Benefits of a Switch from Intermittently Scanned Continuous Glucose Monitoring (isCGM) to Real-Time (rt) CGM in Diabetes Type 1 Suboptimal Controlled Patients in Real-Life: A One-Year Prospective Study § †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Genesis and Objective
2.3. Study Design and Patients
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
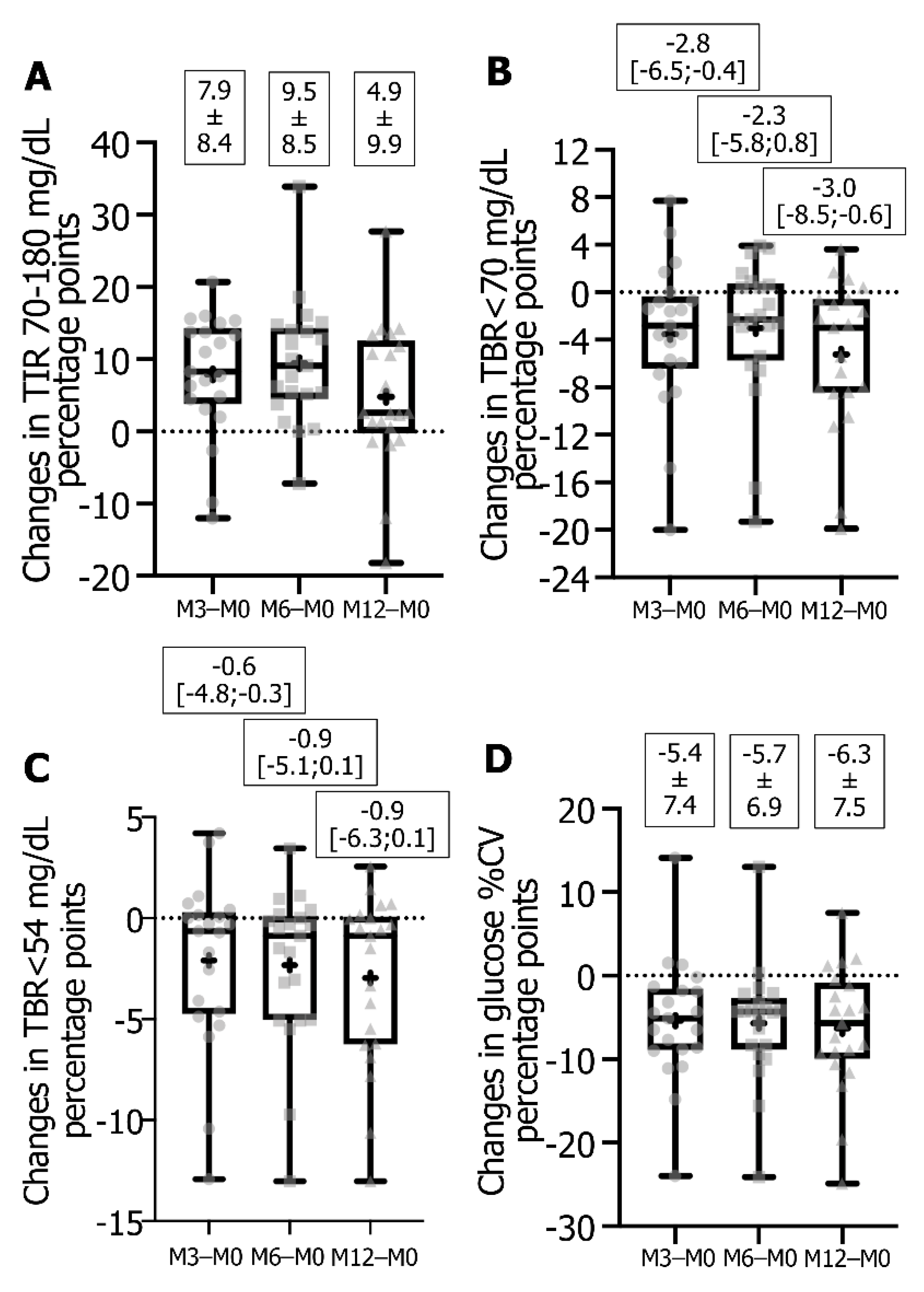
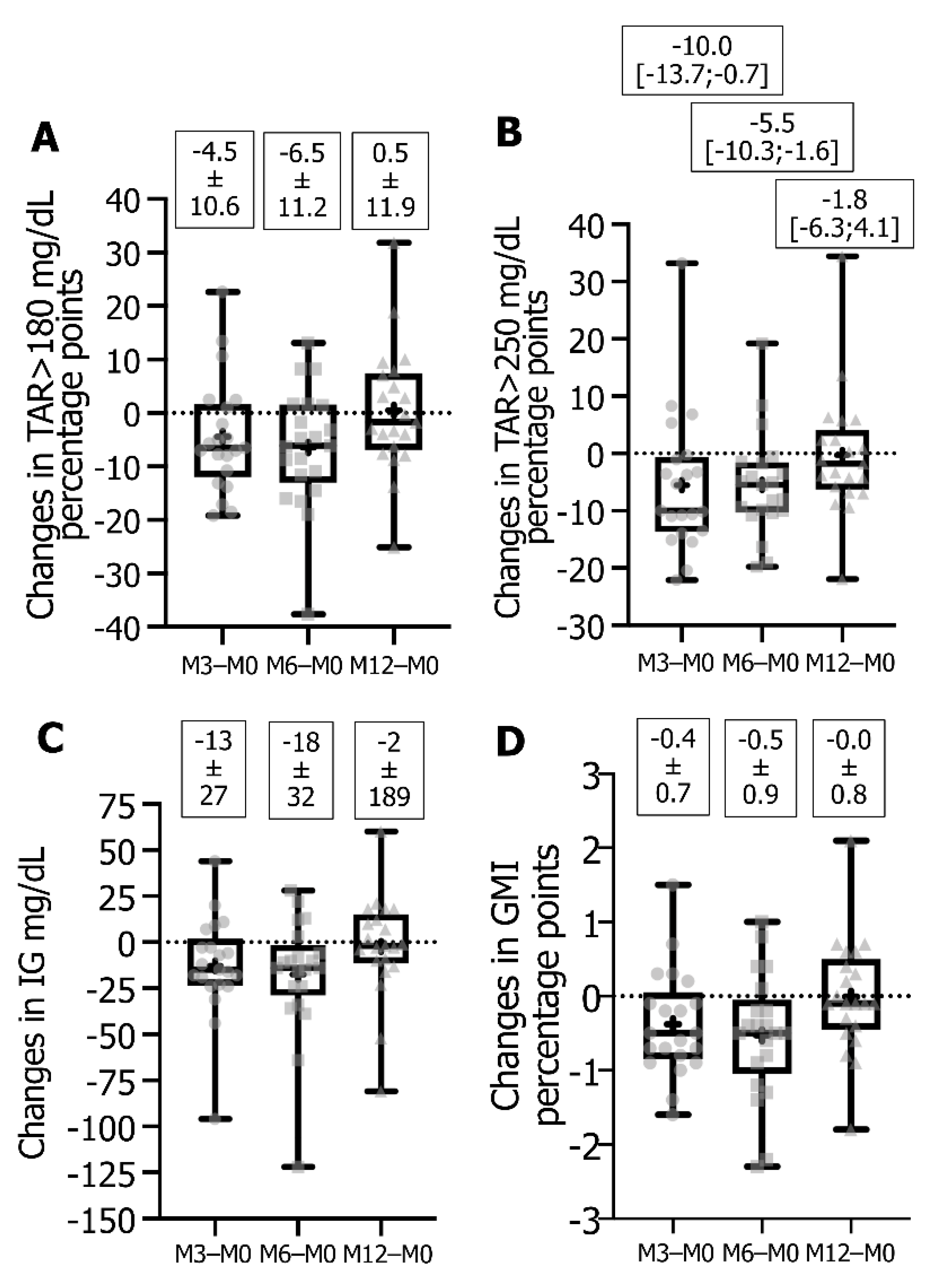
3.2. Impact of Switching from FSL1 to DG4 CGM Sensors on Glucose Metrics and HbA1c
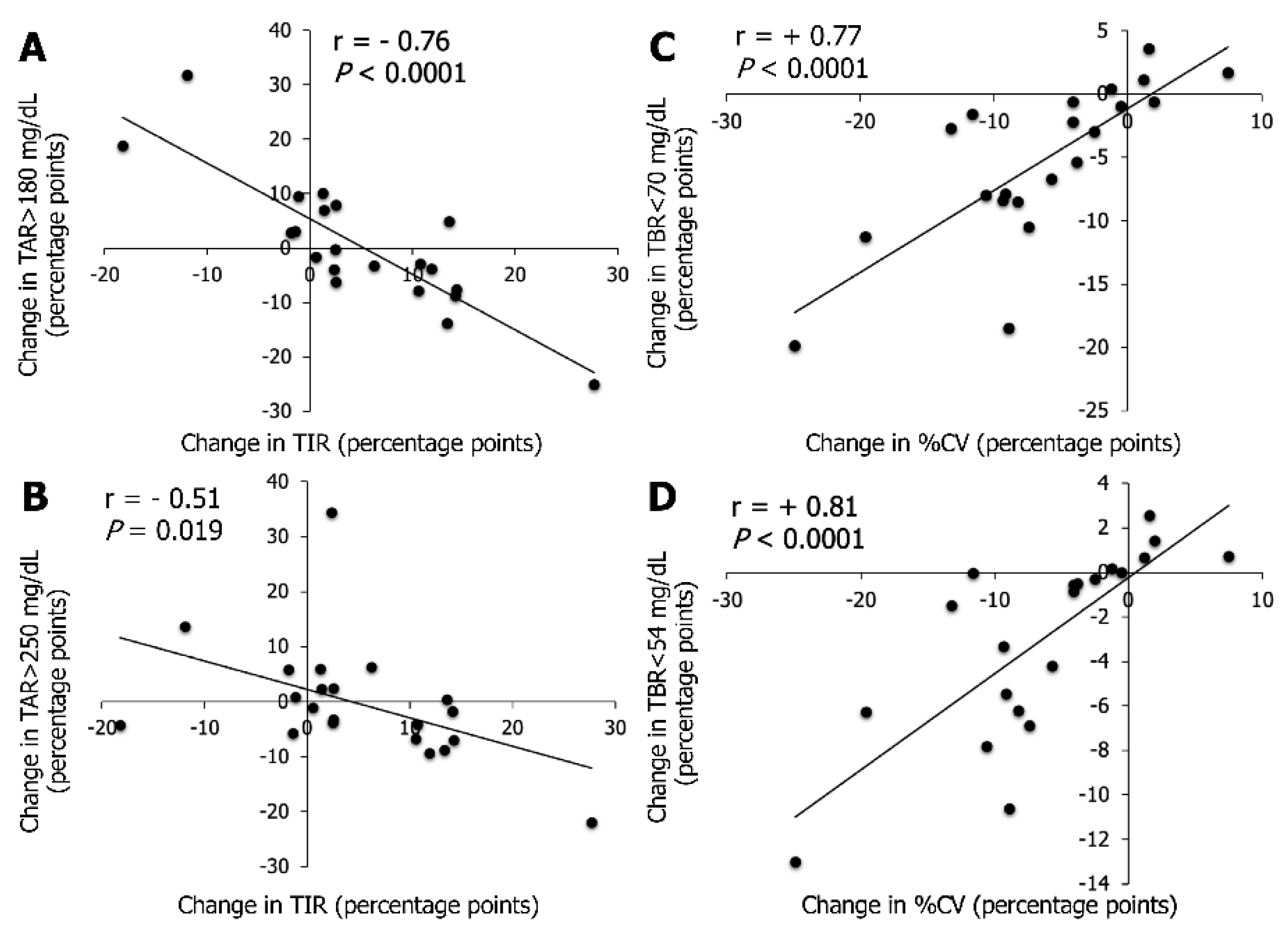
3.3. Association between CGM Metrics
3.4. Association between Patient Characteristics at Baseline and Changes in Metrics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dicembrini, I.; Cosentino, C.; Monami, M.; Mannucci, E.; Pala, L. Effects of real-time continuous glucose monitoring in type 1 diabetes: A meta-analysis of randomized controlled trials. Acta Diabetol. 2020, 58, 401–410. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Signoriello, S.; Maio, A.; Chiodini, P.; Bellastella, G.; Scappaticcio, L.; Longo, M.; Giugliano, D.; Esposito, K. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: A systematic review with meta-analysis of randomized controlled trials. Diabetes Care 2020, 43, 1146–1156. [Google Scholar] [CrossRef]
- Lin, Y.K.; Fisher, S.J.; Pop-Busui, R. Hypoglycemia unawareness and autonomic dysfunction in diabetes: Lessons learned and roles of diabetes technologies. J. Diabetes Investig. 2020, 11, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Charleer, S.; Mathieu, C.; Nobels, F.; De Block, C.; Radermecker, R.P.; Hermans, M.P.; Taes, Y.; Vercammen, C.; T’Sjoen, G.; Crenier, L.; et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: A real-world study. J. Clin. Endocrinol. Metab. 2018, 103, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Roussel, R.; Guerci, B.; Vicaut, E.; Depouvourville, G.; Detournay, B.; Emery, C.; Levrat-Guillen, F.; Riveline, J.-P. Dramatic drop-in ketoacidosis rate after freestyle libre system initiation in type 1 and type 2 diabetes in France, especially in people with low self-monitoring of blood glucose (SMBG): A nationwide study. Diabetes 2020, 69, 68. [Google Scholar] [CrossRef]
- Ranjan, A.G.; Rosenlund, S.V.; Hansen, T.W.; Rossing, P.; Andersen, S.; Nørgaard, K. Improved time in range over 1 year is associated with reduced albuminuria in individuals with sensor-augmented insulin pump–treated type 1 diabetes. Diabetes Care 2020, 43, 2882–2885. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, J.H. Time in range from continuous glucose monitoring: A novel metric for glycemic control. Diabetes Metab. J. 2020, 44, 828–839. [Google Scholar] [CrossRef]
- Reddy, M.; Jugnee, N.; El Laboudi, A.; Spanudakis, E.; Anantharaja, S.; Oliver, N. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with type 1 diabetes and impaired awareness of hypoglycaemia. Diabet. Med. 2017, 35, 483–490. [Google Scholar] [CrossRef]
- Reddy, M.; Jugnee, N.; Anantharaja, S.; Oliver, N. Switching from flash glucose monitoring to continuous glucose monitoring on hypoglycemia in adults with type 1 diabetes at high hypoglycemia risk: The extension phase of the I HART CGM study. Diabetes Technol. Ther. 2018, 20, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Hásková, A.; Radovnická, L.; Petruželková, L.; Parkin, C.G.; Grunberger, G.; Horová, E.; Navrátilová, V.; Kádě, O.; Matoulek, M.; Prázný, M.; et al. Real-time CGM is superior to flash glucose monitoring for glucose control in type 1 diabetes: The CORRIDA randomized controlled trial. Diabetes Care 2020, 43, 2744–2750. [Google Scholar] [CrossRef]
- Préau, Y.; Armand, M.; Galie, S.; Schaepelynck, P.; Raccah, D. Impact of switching from intermittently scanned to real-time continuous glucose monitoring systems in a type 1 diabetes patient French cohort: An observational study of clinical practices. Diabetes Technol. Ther. 2021, 23, 259–267. [Google Scholar] [CrossRef]
- Visser, M.M.; Charleer, S.; Fieuws, S.; De Block, C.; Hilbrands, R.; Van Huffel, L.; Maes, T.; Vanhaverbeke, G.; Dirinck, E.; Myngheer, N.; et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): A 6-month, prospective, multicentre, randomized controlled trial. Lancet 2021, 397, 2275–2283. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Edelman, S.V.; Argento, N.B.; Pettus, J.; Hirsch, I.B. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care 2018, 41, 2265–2274. [Google Scholar] [CrossRef] [Green Version]
- Mian, Z.; Hermayer, K.L.; Jenkins, A. Continuous glucose monitoring: Review of an innovation in diabetes management. Am. J. Med. Sci. 2019, 358, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Kravarusic, J.; Aleppo, G. Diabetes technology use in adults with type 1 and type 2 diabetes. Endocrinol. Metab. Clin. N. Am. 2019, 49, 37–55. [Google Scholar] [CrossRef]
- Choudhary, P.; Bellido, V.; Graner, M.; Altpeter, B.; Cicchetti, A.; Durand-Zaleski, I.; Kristensen, F.B. The challenge of sustainable access to telemonitoring tools for people with diabetes in Europe: Lessons from COVID-19 and beyond. Diabetes Ther. 2021, 12, 2311–2327. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous glucose monitoring sensors for diabetes management: A review of technologies and applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Borot, S.; Benhamou, P.Y.; Atlan, C.; Bismuth, E.; Bonnemaison, E.; Catargi, B.; Charpentier, G.; Farret, A.; Filhol, N.; Franc, S.; et al. Practical implementation, education and interpretation guidelines for continuous glucose monitoring: A French position statement. Diabetes Metab. 2018, 44, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Campbell, F.; Joule, N.; Kar, P.; Diabetes, U.K. A Type 1 diabetes technology pathway: Consensus statement for the use of technology in Type 1 diabetes. Diabet. Med. 2019, 36, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.V.; Erbey, J.R.; Becker, D.; Arslanian, S.; Orchard, T.J. Can clinical factors estimate insulin resistance in type 1 diabetes. Diabetes 2000, 49, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Kietsiriroje, N.; Pearson, S.; Campbell, M.; Robert, A.S.; Ariëns, R.A.S.; Ajjan, R.A. Double diabetes: A distinct high-risk group? Diabetes Obes. Metab. 2019, 21, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Rodbard, D. Glucose time in range, time above range, and time below range depend on mean or median glucose or HbA1c, glucose coefficient of variation, and shape of the glucose distribution. Diabetes Technol. Ther. 2020, 22, 492–500. [Google Scholar] [CrossRef]
- Lin, Y.K.; Groat, D.; Chan, O.; Hung, M.; Sharma, A.; Varner, M.W.; Gouripeddi, R.; Facelli, J.C.; Fisher, S.J. Alarm settings of continuous glucose monitoring systems and associations to glucose outcomes in type 1 diabetes. J. Endocr. Soc. 2019, 4, bvz005. [Google Scholar] [CrossRef] [PubMed]
- Vigersky, R.A.; McMahon, C. The Relationship of Hemoglobin A1C to Time-in-Range in Patients with Diabetes. Diabetes Technol. Ther. 2019, 21, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Bergenstal, R.M.; Cheng, P.; Kollman, C.; Carlson, A.L.; Johnson, M.L.; Rodbard, D. The relationships between time in range, hyperglycemia metrics, and HbA1c. J. Diabetes Sci. Technol. 2019, 13, 614–626. [Google Scholar] [CrossRef]
- Ajjan, R.A.; Cummings, M.H.; Jennings, P.; Leelarathna, L.; Rayman, G.; Wilmot, E.G. Accuracy of flash glucose monitoring and continuous glucose monitoring technologies: Implications for clinical practice. Diabetes Vasc. Dis. Res. 2018, 15, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kirchsteiger, H.; Heinemann, L.; Freckmann, G.; Lodwig, V.; Schmelzeisen-Redeker, G.; Schoemaker, M.; Del Re, L. Performance comparison of CGM systems: MARD values are not always a reliable Indicator of CGM system accuracy. J. Diabetes Sci. Technol. 2015, 9, 1030–1040. [Google Scholar] [CrossRef] [Green Version]
- Freckmann, G.; Pleus, S.; Schauer, S.; Link, M.; Jendrike, N.; Waldenmaier, D.; Haug, C.; Stuhr, A. Choice of continuous glucose monitoring systems may affect metrics: Clinically relevant differences in times in ranges. Exp. Clin. Endocrinol. Diabetes 2021. [Google Scholar] [CrossRef]
- Schrangl, P.; Reiterer, F.; Heinemann, L.; Freckmann, G.; Del Re, L. Limits to the evaluation of the accuracy of continuous glucose monitoring systems by clinical trials. Biosensors 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleus, S.; Stuhr, A.; Link, M.; Haug, C.; Freckmann, G. Variation of mean absolute relative differences of continuous glucose monitoring systems throughout the day. J. Diabetes Sci. Technol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Akturk, H.K.; Giordano, D.; Champakanath, A.; Brackett, S.; Garg, S.; Snell-Bergeon, J. Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes. Metab. 2019, 22, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Breton, M.D.; Kovatchev, B.P. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol. Ther. 2021, 23, 601–608. [Google Scholar] [CrossRef] [PubMed]

| Variables | T1D Patients (n = 21) |
|---|---|
| Female/Male, n (%) | 13/8 (62/38) |
| Age, years (range) | 43.2 ± 15.1 (21–75) |
| Duration of diabetes, years (range) | 25.1 ± 13.6 (7–60) |
| BMI, kg/m2 (range) | 25.3 ± 4.9 (18–39) |
| Complications | |
| Retinopathy, n (%) | 8 (38) |
| Nephropathy, n (%) | 4 (19) |
| Coronary artery disease, n (%) | 1 (5) |
| Carotid macroangiopathy, n (%) | 2 (9.5) |
| Hypertension, n (%) | 8 (38) |
| HbA1c, % (range) | 8.08 ± 1.04 (6.3–10.6) |
| eGDR, mg/kg/min (range) | 7.28 ± 2.22 (1.7–10.4) |
| Severe hypoglycemic episode 1 last 12 months, n (%) | 8 (38) |
| CSII, n (%) | 18 (86) |
| Variables | FSL1 M0 | DG4 M3 | DG4 M6 | DG4 M12 | P M3 vs. M0 | P M6 vs. M0 | P M12 vs. M0 |
|---|---|---|---|---|---|---|---|
| GMI 1, % | 7.98 ± 1.34 | 7.60 ± 1.08 * | 7.45 ± 1.14 * | 7.97 ± 1.15 | 0.0255 | 0.0099 | NS |
| TIR 70–180 mg/dL 1,2, % | 45.4 ± 16.0 | 53.3 ± 16.4 * | 54.8 ± 16.0 * | 50.2 ± 17.1 | 0.0003 | <0.0001 | 0.0365 |
| TBR < 70 mg/dL 1,2, % | 7.0 [4.5;12.5] | 4.6 [2.6;9.9] * | 4.6 [4.6;8.8] * | 2.5 [1.6;5.5] | 0.0153 | 0.0450 | 0.0007 |
| TBR < 54 mg/dL 1,2, % | 2.3 [0.8;7.0] | 1.3 [0.7;4.3] * | 1.4 [0.5;2.7] | 0.7 [0.4;0.8] | 0.0441 | 0.0107 | 0.0073 |
| TAR > 180 mg/dL 1,2, % | 45.4 ± 19.3 | 41.0 ± 17.8 * | 39.0 ± 18.0 * | 45.9 ± 18.2 | NS | 0.0152 | NS |
| TAR > 250 mg/dL 1,2, % | 19.4 [9.3;32.2] | 10.1 [5.8;21.5] * | 10.1 [3.6;25.7] * | 16.2 [8.7;30.5] | 0.0127 | 0.0071 | NS |
| Average IG 1, mg/dL | 184.5 ± 46.2 | 171.7 ± 31.0 * | 166.9 ± 32.7 * | 182.0 ± 33.1 | 0.0433 | 0.0206 | NS |
| CV 1, % | 45.4 ± 8.3 | 40.0 ± 6.0 | 39.7 ± 6.3 | 39.1 ± 5.1 | 0.0032 | 0.0013 | 0.0009 |
| Sensor use rate, % | 78.0 [35.5;91.0] | 90.7 [66.1;94.5] | 82.0 [57.1;95.0] | 84.6 [57.1;92.2] | NS | NS | NS |
| Metrics | Target Values 1 | FSL1 M0 2 | DG4 M6 2 | DG4 M12 2 |
|---|---|---|---|---|
| % CV, n | ≤36% | 2 | 7 | 6 |
| TBR < 54 mg/dL, n | <1% | 6 | 7 | 13 |
| TBR < 70 mg/dL, n | <4% | 4 | 7 | 14 |
| TIR 70–180 mg/dL, n | >70% | 1 | 5 | 3 |
| TAR > 180 mg/dL, n | <25% | 3 | 6 | 3 |
| TAR > 250 mg/dL, n | <5% | 2 | 7 | 4 |
| GMI, n | <7% | 6 | 8 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Préau, Y.; Galie, S.; Schaepelynck, P.; Armand, M.; Raccah, D. Benefits of a Switch from Intermittently Scanned Continuous Glucose Monitoring (isCGM) to Real-Time (rt) CGM in Diabetes Type 1 Suboptimal Controlled Patients in Real-Life: A One-Year Prospective Study §. Sensors 2021, 21, 6131. https://doi.org/10.3390/s21186131
Préau Y, Galie S, Schaepelynck P, Armand M, Raccah D. Benefits of a Switch from Intermittently Scanned Continuous Glucose Monitoring (isCGM) to Real-Time (rt) CGM in Diabetes Type 1 Suboptimal Controlled Patients in Real-Life: A One-Year Prospective Study §. Sensors. 2021; 21(18):6131. https://doi.org/10.3390/s21186131
Chicago/Turabian StylePréau, Yannis, Sébastien Galie, Pauline Schaepelynck, Martine Armand, and Denis Raccah. 2021. "Benefits of a Switch from Intermittently Scanned Continuous Glucose Monitoring (isCGM) to Real-Time (rt) CGM in Diabetes Type 1 Suboptimal Controlled Patients in Real-Life: A One-Year Prospective Study §" Sensors 21, no. 18: 6131. https://doi.org/10.3390/s21186131
APA StylePréau, Y., Galie, S., Schaepelynck, P., Armand, M., & Raccah, D. (2021). Benefits of a Switch from Intermittently Scanned Continuous Glucose Monitoring (isCGM) to Real-Time (rt) CGM in Diabetes Type 1 Suboptimal Controlled Patients in Real-Life: A One-Year Prospective Study §. Sensors, 21(18), 6131. https://doi.org/10.3390/s21186131







