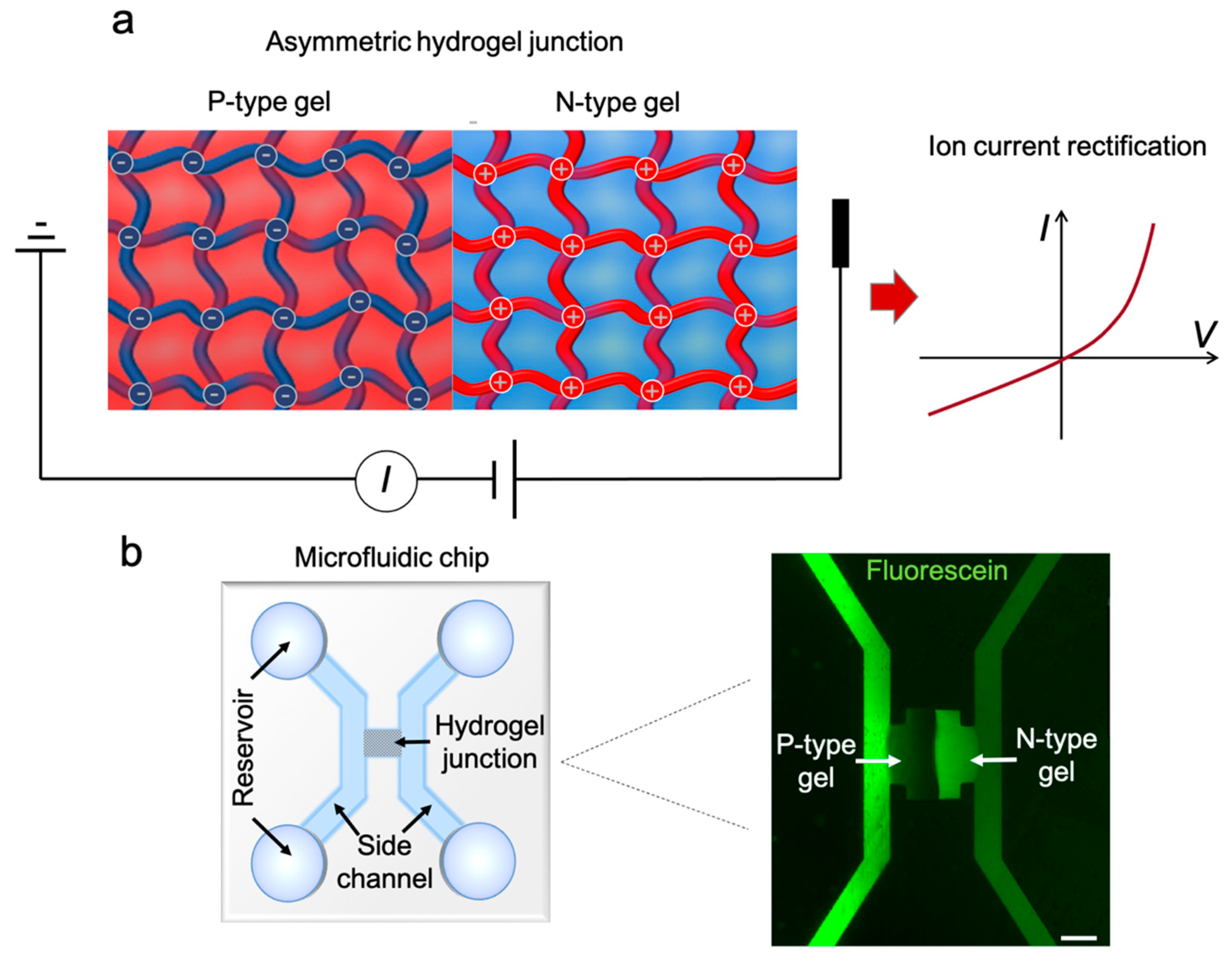
3.1. Experimental Characterization of the PHID Device
The schematic of the complete PHID device is shown in
Figure 1b (left). The device consists of two side channels bridged by a hydrogel PN junction. The fluorescent micrograph (
Figure 1b, right) highlights the junction area. To better visualize the hydrogel, the device was filled with 1 mM fluorescein solution. Because fluorescein molecules are negatively charged, they accumulate at the positively charged N-type gel region, rendering it in bright. On the contrary, the negatively charged P-type gel repels fluorescein and is thus shown in dark. As depicted in the micrograph, the two hydrogel blocks are around 100 μm wide and adjacent to each other without noticeable gaps in between. The two side channels are connected solely via the hydrogel PN junction, evading any potential leaking currents that may cause inferences.
The PHID device, in this study, rectifies ion currents due to electrostatic field effect; charged hydrogel backbones attract counterions in the electrolyte solution within the electrical double layers, which lead to a lopsided distribution of charge carriers (positive and negative ions) in either type of PE gel. Therefore, the ICR behavior should be sensitive to the bulk electrolyte strength, which determines the characteristic length of electrical double layers. For other types of ionic diodes, i.e., 70 nm conical nanochannels, the rectification level is reversely correlated with the ionic strength until saturation is reached (at micromolar scale). For the PHID, however, we expect the trend to be different, as the hydrogels ought to contain more confined and irregular nanopores. In fact, the relation between the rectification level and ionic strength for PHIDs has not been explored. Therefore, to evaluate this, first, we monitored the I-V response of a representative PHID device at micromolar ionic strengths,
Figure 2a. Here, we define the logarithmic rectification ratio (
R), i.e.,
, where
and
refer to ionic current at maximum positive and negative biases, respectively. The same definition has also been used in previous reports to quantify the ICR of nanofluidic diodes [
17,
18]. As seen, only a slight change in
R, i.e., 0.4, is observed from 1 to 100 μM, indicating that the rectification reached saturation at a micromolar scale. The results suggest that unlike other ionic diodes the ionic behavior of PHIDs should be sensitive to higher ionic strength, for example, in the range of millimolar, possibly owing to more confined geometry and enhanced electrostatic field effect. Indeed, we found a higher rectification in a later experiment (
Figure 2b, 1X) in which the ionic strength was elevated to 10 mM with other conditions unchanged.
Next, we investigated whether tuning the hydrogel confinement level would affect the ionic rectification behavior. In this experiment, we lowered the original (1X) concentration of the N-type gel precursor solution. The SEM images in
Figure 2c show that the mesh size of N-type gel is significantly enlarged when diluting the precursor solution. The average pore size is 0.23, 1.47, and 2.96 μm for 1X, 0.5X, and 0.25X N-type gels, respectively (n = 15). Here, it is worthwhile noting that the measured pore sizes may not represent the actual pore sizes of the hydrogel, as the pores might experience swelling during the sample preparation procedure for SEM. The plots in
Figure 2b clearly show that the highest rectification level, i.e., R = −4.4, is obtained with 1X N-type gel which possesses the highest confinement. These results confirm the ideal working condition for a PHID device to obtain a reasonably high and sensitive rectification.
3.2. Numerical Study of the Ion Transport Behavior of PHID
In addition to testing the device experimentally, we performed a numerical analysis to further study the ionic rectification characteristics. In the model, hydrogels are simplified to 2D nanopillar arrays (
Figure S1, Supplementary Information) and the electrokinetic effect is neglected.
Figure 3a displays the electrical potential distribution around the junction center with no external bias applied at three distinct ionic levels. Note that in all three conditions, the Debye length
(30.3–303.2 nm) is greater than the nanopore size (fixed at 20 nm). It can be seen that the potential profile in the nanoporous region does not align with that in the reservoirs and is rather dominated by the charged pillar surfaces as the electrical double layers overlap extensively. Interestingly, although the surface charge density is fixed (±2 mC/m
2), the electrical potential on the pillar surfaces seems to decay with an increase in ionic strength. For 1, 10, and 100 μM KCl solutions, the maximum absolute value of potentials on the pillar surfaces are 0.186, 0.126, and 0.066 V, respectively. Under such a condition, the electrical double layers around a single pillar interact intensively with the double layers of surrounding ones which may affect the electrical potential on pillar surfaces when equilibrium is reached. This may help explain the increasing conductance of nanochannels under extremely low ionic strengths experimentally observed in a previous work [
24]. Moreover, it should be noted that this tendency could be specific to the condition where
is greater than the critical length of the geometry. Indeed, an opposite trend was found in a previous numerical study, in which the potential on the surfaces of a conical nanopore was boosted by increasing the ionic strength under the condition that
was smaller than the pore size [
14].
Next, we applied external potentials to the junction to monitor its ICR behavior.
Figure 3b shows the numerically obtained conductivity profile in the junction under different bias polarities at an ionic strength of 100 μM. It can be observed that at a
Vap of 1 V, the conductivity is significantly increased, especially around the P-N gel interface, owing to the localized enrichment of ions. In this scenario, the ionic diode experiences a forward bias. At a
Vap of −1 V, however, ions are depleted from the P-N gel interface and the conductivity is much lower than the bulk value, leading to a reverse bias in which the ionic diode prevents the ionic current from flowing through. To visualize the relationship between conductivity and ionic strength, we plotted the ionic conductivity along the center z-axis AD (
Figure S1) under different bias conditions. As shown in
Figure 3c, under forward biases, the conductivity values in the junction area are markedly lifted over the bulk. Moreover, the values are positively correlated to the ionic strength. Under reverse biases, however, the conductivity values in the junction are rather independent of ionic strength except for the depletion zone near the P-N gel interface. In the depletion zone, the conductivity can be as low as ~
,
, and
mS/m for the ionic strengths of 100, 10, and 1 μM, respectively. The conductivity profile of the hydrogel junction is closely related to its overall voltage-current response, as shown in
Figure 3e. As can be seen in
Figure 3f, R values do not exhibit a noticeable tendency, which is in strong agreement with the experimental results (
Figure 2a). These results suggest that the ICR behavior may not be directly dependent on ionic strength when
is greater than the critical dimension. Instead, one may consider the trade-off between
and pore size. In fact, the saturation of R was also observed in previous experimental studies of nanopores, which was explained by the saturation of counterions governed by the charged surfaces [
14,
25]. Although the simulated results qualitatively agree with the experiments, it is worthwhile noting that the quantitative R values are not strictly aligned. The discrepancy, here, can arise from leaking currents in the experiments which might lead to enlarged current especially at reverse biases and errors in the modeling process such as inaccurate modeling of hydrogel porous structure.
Next, we examined the effect of nanopore size on the ionic transport characteristics of the PHID. In the process, we fixed the ionic strength to 100 μM and the pillar surface charge density to ±2 mC/m
2. Under such a condition, the potential profile in the junction was more or less maintained (data not shown). Therefore, we directly jumped to inspect the ionic transport characteristic.
Figure 4 plots the I-V curves and R values for varying pore sizes. It can be seen that the ionic currents at forward biases (
Figure 4a) were almost identical and independent of pore size, which was also observed in our previous experiments (
Figure 2b). However, the current at reverse biases are significantly different (inset), which leads to considerable changes in the rectification ratio (
Figure 4b). The change of rectification ratio strictly follows the trend that R decreases with an increase in pore size, which aligns with the experimental results in
Figure 2b. The trend can be explained by the intensified electrical double layer overlapping when reducing the pore size, which leads to severer depletion around the P-N gel interface at reverse biases.
With extensive discussions about the effect of ionic strength and pore size on the ion transport behavior of a PHID, we continued to ask whether ICR could be described by a simple dimensionless number. The ionic strength term can be represented by the characteristic length of electrical double layers,
, using Equation (3). As we considered, the ratio between
and the pore size w could be a promising candidate to monitor the overall rectification behavior, because it reflects the degree of double-layer overlapping in nanopores, and thus the degree of surface-governed ion transport. In fact, existing experiments have shown that ICR increases with increasing
to a certain extent, for the case of conical nanopores [
25]. To explore whether the trend could be numerically obtained, we plotted the rectification ratio R against
for various ionic strengths and pore sizes, as shown in
Figure 5. Excitingly, we found that R indeed increased with
, when
was comparable to w (0.5–2 for
, highlighted by the blue background color in
Figure 5), which partially verified our hypothesis that ICR can be characterized by the degree of double layer if overlapped in a moderate range. When
is considerably higher than w, however, the trend no longer exists (highlighted by grey background color), and R seems to reach a saturation stage. This could be explained, in part, by the ion saturation effect, i.e., with a decrease in the ionic strength, the electrical double layers nearly occupy the entire nanopore. In this case, the conductivity solely relies on the transport of counterions which is governed by the surface charge. As a result, further expanding the electrical double layers would not possess significant changes on the ICR since it is saturated. Further efforts are required to resolve the ICR characteristics at the saturation region.
After understanding the role of ionic strength and pore size on rectification, next, we investigated the effect of surface charge density of the polyelectrolyte backbone on ICR behavior. This is important because it can predict whether the PHID device can be practically used to detect biomolecules (e.g., DNA and protein) based on their intrinsic charge. Here, to simulate the process of negatively charged DNA molecules captured within N-type gel, we fixed the charge density (σ) of the P-type gel and varied that of the N-type gel from 1 to 3 mC/m
2. Similar surface charge densities have been frequently used in previous studies of ICR [
24]. The ionic strength was fixed to 100 μM with the fixed pore size of 20 nm. First, we increased the amplitude of σ and observed increased conductivities in both forward (
Figure 6a) and reverse biases (
Figure 6b). Importantly, the conductivities increased most significantly around the pillar surfaces, especially noticeable at reverse biases. This suggests that one can expect a reasonable surface current effect with higher surface charges, which may lead to enhanced rectification. Indeed, both the ionic currents and the rectification ratio experienced a noticeable increase with higher σ values, as shown in
Figure 6c,d. These results predict that the ICR of our PHID device is sensitive to surface charge and can be potentially applied to charge-based biosensing.
3.3. Detection of DNA with the PHID Device
With the insights gained from the simulation results, we experimentally tested our PHID device to determine if it could respond to the presence of DNA molecules in the environment. In the experiments, the N-type gel side of the hydrogel heterojunction was exposed to a DNA solution (500 bp double-stranded DNA). The DNA molecules in the solution were expected to be immobilized on the N-type gel backbone due to electrostatic interactions, and hence change the surface charge. As shown, the I-V characteristic was shifted slightly from its original state (0 ng/μL) upon exposure to DNA (
Figure 7a). The rectification ratio decreased by 0.25 and 0.52, respectively, for 1 and 2 ng/μL DNA (
Figure 7b). Such an exhibited trend is in qualitative agreement with the simulation results in
Figure 6d. The results verify that the DNA molecules do interact with the N-type gel surfaces and, importantly, such electrostatic interactions are prominent enough to modulate the overall ionic current behavior of the device. Moreover, the results show that the change in
R is relevant to DNA concentration, suggesting that the device can be applied to quantitatively detect DNA with a sensitivity of <1 ng/μL.
It should be noted that the PHID device presented here lacks sequence specificity to DNA molecules. That means the device detects the mass concentration of DNAs regardless of their base pair information. In fact, it should indistinctively detect any (negatively) charged macromolecules. An ideal application scenario for the device would be monitoring the amplification of nucleic acids where only specific product can be obtained. Whether the device is sensitive to the length of DNAs is yet to be explored. A smaller geometry of the hydrogel heterojunction would lead to enhanced sensitivity. Compared to other ionic diodes (e.g., nanopipettes), PHID devices are fully integrated, and thus more robust and amenable to fluidic integration and automation. Therefore, we anticipate that such novel ionic diode devices would offer interesting possibilities in the field of biosensing.
In conclusion, we have performed a systematic characterization of a polyelectrolyte hydrogel ionic diode, both experimentally and numerically. Our study has dissected major physical quantities that affect ionic rectification behavior. According to the results of charge sensitivity and field effect, the rectification has been verified to be responsive to charged biomolecules. Current efforts are underway to develop PHID-based biosensors for sensitive and multiplexed detection of important biomarkers.