Highly Sensitive Amperometric Detection of Hydrogen Peroxide in Saliva Based on N-Doped Graphene Nanoribbons and MnO2 Modified Carbon Paste Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Synthesis of Graphene-Based Nanomaterials
2.3. Preparation of the Electrode Surface
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of Graphene-Based Nanomaterials
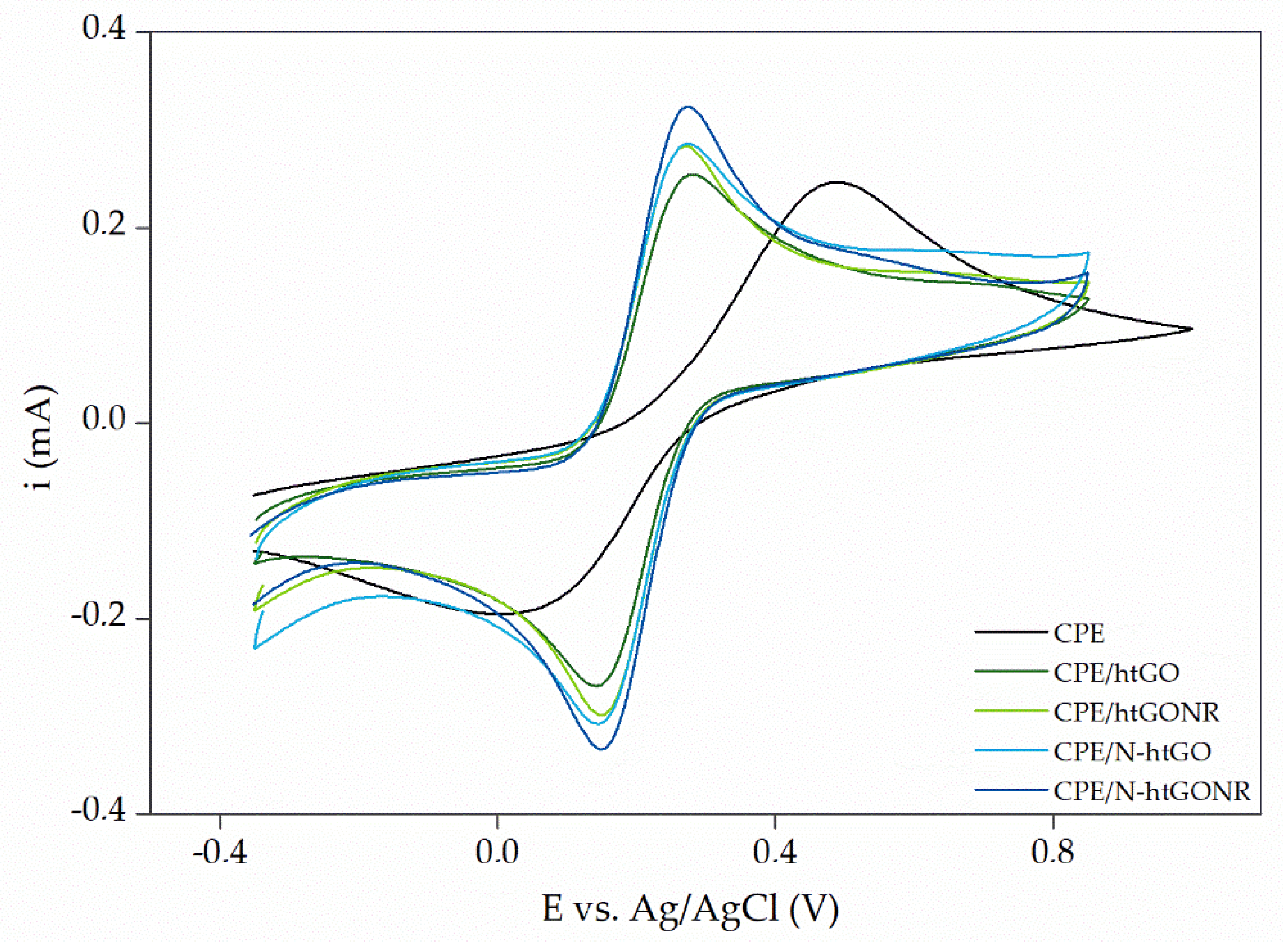
3.2. Electrode Preparation, Characterization, and Optimization
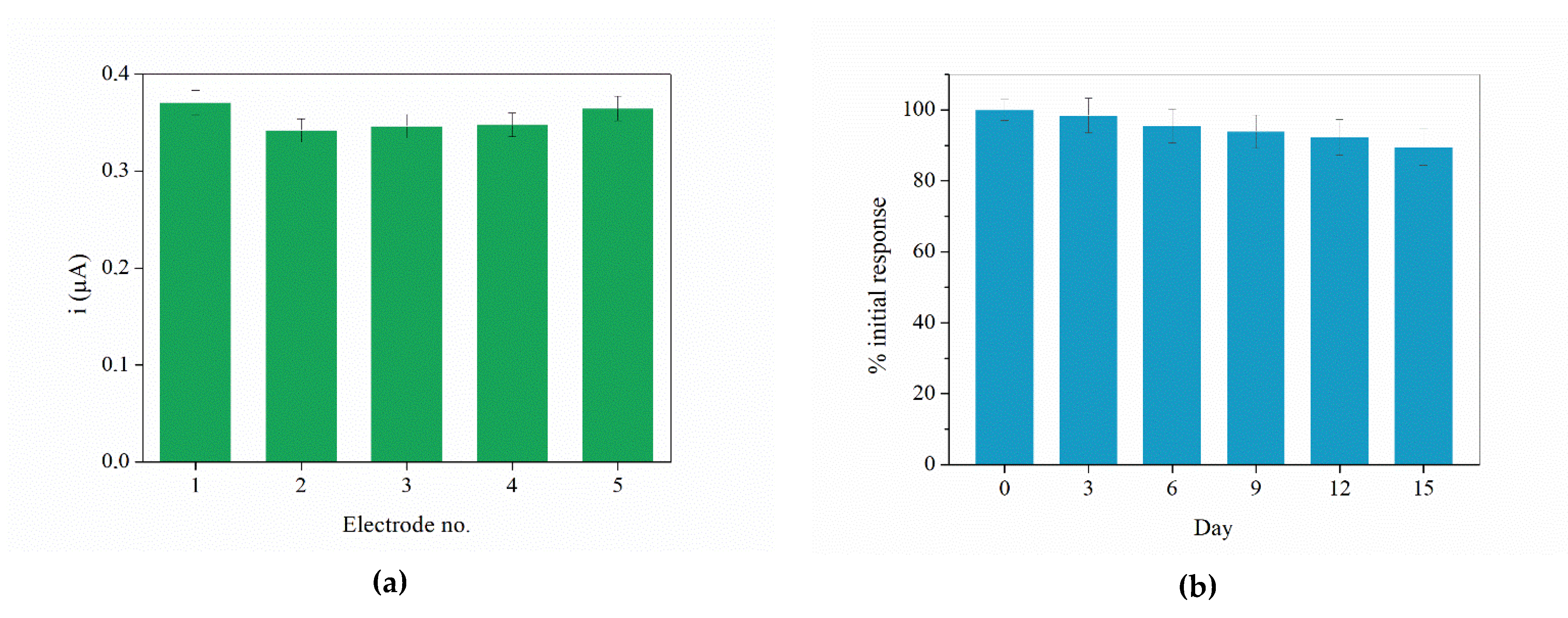
3.3. Linear Range, Sensitivity, Durability, Reproducibility, and Selectivity Studies
3.4. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Steinberg, S.M.; Johnson, B.J. A high performance liquid chromatography method for determination of gas-phase hydrogen peroxide in ambient air using Fenton’s chemistry. Chemosphere 2003, 52, 815–823. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; Chen, B.; Wang, L. A Facile, Nonreactive Hydrogen Peroxide (H2O2) Detection Method Enabled by Ion Chromatography with UV Detector. Anal. Chem. 2017, 89, 11537–11544. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.M. High-performance liquid chromatography method for determination of hydrogen peroxide in aqueous solution and application to simulated Martian soil and related materials. Environ. Monit. Assess. 2013, 185, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Khaja, S.; Velasquez-Castano, J.C.; Dasari, M.; Sun, C.; Petros, J.; Taylor, W.R.; Murthy, N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769. [Google Scholar] [CrossRef]
- Rivero, P.J.; Ibañez, E.; Goicoechea, J.; Urrutia, A.; Matias, I.R.; Arregui, F.J. A self-referenced optical colorimetric sensor based on silver and gold nanoparticles for quantitative determination of hydrogen peroxide. Sens. Actuators B Chem. 2017, 251, 624–631. [Google Scholar] [CrossRef]
- Domínguez-Henao, L.; Turolla, A.; Monticelli, D.; Antonelli, M. Assessment of a colorimetric method for the measurement of low concentrations of peracetic acid and hydrogen peroxide in water. Talanta 2018, 183, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. H2O2 Determination by the I3– Method and by KMnO4 Titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Shamkhalichenar, H.; Choi, J.-W. Non-Enzymatic Hydrogen Peroxide Electrochemical Sensors Based on Reduced Graphene Oxide. J. Electrochem. Soc. 2020, 167, 037531. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Recent advances in electrochemical nonenzymatic hydrogen peroxide sensors based on nanomaterials: A review. J. Mater. Sci. 2019, 54, 12319–12357. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, C.; Ge, S.; Yu, J.; Yan, M. Platelike WO3 sensitized with CdS quantum dots heterostructures for photoelectrochemical dynamic sensing of H2O2 based on enzymatic etching. Biosens. Bioelectron. 2016, 85, 205–211. [Google Scholar] [CrossRef]
- Mercante, L.A.; Facure, M.H.M.; Sanfelice, R.C.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. One-pot preparation of PEDOT:PSS-reduced graphene decorated with Au nanoparticles for enzymatic electrochemical sensing of H2O2. Appl. Surf. Sci. 2017, 407, 162–170. [Google Scholar] [CrossRef]
- Stanković, V.; Đurđić, S.; Ognjanović, M.; Mutić, J.; Kalcher, K.; Stanković, D.M. A novel nonenzymatic hydrogen peroxide amperometric sensor based on AgNp@GNR nanocomposites modified screen-printed carbon electrode. J. Electroanal. Chem. 2020, 876, 114487. [Google Scholar] [CrossRef]
- Prasertying, P.; Yamkesorn, M.; Chimsaard, K.; Thepsuparungsikul, N.; Chaneam, S.; Kalcher, K.; Chaisuksant, R. Modified pencil graphite electrode as a low-cost glucose sensor. J. Sci. Adv. Mater. Devices 2020, 5, 330–336. [Google Scholar] [CrossRef]
- Svancara, I.; Kalcher, K.; Walcarius, A.; Vytras, K. Electroanal. Carbon Paste Electrodes; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012; ISBN 9781439830208. [Google Scholar]
- Sheng, Q.; Zhang, D.; Wu, Q.; Zheng, J.; Tang, H. Electrodeposition of Prussian blue nanoparticles on polyaniline coated halloysite nanotubes for nonenzymatic hydrogen peroxide sensing. Anal. Methods 2015, 7, 6896–6903. [Google Scholar] [CrossRef]
- Pournaghi-Azar, M.H.; Ahour, F.; Pournaghi-Azar, F. Simple and rapid amperometric monitoring of hydrogen peroxide in salivary samples of dentistry patients exploiting its electro-reduction on the modified/palladized aluminum electrode as an improved electrocatalyst. Sens. Actuators B Chem. 2010, 145, 334–339. [Google Scholar] [CrossRef]
- Haghighi, B.; Varma, S.; Alizadeh Sh., F. M.; Yigzaw, Y.; Gorton, L. Prussian blue modified glassy carbon electrodes–Study on operational stability and its application as a sucrose biosensor. Talanta 2004, 64, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bo, X.; Zhao, Z.; Guo, L. Highly exposed Pt nanoparticles supported on porous graphene for electrochemical detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2015, 74, 71–77. [Google Scholar] [CrossRef]
- Anojčić, J.; Guzsvány, V.; Vajdle, O.; Madarász, D.; Rónavári, A.; Kónya, Z.; Kalcher, K. Hydrodynamic chronoamperometric determination of hydrogen peroxide using carbon paste electrodes coated by multiwalled carbon nanotubes decorated with MnO2 or Pt particles. Sens. Actuators B Chem. 2016, 233, 83–92. [Google Scholar] [CrossRef]
- Trujillo, R.M.; Barraza, D.E.; Zamora, M.L.; Cattani-Scholz, A.; Madrid, R.E. Nanostructures in hydrogen peroxide sensing. Sensors 2021, 21, 2204. [Google Scholar] [CrossRef] [PubMed]
- Schachl, K.; Alemu, H.; Kalcher, K.; Moderegger, H.; Svancara, I.; Vytras, K. Amperometric determination of hydrogen peroxide with a manganese dioxide film-modified screen printed carbon electrode. Fresenius. J. Anal. Chem. 1998, 362, 194–200. [Google Scholar] [CrossRef]
- He, S.; Zhang, B.; Liu, M.; Chen, W. Non-enzymatic hydrogen peroxide electrochemical sensor based on a three-dimensional MnO2 nanosheets/carbon foam composite. RSC Adv. 2014, 4, 49315–49323. [Google Scholar] [CrossRef]
- Mailart, M.C.; Sakassegawa, P.A.; Torres, C.R.G.; Palo, R.M.; Borges, A.B. Assessment of peroxide in saliva during and after at-home bleaching with 10% carbamide and hydrogen peroxide gels: A clinical crossover trial. Oper. Dent. 2020, 45, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Watt, B.E.; Proudfoot, A.T.; Vale, J.A. Hydrogen Peroxide Poisoning. Toxicol. Rev. 2004, 23, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Moretto, L.M.; Metelka, R.; Scopece, P. Carbon Nanomaterials in Electrochemical Detection. In Carbon-Based Nanomaterials in Analytical Chemistry; Royal Society of Chemistry: London, UK, 2019; pp. 150–199. [Google Scholar]
- Genorio, B.; Znidarsic, A. Functionalization of graphene nanoribbons. J. Phys. D Appl. Phys. 2014, 47, 094012. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Woo, I.; Jung, Y. On the mechanism of enhanced oxygen reduction reaction in nitrogen-doped graphene nanoribbons. Phys. Chem. Chem. Phys. 2011, 13, 17505–17510. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Li, C.K.; Yu, J.G.; Chen, X.Q. MnO2/reduced graphene oxide nanoribbons: Facile hydrothermal preparation and their application in amperometric detection of hydrogen peroxide. Sens. Actuators B Chem. 2017, 239, 544–552. [Google Scholar] [CrossRef]
- Shi, L.; Niu, X.; Liu, T.; Zhao, H.; Lan, M. Electrocatalytic sensing of hydrogen peroxide using a screen printed carbon electrode modified with nitrogen-doped graphene nanoribbons. Microchim. Acta 2015, 182, 2485–2493. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.S.; Alemany, L.B.; Lu, W.; Tour, J.M. Correction to Improved Synthesis of Graphene. ACS Nano 2018, 12, 2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosan, M.; Mario, L.; Jerman, I.; Kolar, M.; Katsounaros, I.; Genorio, B. Understanding the Oxygen Reduction Reaction Activity of Quasi-1D and 2D N-Doped Heat-Treated Graphene Oxide Catalysts with Inherent Metal Impurities. ACS Appl. Energy Mater. 2021, 4, 3593–3603. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The Role of Oxygen during Thermal Reduction of Graphene Oxide Studied by Infrared Absorption Spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Shornikova, O.N.; Kogan, E.V.; Sorokina, N.E.; Avdeev, V. V The Specific Surface Area and Porous Structure of Graphite Materials. Russ. J. Phys. Chem. A 2009, 83, 1022–1025. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active Sites and Mechanism of Oxygen Reduction Reaction Electrocatalysis on Nitrogen-Doped Carbon Materials. Adv. Mater. 2019, 31, 1804297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Zhang, N.; Kong, F.; Liu, H.; Yin, G. Role of Pt-pyridinic nitrogen sites in methanol oxidation on Pt/polypyrrole-carbon black Catalyst. J. Power Sources 2012, 197, 44–49. [Google Scholar] [CrossRef]
- Brycht, M.; Leniart, A.; Zavašnik, J.; Nosal-Wiercińska, A.; Wasiński, K.; Półrolniczak, P.; Skrzypek, S.; Kalcher, K. Paste electrode based on the thermally reduced graphene oxide in ambient air–Its characterization and analytical application for analysis of 4–chloro–3,5–dimethylphenol. Electrochim. Acta 2018, 282, 233–241. [Google Scholar] [CrossRef]
- Moreira, F.; de Andrade Maranhão, T.; Spinelli, A. Carbon paste electrode modified with Fe3O4 nanoparticles and BMI.PF6 ionic liquid for determination of estrone by square-wave voltammetry. J. Solid State Electrochem. 2018, 22, 1303–1313. [Google Scholar] [CrossRef]
- Benvidi, A.; Nafar, M.T.; Jahanbani, S.; Tezerjani, M.D.; Rezaeinasab, M.; Dalirnasab, S. Developing an electrochemical sensor based on a carbon paste electrode modified with nano-composite of reduced graphene oxide and CuFe2O4 nanoparticles for determination of hydrogen peroxide. Mater. Sci. Eng. C 2017, 75, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Radić, J.; Buljac, M.; Genorio, B.; Gričar, E.; Kolar, M. A novel reduced graphene oxide modified carbon paste electrode for potentiometric determination of trihexyphenidyl hydrochloride in pharmaceutical and biological matrices. Sensors 2021, 21, 2955. [Google Scholar] [CrossRef]
- El-Kosasy, A.M.; Abdel Rahman, M.H.; Abdelaal, S.H. Graphene nanoplatelets in potentiometry: A nanocomposite carbon paste and PVC based membrane sensors for analysis of Vilazodone HCl in plasma and milk samples. Talanta 2019, 193, 9–14. [Google Scholar] [CrossRef] [PubMed]
- AlAqad, K.M.; Suleiman, R.; Al Hamouz, O.C.S.; Saleh, T.A. Novel graphene modified carbon-paste electrode for promazine detection by square wave voltammetry. J. Mol. Liq. 2018, 252, 75–82. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Swartz, M.; Krull, I.S. Analytical Method Development and Validation for the Academic Researcher; Taylor & Francis: Abingdon, UK, 1999. [Google Scholar]
- Bohlooli, F.; Yamatogi, A.; Mori, S. Manganese oxides/carbon nanowall nanocomposite electrode as an efficient non-enzymatic electrochemical sensor for hydrogen peroxide. Sens. Bio-Sensing Res. 2021, 31, 100392. [Google Scholar] [CrossRef]
- Li, Y. Safety and Toxicologic Consideratios for Tooth Bleaching. In Tooth Whitening Techniques; Greenwall, L., Ed.; CRC Press Taylor & Francis Group: London, UK, 2017; pp. 307–316. [Google Scholar]
- Kawanishi, S.; Inoue, S.; Yamamoto, K. Hydroxyl radical and singlet oxygen production and DNA damage induced by carcinogenic metal compounds and hydrogen peroxide. Biol. Trace Elem. Res. 1989, 21, 367–372. [Google Scholar] [CrossRef] [PubMed]
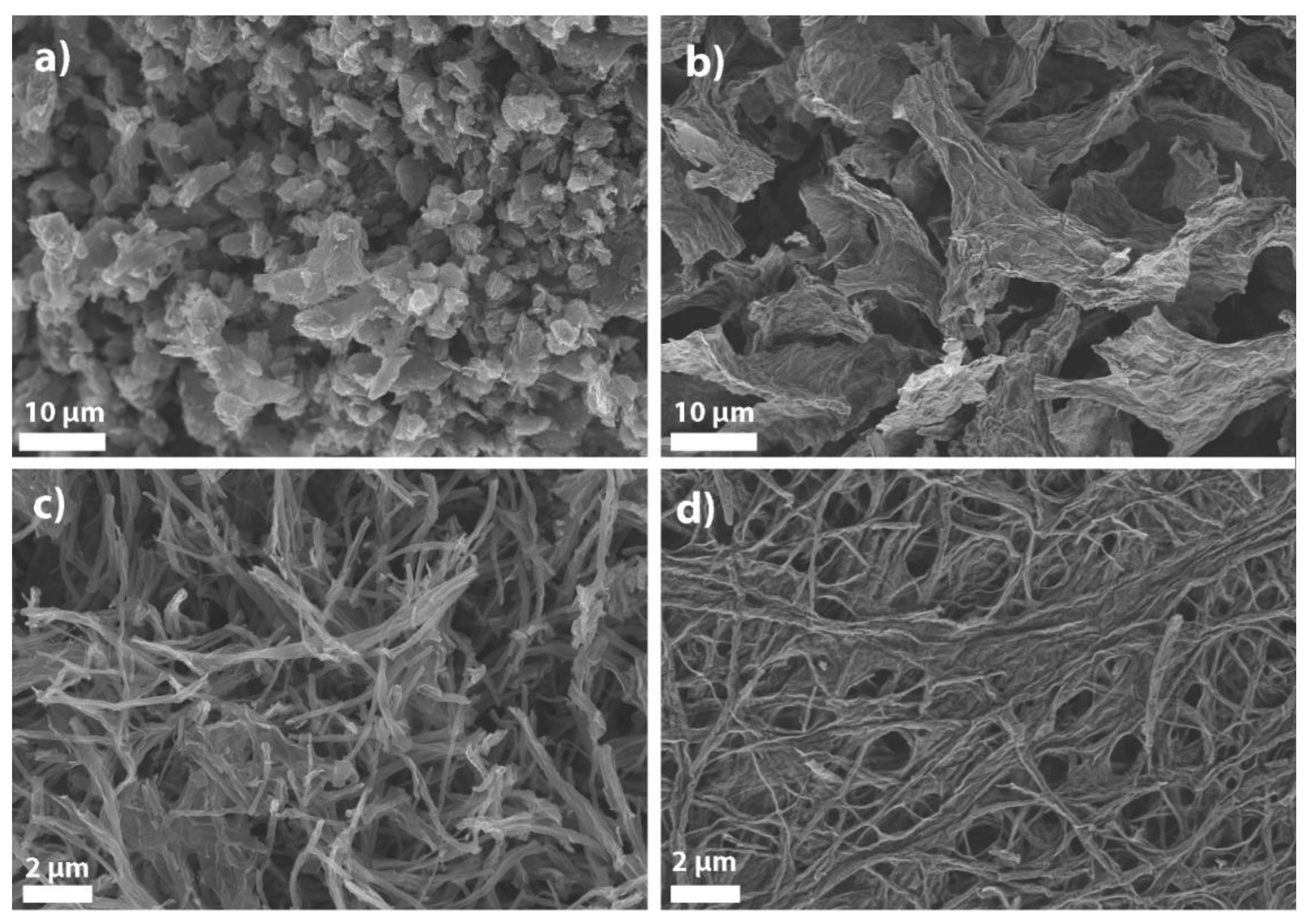


| Material | C (at %) | H (at %) | N (at %) | Pyridinic N (at %) | SBET (m2/g) | w (Mn) (mg/g) |
|---|---|---|---|---|---|---|
| htGO | 76.9 | 12.7 | 0.0 | 0.0 | 602.1 | 4.1 |
| htGONR | 72.3 | 20.1 | 0.0 | 0.0 | 296.5 | 1.6 |
| N-htGO | 75.2 | 9.1 | 8.2 | 3.62 | 129.7 | 2.9 |
| N-htGONR | 73.1 | 9.2 | 7.2 | 2.99 | 85.4 | 1.2 |
| Electrode Composition | Linear Range (µM) | Slope (µA/µM) | LOD (µM) | Ref. |
|---|---|---|---|---|
| CPE/N-htGONR/MnO2/Nafion | 1.0–300 | 0.0677 | 0.08 | This work |
| MnO2-MWCNT/CPE | – | 0.0018 | 70.6 | [19] |
| MnOx/CNW | 40–10230 | 0.439 | 0.55 | [44] |
| MnO2/rGONR | 0.25–2455 | 0.0142 | 0.071 | [28] |
| PB/Pd-Al | 5–34 | 0.0508 | 4 | [16] |
| Measurement No. | Analyte Found (%) | Recovery (%) |
|---|---|---|
| 1 | 2.83 | 94.3 |
| 2 | 2.94 | 98.0 |
| 3 | 2.88 | 96.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gričar, E.; Kalcher, K.; Genorio, B.; Kolar, M. Highly Sensitive Amperometric Detection of Hydrogen Peroxide in Saliva Based on N-Doped Graphene Nanoribbons and MnO2 Modified Carbon Paste Electrodes. Sensors 2021, 21, 8301. https://doi.org/10.3390/s21248301
Gričar E, Kalcher K, Genorio B, Kolar M. Highly Sensitive Amperometric Detection of Hydrogen Peroxide in Saliva Based on N-Doped Graphene Nanoribbons and MnO2 Modified Carbon Paste Electrodes. Sensors. 2021; 21(24):8301. https://doi.org/10.3390/s21248301
Chicago/Turabian StyleGričar, Ema, Kurt Kalcher, Boštjan Genorio, and Mitja Kolar. 2021. "Highly Sensitive Amperometric Detection of Hydrogen Peroxide in Saliva Based on N-Doped Graphene Nanoribbons and MnO2 Modified Carbon Paste Electrodes" Sensors 21, no. 24: 8301. https://doi.org/10.3390/s21248301








