health Habit Scoring: A Retrospective Case Study. Sensors 2021, 21, 5774. https://doi.org/10.3390/s21175774
Oh SH, Choi J-Y, Lee SR, Kim S-H. Evaluation of Periodontal Risk Factors with Quantitative Light-Induced Fluorescence Based Fluorescent Plaque Index, in Comparison to Radiographic and Oral Health Habit Scoring: A Retrospective Case Study. Sensors. 2021; 21(17):5774. https://doi.org/10.3390/s21175774
Chicago/Turabian StyleOh, Song Hee, Jin-Young Choi, Sae Rom Lee, and Seong-Hun Kim. 2021. "Evaluation of Periodontal Risk Factors with Quantitative Light-Induced Fluorescence Based Fluorescent Plaque Index, in Comparison to Radiographic and Oral Health Habit Scoring: A Retrospective Case Study" Sensors 21, no. 17: 5774. https://doi.org/10.3390/s21175774
APA StyleOh, S. H., Choi, J.-Y., Lee, S. R., & Kim, S.-H. (2021). Evaluation of Periodontal Risk Factors with Quantitative Light-Induced Fluorescence Based Fluorescent Plaque Index, in Comparison to Radiographic and Oral Health Habit Scoring: A Retrospective Case Study. Sensors, 21(17), 5774. https://doi.org/10.3390/s21175774







