Orthogonality-Constrained CNMF-Based Noise Reduction with Reduced Degradation of Biological Sound
Abstract
:1. Introduction
2. Biological Sound Sensor
2.1. Vascular Sound
2.2. Respiratory Sound
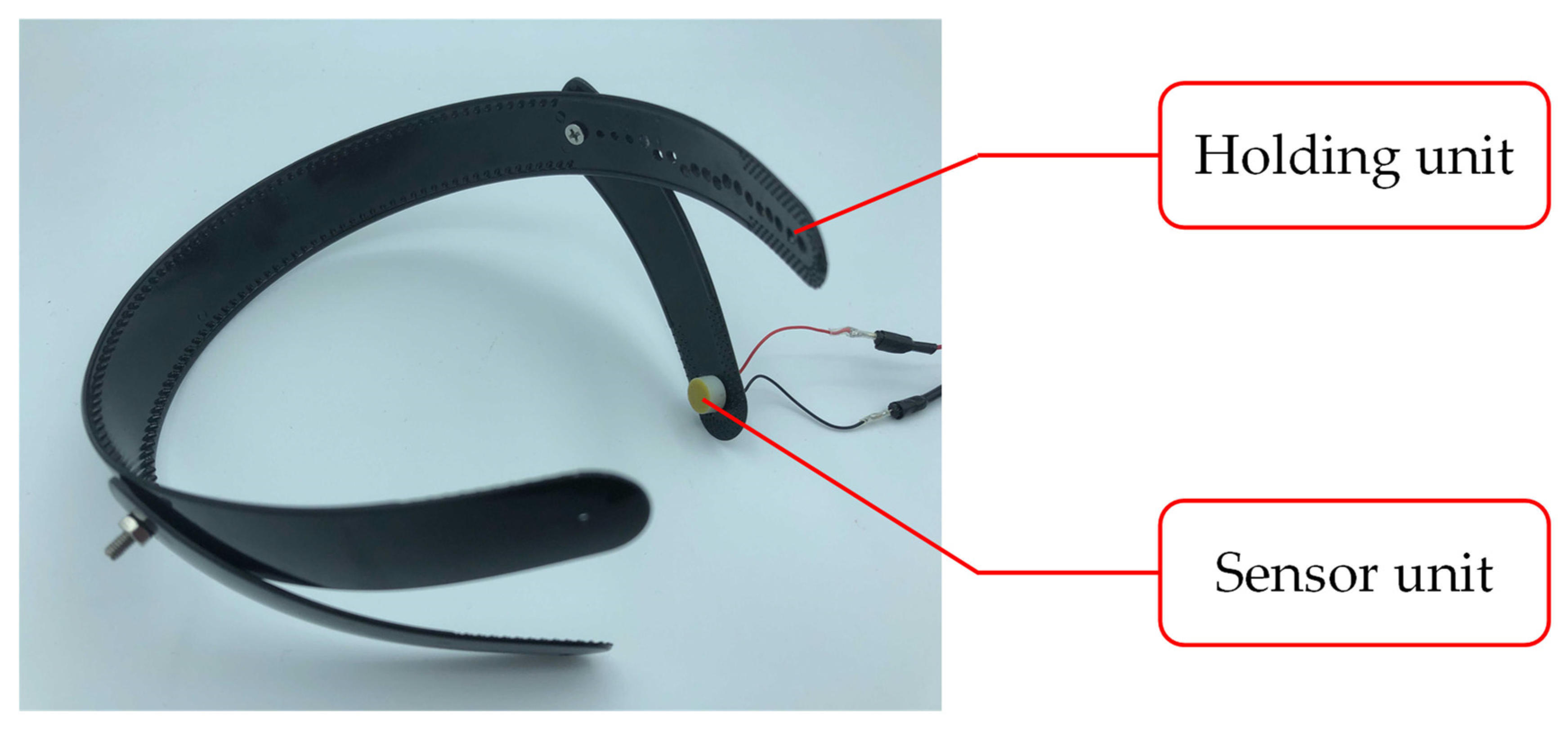
2.3. Biological Sound Sensor
3. Noise Reduction Methods
3.1. Related Research
3.1.1. NMF and Semi-Supervised NMF
3.1.2. Convolutive NMF and Semi-Supervised Convolutive NMF
3.1.3. Orthogonality-Constrained NMF
3.2. Proposed Method
3.2.1. Preprocessing with BPF and HPSS
3.2.2. Noise Reduction with OCNMF
| Algorithm 1. Signal basis training using CNMF |
| Input: Spectrogram of signal dataset , priori basis number R, shift length of CNMF K, type of β-divergence β |
| number of iterations in CNMF |
| Output: The basis matrix of signal |
| 1: Initialize and with random non-negative values |
| 2: Normalize columns of |
| 3: for i = 1, , do |
| 4: for = 0, , K − 1 do |
| 5: Compute Z |
| 6: Update and using Equations (21) and (22) |
| 7: Normalize columns of |
| 8: end for |
| 9: end for |
| Algorithm 2. Noise analysis and reduction using OCNMF |
| Input: Spectrogram of an input signal , priori basis number R, an undesired basis number J, type of β-divergence β, |
| number of iterations in OCNMF , shift length of CNMF K, signal basis matrix , |
| weight parameter , phase matrix of the input signal |
| Output: Noise reduced signal |
| 1: Initialize , U, and G with random non-negative values |
| 2: Normalize columns of |
| 3: for i = 1, , do |
| 4: for = 0, , R − 1 do |
| 5: Compute Z |
| 6: Update , U, and G using Equations (15), (16) and (23) |
| 7: Normalize columns of |
| 8: end for |
| 9: end for |
| 10: Compute M using Equation (19) and = M |
| 11: ISTFT ( ) |
4. Experimental Verification
4.1. Setup
4.2. Evaluation Methodology
4.3. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Subject | SNR [dB] | SDR [dB] | ||
|---|---|---|---|---|
| SCNMF | OCNMF | SCNMF | OCNMF | |
| A | 20.5 | 20.5 | 15.9 | 18.1 |
| B | 23.4 | 24.3 | 14.8 | 15.0 |
| C | 22.7 | 24.2 | 11.3 | 16.7 |
| D | 21.1 | 22.8 | 12.4 | 13.2 |
| E | 22.3 | 25.7 | 13.2 | 18.7 |
| F | 21.8 | 21.6 | 11.5 | 11.9 |
| G | 20.9 | 22.3 | 11.2 | 12.3 |
| H | 21.2 | 22.4 | 15.7 | 17.2 |
| I | 21.1 | 24.1 | 11.2 | 14.2 |
| J | 22.6 | 23.2 | 14.1 | 16.7 |
| K | 22.0 | 24.9 | 12.1 | 14.8 |
| L | 21.4 | 22.3 | 11.5 | 13.9 |
| M | 23.2 | 24.1 | 12.3 | 12.6 |
| N | 20.1 | 21.2 | 14.2 | 17.1 |
| O | 20.3 | 21.5 | 14.8 | 15.7 |
| P | 20.6 | 22.4 | 12.0 | 13.4 |
| Q | 21.2 | 24.3 | 11.9 | 14.8 |
| R | 21.4 | 22.6 | 13.1 | 16.2 |
| S | 21.2 | 22.9 | 14.1 | 17.1 |
| T | 20.7 | 22.1 | 12.6 | 15.3 |
| U | 21.3 | 21.4 | 13.2 | 14.2 |
| Subject | SNR [dB] | SDR [dB] | ||
|---|---|---|---|---|
| SCNMF | OCNMF | SCNMF | OCNMF | |
| A | 19.6 | 20.8 | 13.8 | 16.0 |
| B | 21.7 | 22.4 | 14.1 | 13.3 |
| C | 21.5 | 23.5 | 9.0 | 16.1 |
| D | 18.9 | 23.7 | 10.2 | 11.6 |
| E | 20.9 | 22.9 | 13.9 | 18.5 |
| F | 20.4 | 20.9 | 9.1 | 12.4 |
| G | 21.6 | 22.4 | 10.2 | 10.1 |
| H | 21.2 | 23.5 | 15.1 | 17.7 |
| I | 21.3 | 22.8 | 10.6 | 14.0 |
| J | 22.5 | 22.5 | 12.7 | 14.7 |
| K | 22.3 | 23.4 | 11.2 | 13.4 |
| L | 22.5 | 24.1 | 11.7 | 12.4 |
| M | 22.3 | 21.0 | 10.4 | 12.6 |
| N | 21.2 | 20.8 | 13.1 | 14.7 |
| O | 18.6 | 20.7 | 13.5 | 15.8 |
| P | 19.4 | 22.9 | 11.0 | 12.0 |
| Q | 20.6 | 21.5 | 12.0 | 13.0 |
| R | 20.1 | 22.0 | 13.2 | 16.9 |
| S | 19.8 | 22.3 | 14.3 | 17.1 |
| T | 22.0 | 23.3 | 11.5 | 12.8 |
| U | 20.6 | 20.4 | 13.8 | 13.1 |
References
- Şahin, B.; ligun, G. Risk factors of deaths related to cardiovascular diseases in World Health Organization (WHO) member countries. Health Soc. Care Community 2020. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S. New ideas about atrial fibrillation 50 years on. Nature 2002, 415, 219–226. [Google Scholar] [PubMed]
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2020, 7, 574–582. [Google Scholar] [CrossRef]
- Williams, B.A.; Chamberlain, A.M.; Blankenship, J.C.; Hylek, E.M.; Voyce, S. Trends in atrial fibrillation incidence rates within an integrated health care delivery system, 2006 to 2018. JAMA Netw. Open 2020, 3, e2014874. [Google Scholar] [CrossRef] [PubMed]
- Zulkifly, H.; Lip, G.Y.H.; Lane, D.A. Epidemiology of atrial fibrillation. Int. J. Clin. Pract. 2018, 72, e13070. [Google Scholar] [CrossRef] [Green Version]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of atrial fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Harju, J.; Tarniceriu, A.; Parak, J.; Vehkaoja, A.; Yli-Hankala, A.; Korhonen, I. Monitoring of heart rate and inter-beat intervals with wrist plethysmography in patients with atrial fibrillation. Physiol. Meas. 2018, 39, 065007. [Google Scholar] [CrossRef]
- MacNee, W. Pathogenesis of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 22 October 2021).
- World Health Organization. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.who.int/nmh/publications/ncd_report_full_en.pdf (accessed on 22 October 2021).
- Miravitlles, M.; Ribera, A. Understanding the impact of symptoms on the burden of COPD. Respir. Res. 2017, 18, 67. [Google Scholar] [CrossRef] [Green Version]
- Pelter, M.M.; Mortara, D.; Badilini, F. Computer Assisted Patient Monitoring: Associated Patient, Clinical and ECG Characteristics and Strategy to Minimize False Alarms. Hearts 2021, 2, 459–471. [Google Scholar] [CrossRef]
- Asmare, M.H.; Filtjens, B.; Woldehanna, F.; Janssens, L.; Vanrumste, B. Rheumatic Heart Disease Screening Based on Phonocardiogram. Sensors 2021, 21, 6558. [Google Scholar] [CrossRef] [PubMed]
- Torii, K.; Nakashima, S.; Nakamura, H.; Ooyagi, T.; Tanaka, K.; Yagami, H. Distinction of heart sound and respiratory sound using body conduction sound sensor based on HPSS. In Proceedings of the 7th ACIS International Conference on Applied Computing & Information Technology, Honolulu, HI, USA, 31 May 2019; pp. 178–183. [Google Scholar]
- Leng, X.; Chen, J.; Cohen, I.; Benesty, J. Study of widely linear multichannel Wiener filter for binaural noise reduction. In Proceedings of the 2017 25th European Signal Processing, Kos, Greece, 28 August–2 September 2017. [Google Scholar]
- Yu, L.; Antoni, J.; Wu, H.; Jiang, W. Reconstruction of cyclostationary sound source based on a back-propagating cyclic wiener filter. J. Sound Vibr. 2019, 442, 787–799. [Google Scholar] [CrossRef]
- Balaji, V.R.; Maheswaran, S.; Babu, M.R.; Kowsigan, M.; Prabhu, E.; Venkatachalam, K. Combining statistical models using modified spectral subtraction method for embedded system. Microprocess. Microsyst. 2020, 73, 102957. [Google Scholar] [CrossRef]
- Wang, L.; Odani, K.; Kai, A. Dereverberation and denoising based on generalized spectral subtraction by multi-channel LMS algorithm using a small-scale microphone array. EURASIP J. Adv. Signal Process. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Salman, A.H.; Ahmadi, N.; Mengko, R.; Langi, A.Z.R.; Mengko, T.L.R. Empirical Mode Decomposition (EMD) Based Denoising Method for Heart Sound Signal and Its Performance Analysis. Int. J. Electr. Comput. Eng. Syst. 2016, 6, 1–8. [Google Scholar]
- Wang, H.; Wang, M.; Li, J.; Song, L.; Hao, Y. A Novel Signal Separation Method Based on Improved Sparse Non-Negative Matrix Factorization. Entropy 2019, 21, 445. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Kang, Z.; Hu, Y.; Cheng, J.; Cheng, Q. Nonnegative matrix factorization with integrated graph and feature learning. ACM Trans. Intell. Syst. Technol. 2017, 8, 42. [Google Scholar] [CrossRef]
- Ishizuka, R.; Nishikimi, R.; Nakamura, E.; Yoshii, K. Tatum-level drum transcription based on a convolutional recurrent neural network with language model-based regularized training. In Proceedings of the APSIPA2020, Auckland, New Zealand, 7–10 December 2020; pp. 359–364. [Google Scholar]
- Selim, I.M.; Keshk, A.E.; El Shourbugy, B.M. Galaxy image classification using non-negative matrix factorization. IJCA 2016, 137, 4–8. [Google Scholar] [CrossRef]
- Murakami, N.; Torii, K.; Nakashima, S. Biological sound sensor robust to air conduction noise. In Proceedings of the International Symposium on Computer, Consumer and Control, Online, 13–16 November 2020; pp. 537–540. [Google Scholar]
- Shuvo, S.B.; Ali, S.N.; Swapnil, S.I.; Al-Rakhami, M.S.; Gumaei, A. CardioXNet: A novel lightweight deep learning framework for cardiovascular disease classification using heart sound recordings. IEEE Access 2021, 9, 36955–36967. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Zhang, L.; Wang, J.; Damaševičius, R.; Wang, H.; Wang, G.; Zhang, X.; Yuan, J.; Woźniak, M. Risk Assessment of Hypertension in Steel Workers Based on LVQ and Fisher-SVM Deep Excavation. IEEE Access 2019, 7, 23109–23119. [Google Scholar] [CrossRef]
- Choi, S.; Jiang, Z. Comparison of envelope extraction algorithms for cardiac sound signal segmentation. Expert Syst. Appl. 2008, 34, 1056–1069. [Google Scholar] [CrossRef]
- Choi, S.; Jiang, Z. Cardiac sound murmurs classification with autoregressive spectral analysis and multi-support vector machine technique. Comput. Biol. Med. 2010, 40, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Reichert, S.; Gass, R.; Brandt, C.; Andrès, E. Analysis of respiratory sounds: State of the art. Clin. Med. Insights: Circ. Respirat. Pulm. Med. 2008, 2, 45–58. [Google Scholar] [CrossRef]
- Kompis, M.; Pasterkamp, H.; Oh, Y.; Wodicka, G.R. Distribution of inspiratory and expiratory respiratory sound intensity on the surface of the human thorax. In Proceedings of the 19th International Conference—IEEE/EMBS, Chicago, IL, USA, 30 October–2 November 1997; pp. 2047–2050. [Google Scholar]
- Hagiwara, K.; Goto, M.; Iguchi, Y.; Yasuno, Y.; Kodama, H.; Kidokoro, K.; Tajima, T. Soft X-ray charging method for a silicon electret condenser microphone. Appl. Phys. Exp. 2010, 3, 091502. [Google Scholar] [CrossRef]
- Petaros, A.; Sholts, S.B.; Slaus, M.; Bosnar, A.; Wärmländer, S.K. Evaluating sexual dimorphism in the human mastoid process: A viewpoint on the methodology. Clin. Anat. 2015, 28, 593–601. [Google Scholar] [CrossRef]
- Nakano, M.; Kameoka, H.; Le Roux, J.; Kitano, Y.; Ono, N.; Sagayama, S. Convergence-guaranteed multiplicative algorithms for nonnegative matrix factorization with β-divergence. In Proceedings of the 2010 IEEE International Workshop on Machine Learning for Signal Processing, Kittila, Finland, 29 August–1 September 2010; pp. 283–288. [Google Scholar]
- Févotte, C.; Cemgil, A.T. Nonnegative matrix factorizations as probabilistic inference in composite models. In Proceedings of the 2009 17th European Signal Processing Conference, Glasgow, Scotland, 24–28 August 2009; pp. 1913–1917. [Google Scholar]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.K.; Chin, C.S.; Li, Y. Semi-supervised NMF-CNN for sound event detection. IEEE Access 2021, 9, 130529–130542. [Google Scholar] [CrossRef]
- Lefevre, A.; Bach, F.; Févotte, C. Semi-supervised NMF with time-frequency annotations for single-channel source separation. In Proceedings of the 13th International Society for Music Information Retrieval Conference, Porto, Portugal, 8–12 October 2012; pp. 115–120. [Google Scholar]
- Joder, C.; Weninger, F.; Eyben, F.; Virette, D.; Schuller, B. Real-time speech separation by semi-supervised nonnegative matrix factorization. In Proceedings of the 10th International Conference on Latent Variable Analysis and Signal Separation, Tel Aviv, Israel, 12–15 March 2012; pp. 322–329. [Google Scholar]
- Smaragdis, P. Convolutive speech bases and their application to supervised speech separation. IEEE Trans. Audio Speech Lang. Process. 2007, 15, 1–12. [Google Scholar] [CrossRef]
- Wang, W.; Cichocki, A.; Chambers, J.A. A multiplicative algorithm for convolutive non-negative matrix factorization based on squared Euclidean distance. IEEE Trans. Signal Process. 2009, 57, 2858–2864. [Google Scholar] [CrossRef] [Green Version]
- Yagi, K.; Takahashi, Y.; Saruwatari, H.; Shikano, K.; Kondo, K. Music signal separation by orthogonality and maximum-distance constrained nonnegative matrix factorization with target signal information. In Proceedings of the 45th International Conference on Applications of Time-Frequency Processing in Audio, Helsinki, Finland, 1–4 March 2012; pp. 142–147. [Google Scholar]
- Fitzgerald, D. Harmonic/percussive separation using median filtering. In Proceedings of the 13th International Conference on Digital Audio Effects, Graz, Austria, 6–10 September 2010; pp. 10–13. [Google Scholar]
- Oyabu, S.; Kitamura, D.; Yatabe, K. Linear multichannel blind source separation based on time-frequency mask obtained by harmonic/percussive sound separation. In Proceedings of the ICASSP 2021–2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Toronto, ON, Canada, 6–11 June 2021. [Google Scholar]
- Cavalcanti, Y.C.; Oberlin, T.; Dobigeon, N.; Févotte, C.; Stute, S.; Ribeiro, M.J.; Tauber, C. Factor analysis of dynamic PET images: Beyond Gaussian noise. IEEE Trans. Med. Imaging 2019, 38, 2231–2241. [Google Scholar] [CrossRef] [Green Version]
- Chuan, T.C.; Darsono, A.M.; Saat, M.S.M.; Isa, A.A.M.; Hashim, N.M.Z. Blind source separation on biomedical field by using nonnegative matrix factorization. ARPN J. Eng. Appl. Sci. 2016, 11, 8200–8206. [Google Scholar]
- Vincent, E.; Gribonval, R.; Févotte, C. Performance measurement in blind audio source separation. IEEE Trans. Audio Speech Lang. Process. 2006, 14, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, A.; Fitzgerald, D.; Barry, D.; Coyle, E.; Rickard, S. Clustering NMF basis functions using shifted NMF for monaural sound source separation. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Prague, Czech Republic, 22–27 May 2011; pp. 245–248. [Google Scholar]
- Cawley, G.C. Leave-one-out cross-validation based model selection criteria for weighted LS-SVMs. In Proceedings of the 2006 IEEE International Joint Conference on Neural Network Proceedings, Vancouver, BC, Canada, 16–21 July 2006; pp. 1661–1668. [Google Scholar]
- Badjatiya, S.; Mhetre, M.; Kodgule, R.; Abhyankar, H. Breathe sound analysis: A new approach for diagnosis. IJCT 2017, 4, 1–8. [Google Scholar]
- Sanchez, I.; Vizcaya, C. Tracheal and lung sounds repeatability in normal adults. Respir. Med. 2003, 97, 1257–1260. [Google Scholar] [CrossRef] [Green Version]







| Target | Frequency [Hz] | Response | Filter | Order |
|---|---|---|---|---|
| VSS | 75–200 | Infinite impulse response | Butterworth | 12 |
| RSS | 200–2000 |
| Target | Age [years] | Gender | Disease |
|---|---|---|---|
| A | 73 | Male | Asthma and chronic obstructive pulmonary disease |
| B | 74 | Male | Chronic obstructive pulmonary disease |
| C | 73 | Male | Asthma |
| D | 72 | Male | Chronic obstructive pulmonary disease |
| E | 72 | Female | Asthma |
| F | 74 | Male | Asthma |
| G | 77 | Male | Chronic obstructive pulmonary disease |
| H | 87 | Male | Chronic obstructive pulmonary disease |
| I | 62 | Female | Asthma |
| J | 58 | Female | Asthma and Chronic obstructive pulmonary disease |
| K | 65 | Female | Asthma |
| L | 72 | Female | Asthma and chronic bronchitis |
| M | 72 | Male | Chronic obstructive pulmonary disease |
| N | 63 | Male | Asthma and chronic bronchitis |
| O | 56 | Male | Asthma and chronic bronchitis |
| P | 81 | Female | Asthma |
| Q | 57 | Female | Chronic obstructive pulmonary disease |
| R | 24 | Male | No disease |
| S | 24 | Male | No disease |
| T | 24 | Male | No disease |
| U | 22 | Male | No disease |
| Sampling frequency | 44.1 kHz |
| Bit depth | 16 bits |
| Window function in STFT | Hann window |
| Window length in STFT | 1024 points |
| Shift length in STFT | 512 points |
| Input SNR | 0 dB |
| Parameters in HPSS | = 23 |
| Parameters in CNMF | R = 30, K = 10, = 200, β = 2 |
| Parameters in SCNMF | J = 15, K = 10, = 200, β = 2 |
| Parameters in OCNMF | J = 15, K = 10, = 200, β = 2, μ = 1.0 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, N.; Nakashima, S.; Fujimoto, K.; Makihira, S.; Nishifuji, S.; Doi, K.; Li, X.; Hirano, T.; Matsunaga, K. Orthogonality-Constrained CNMF-Based Noise Reduction with Reduced Degradation of Biological Sound. Sensors 2021, 21, 7981. https://doi.org/10.3390/s21237981
Murakami N, Nakashima S, Fujimoto K, Makihira S, Nishifuji S, Doi K, Li X, Hirano T, Matsunaga K. Orthogonality-Constrained CNMF-Based Noise Reduction with Reduced Degradation of Biological Sound. Sensors. 2021; 21(23):7981. https://doi.org/10.3390/s21237981
Chicago/Turabian StyleMurakami, Naoto, Shota Nakashima, Katsuma Fujimoto, Shoya Makihira, Seiji Nishifuji, Keiko Doi, Xianghong Li, Tsunahiko Hirano, and Kazuto Matsunaga. 2021. "Orthogonality-Constrained CNMF-Based Noise Reduction with Reduced Degradation of Biological Sound" Sensors 21, no. 23: 7981. https://doi.org/10.3390/s21237981
APA StyleMurakami, N., Nakashima, S., Fujimoto, K., Makihira, S., Nishifuji, S., Doi, K., Li, X., Hirano, T., & Matsunaga, K. (2021). Orthogonality-Constrained CNMF-Based Noise Reduction with Reduced Degradation of Biological Sound. Sensors, 21(23), 7981. https://doi.org/10.3390/s21237981






