Dissolved Organic Carbon Source Attribution in the Changjiang Outflow Region of the East China Sea
Abstract
1. Introduction
2. Materials and Methods
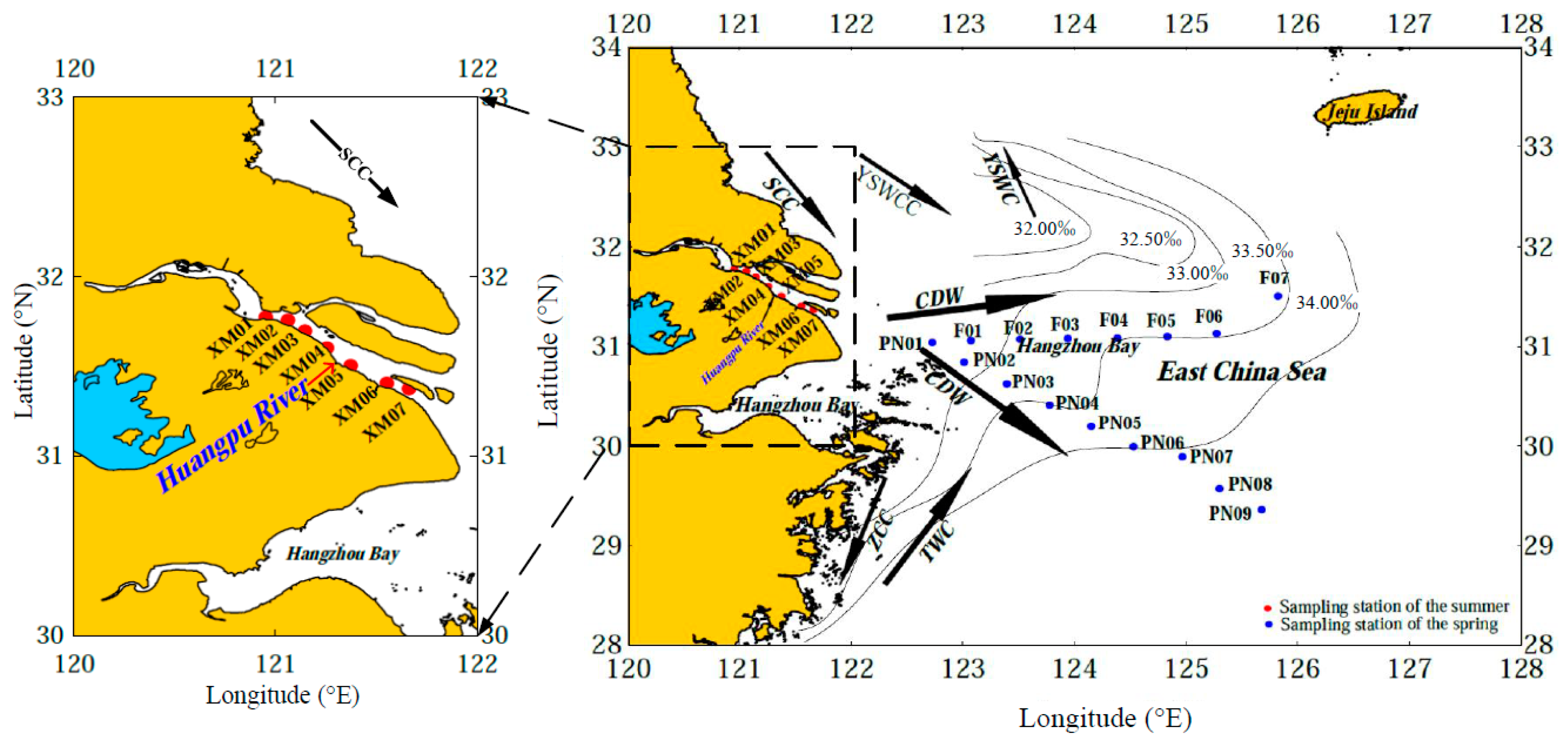
2.1. Hydrodynamic Environment and Sampling
2.2. Methods
2.2.1. Absorption Spectroscopy Analysis
2.2.2. Fluorescence Spectroscopy Analysis and PARAFAC Analysis
2.2.3. DOC Measurements
2.2.4. Measurements of Chlorophyll-a, Suspended Sediments, and Salinity
3. Results and Discussion
3.1. DOC Distribution
3.2. CDOM Absorption Properties
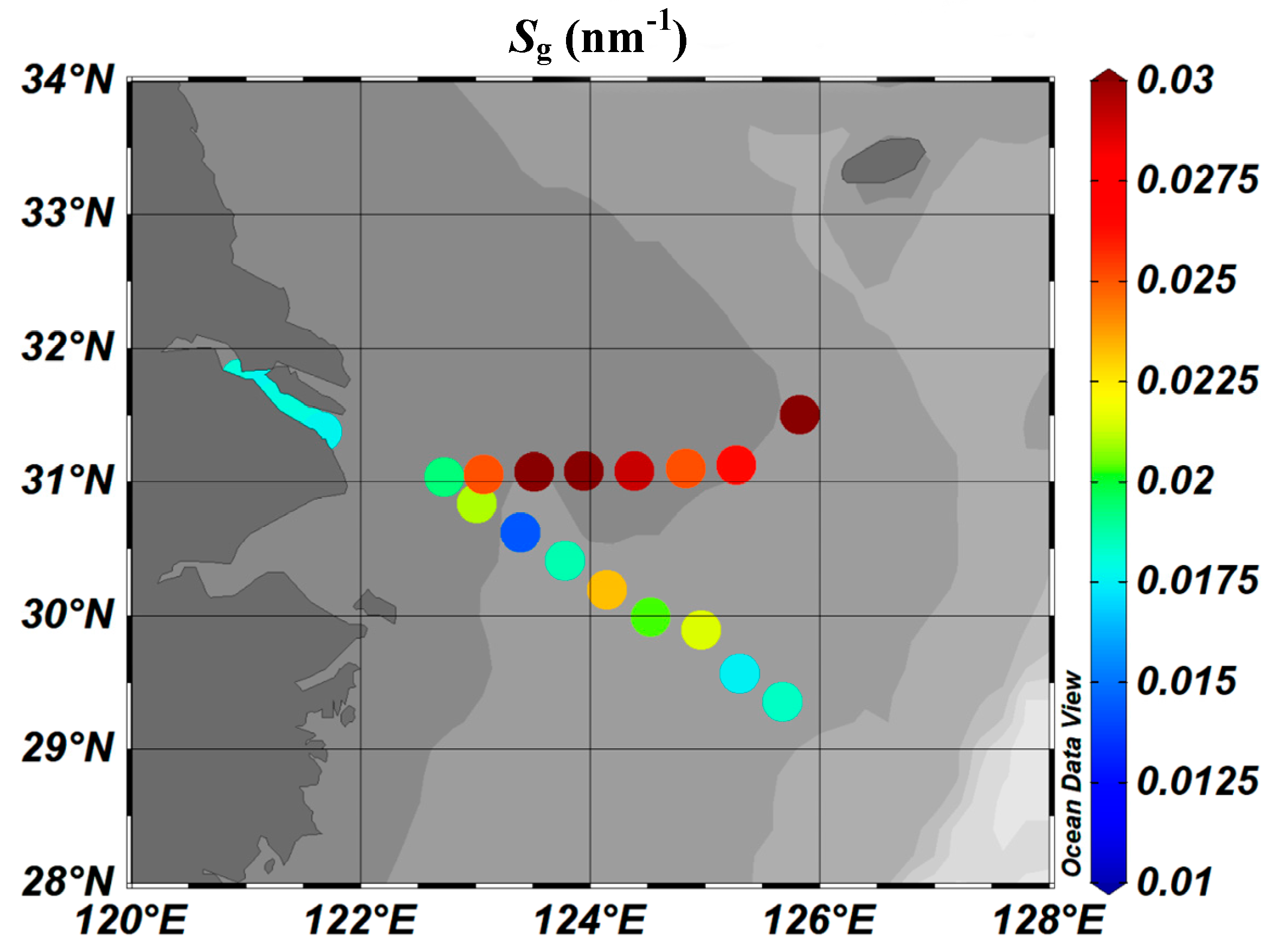
3.2.1. Absorption Spectrum
3.2.2.
3.2.3.
3.3. CDOM Fluorescence Properties
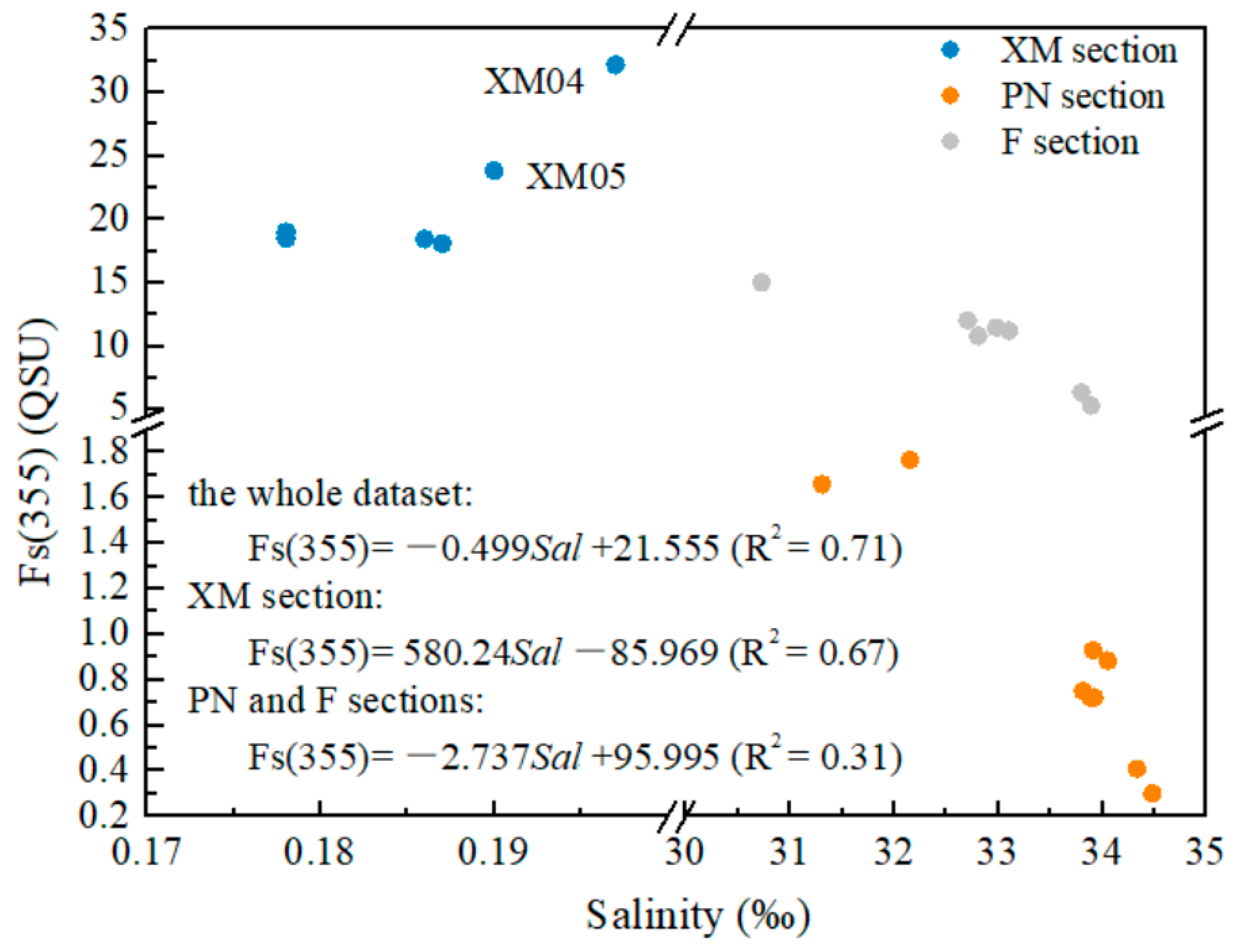
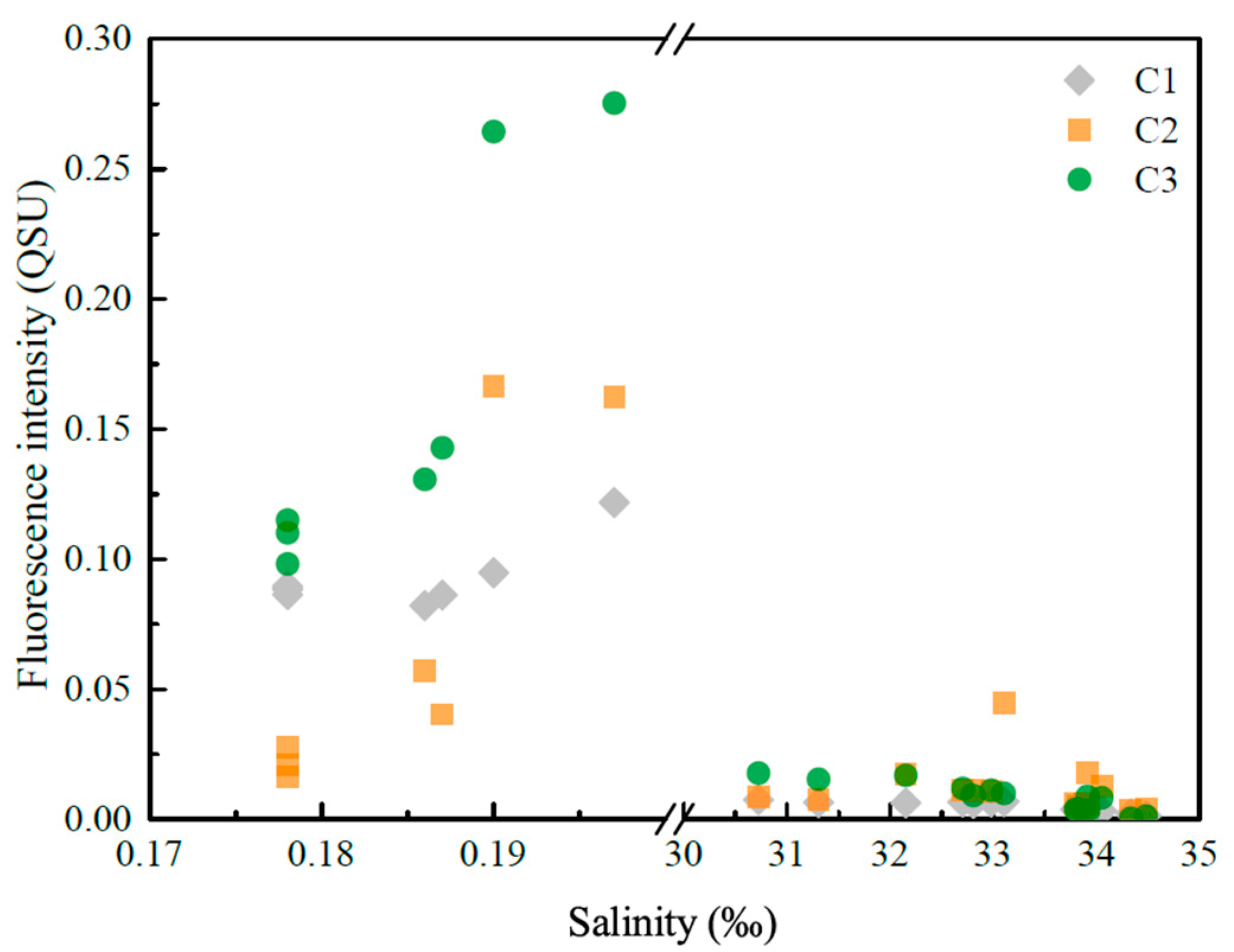
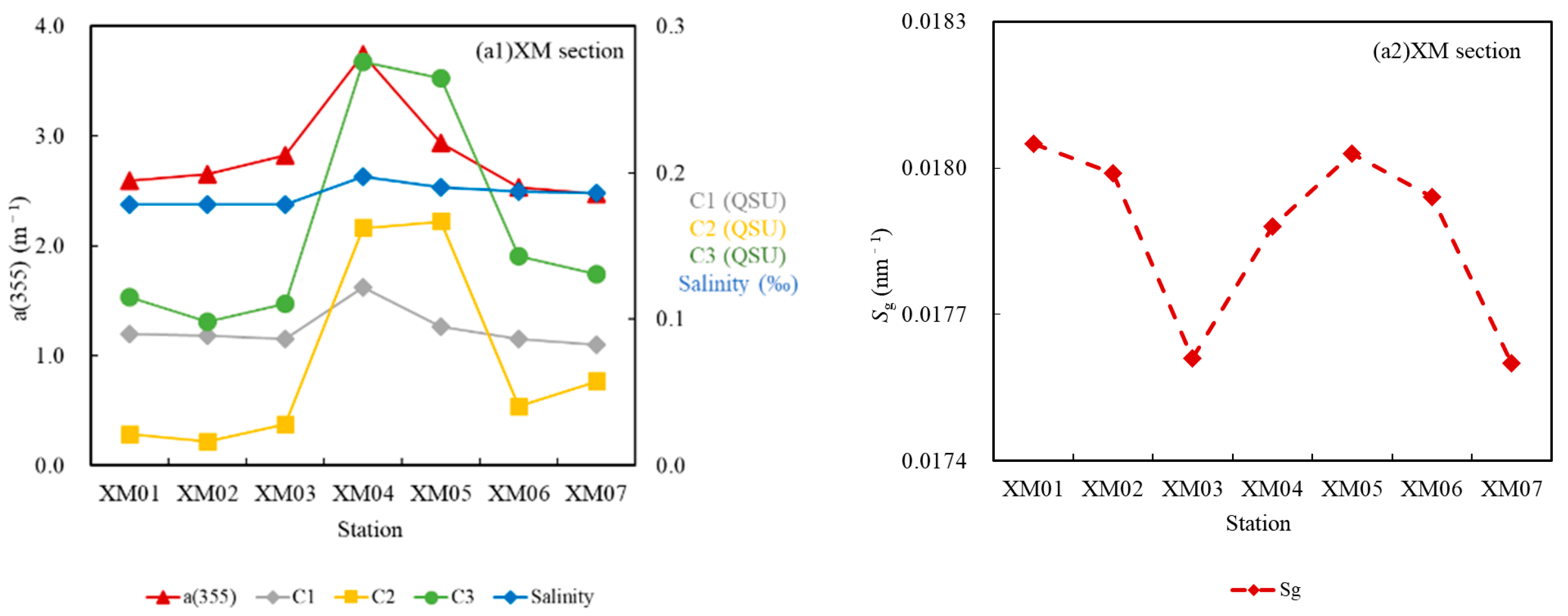
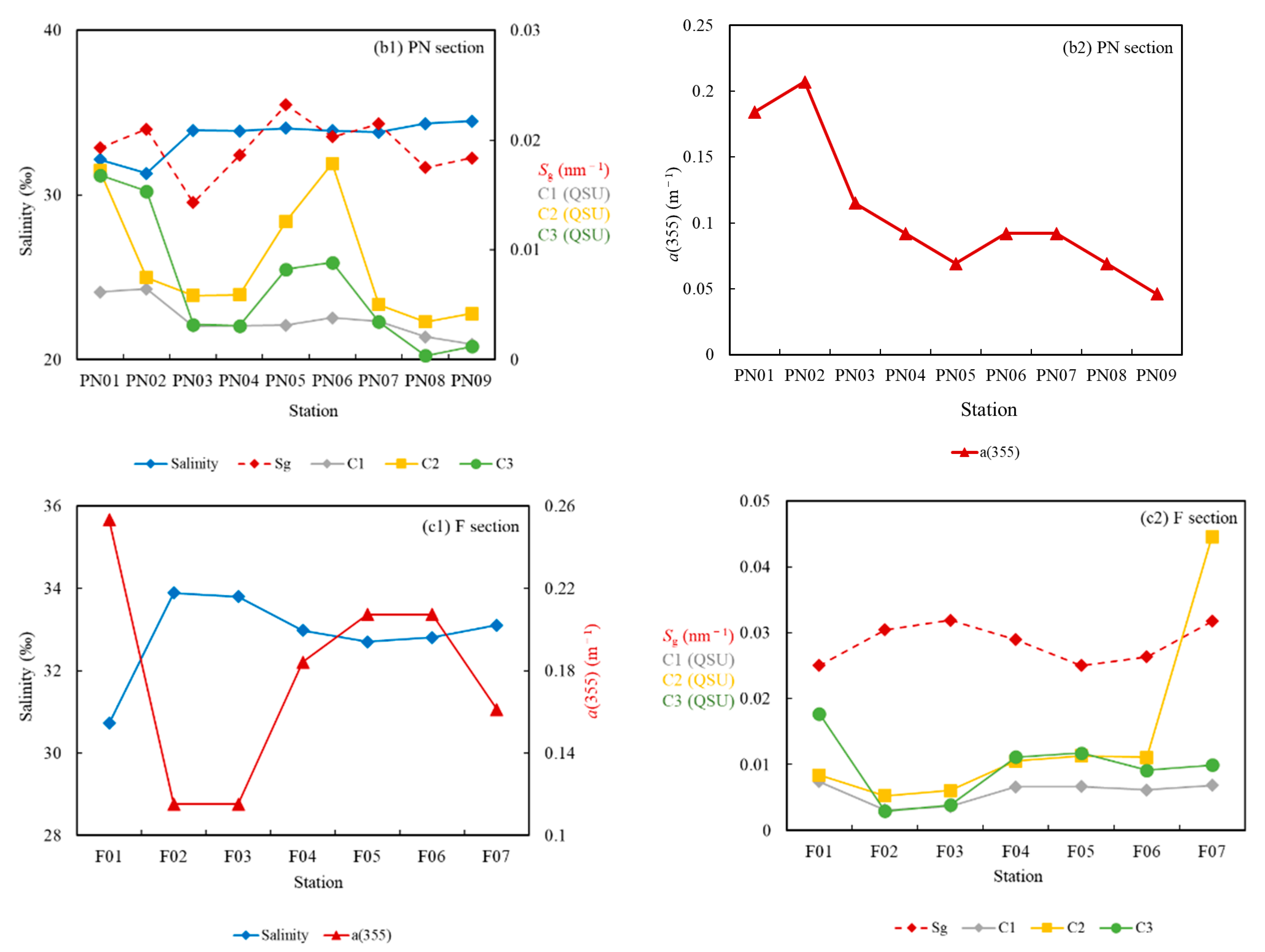
3.3.1. Fluorescence Intensity Fs(355)
3.3.2. CDOM Excitation–Emission Matrix Spectroscopy (EEMS)
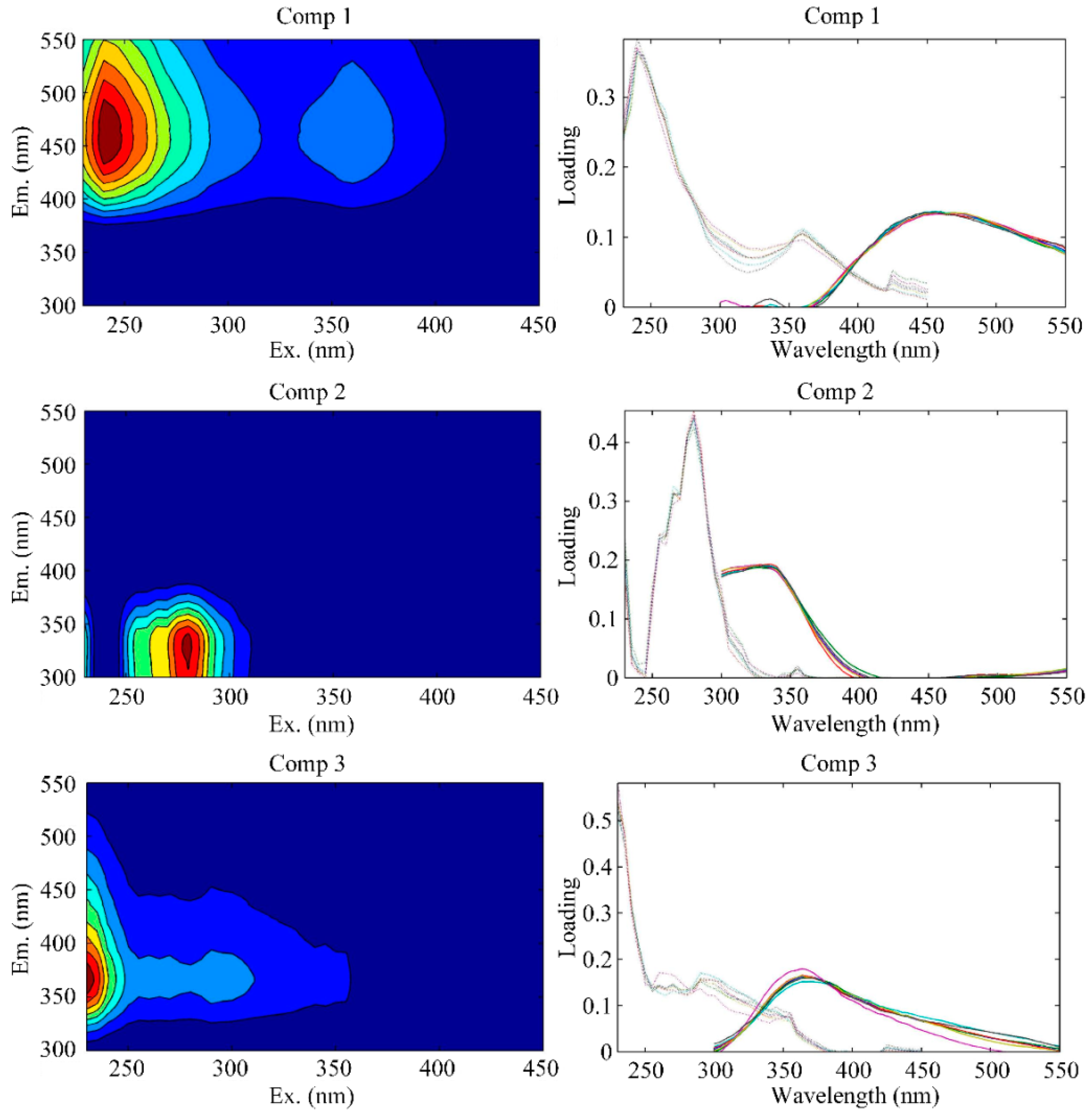
3.3.3. Fluorescent Components
4. Discussion
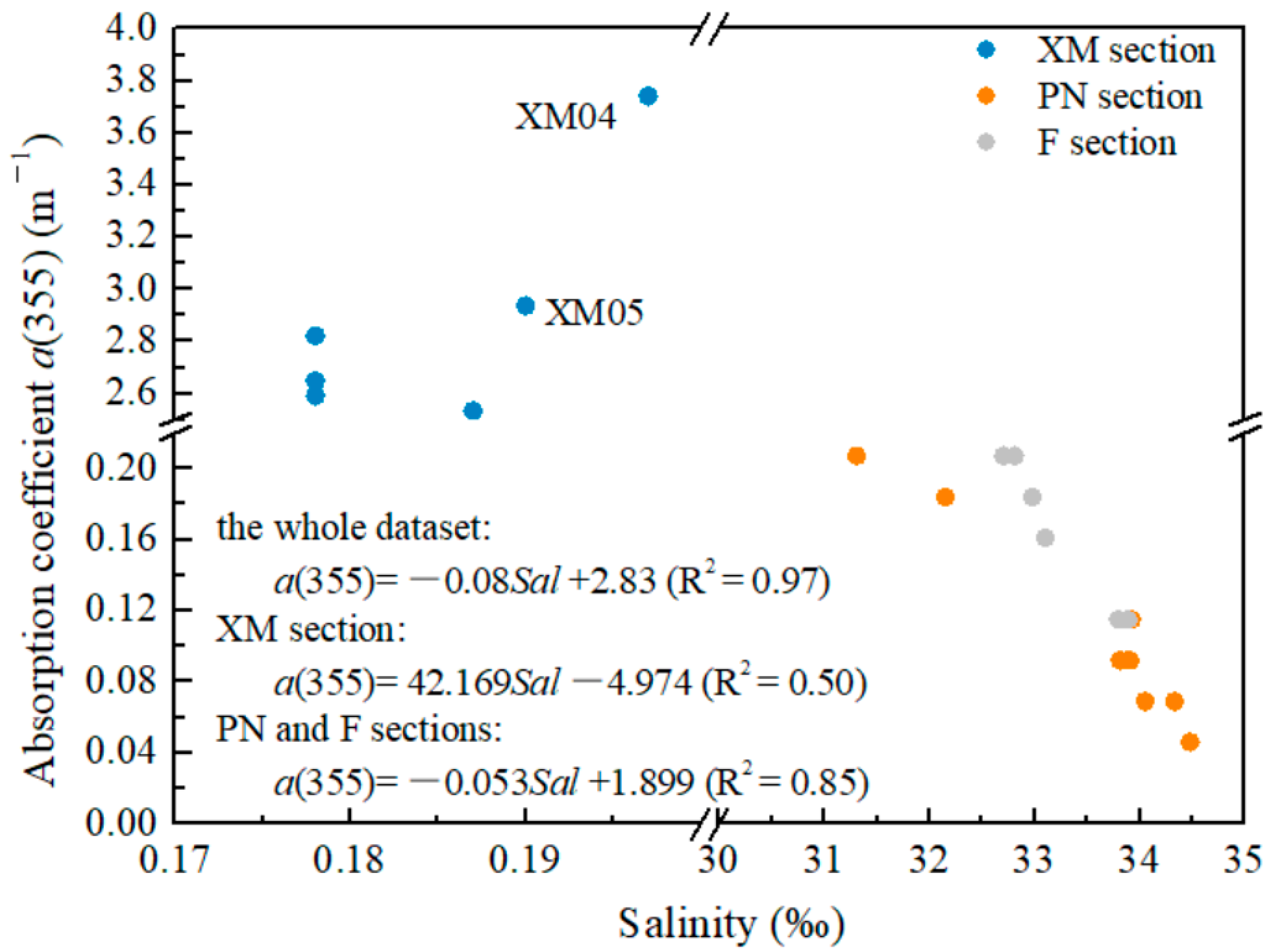
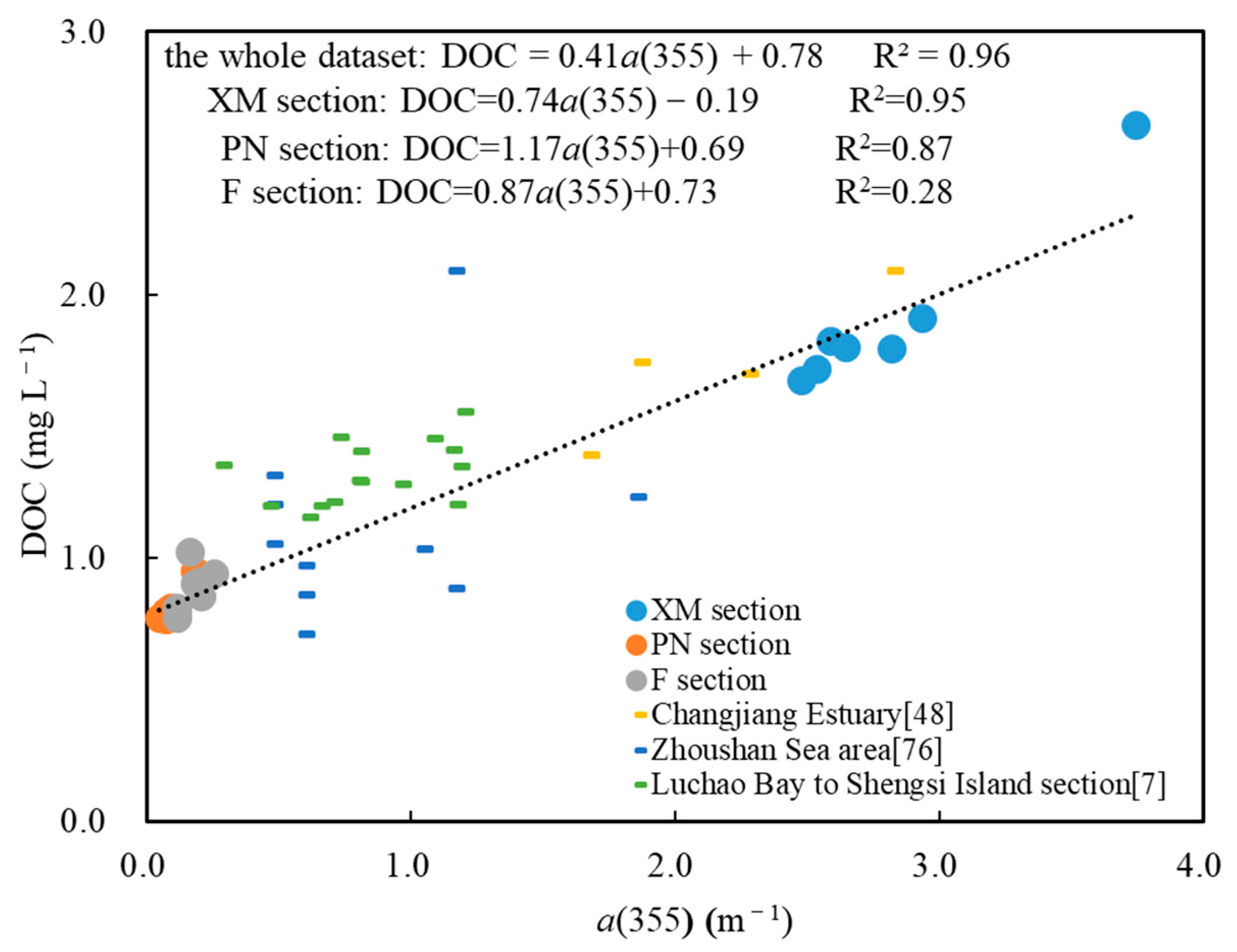
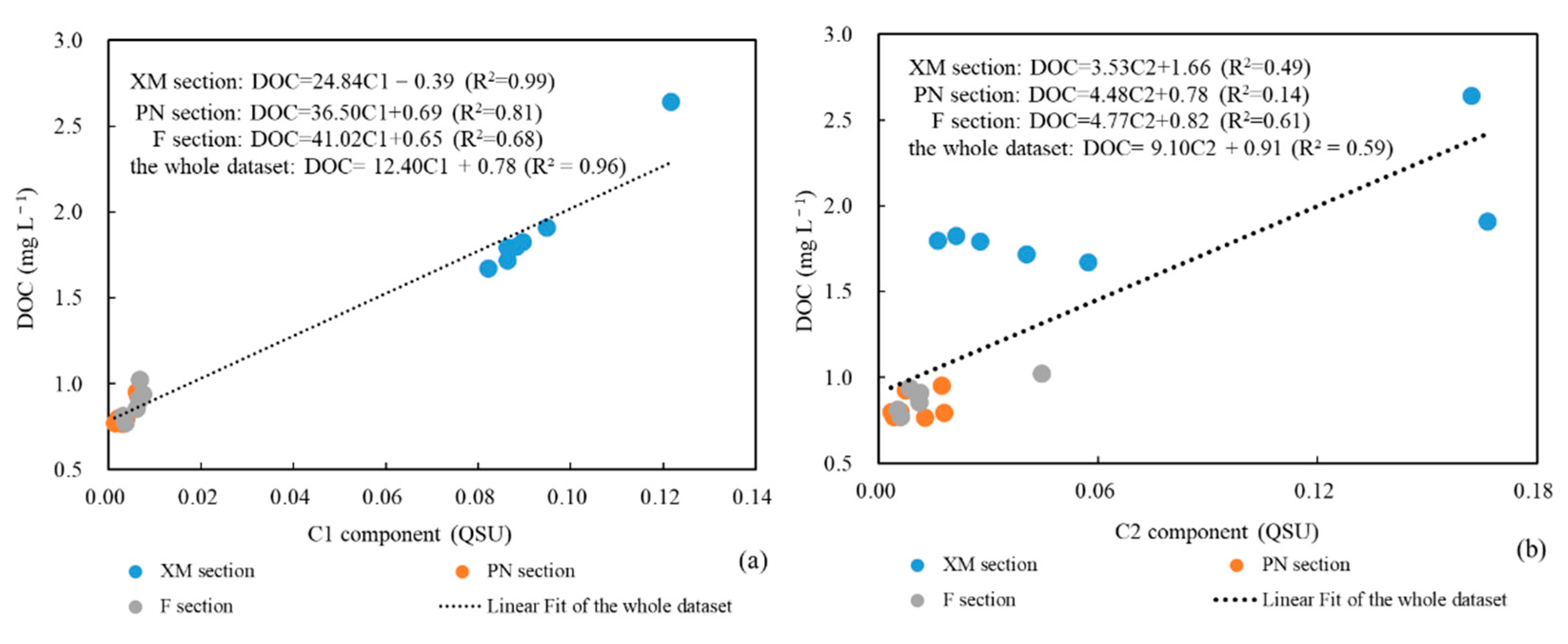
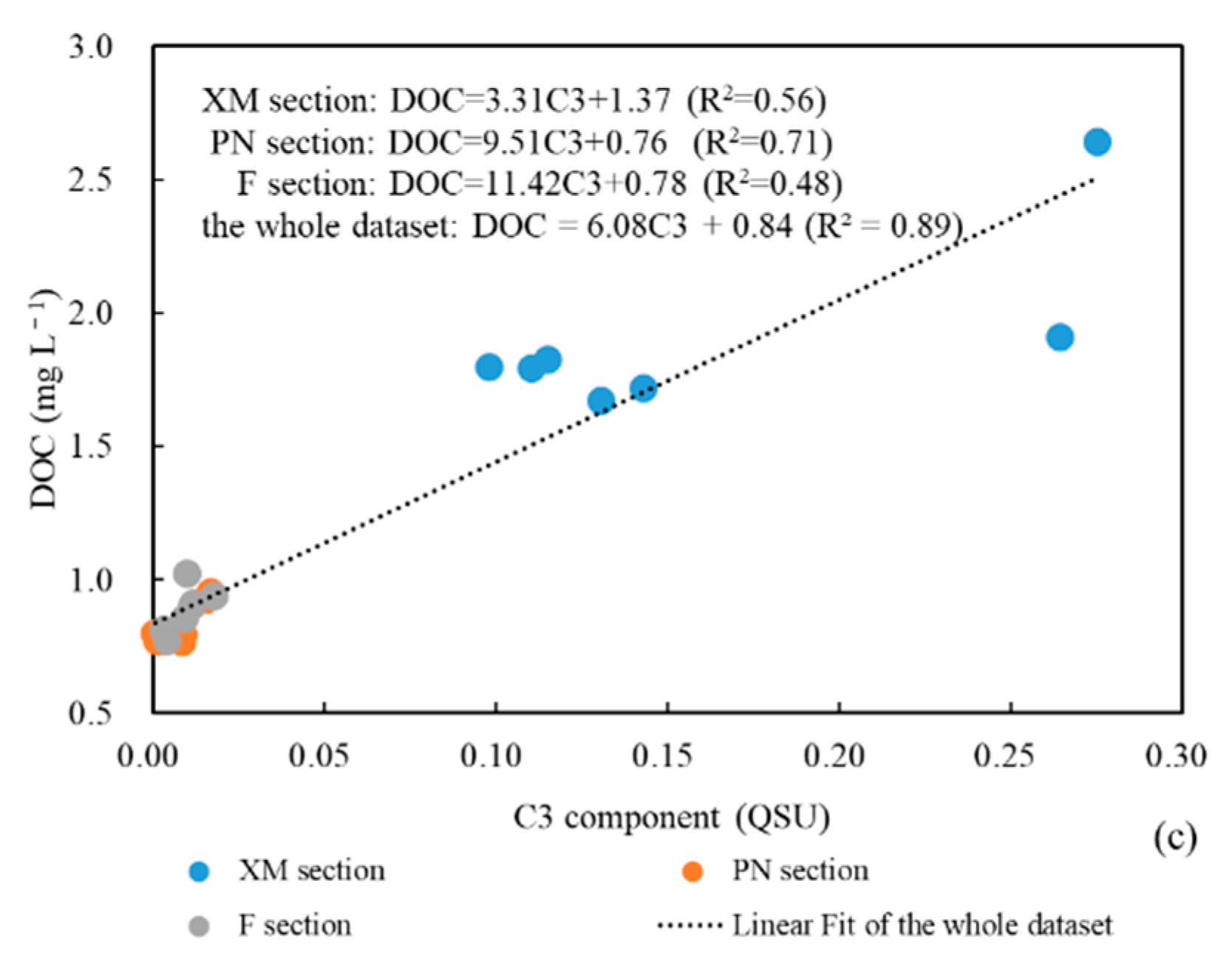
4.1. Relationship between CDOM and DOC
4.2. DOC Source Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
Abbreviations
| CDOM | Chromophoric Dissolved Organic Matter |
| CDW | Changjiang Diluted Water |
| chlorophyll-a | Chl-a |
| DOC | Dissolved Organic Carbon |
| DOM | Dissolved Organic Matter |
| ECS | East China Sea |
| EEMs | Excitation Emission Matrix Fluorescence spectroscopy |
| HTCO | High-Temperature Catalytic Oxidation |
| MTZ | Maximum Turbidity Zone |
| PARAFAC | Parallel Factor Analysis |
| SCC | Subei Coastal Current |
| SS | Suspended Sediments |
| TWC | Taiwan Warm Current |
| YSWC | Yellow Sea Warm Current |
| YSWCC | Yellow Sea Western Coastal Current |
| ZCC | Zhejiang Coastal Current |
References
- John, A.H.; Nina, C.; Sybil, P.S. Global patterns and sources of dissolved organic matter export to the coastal zone: Results from a spatially explicit, global model. Glob. Biogeochem. Cycles 2005, 19, 367–384. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.Y.; Lei, H. Optical absorption properties of CDOM and tracing implication of DOC in the Changjiang Estuary. Mar. Environ. Sci. 2012, 31, 625–630. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Wang, C.; Wang, P.F.; Hou, J.; Ao, Y.H. Absorption and fluorescence characteristics of chromophoric dissolved organic matter in the Yangtze Estuary. Environ. Sci. Pollut. Res. 2014, 21, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, G.; Hu, S.; Xie, H. Optical characterization, distribution and sources of chromophoric dissolved organic material (CDOM) in the Changjiang river estuary in July 2014. Oceanol. Limnol. Sin. 2015, 46, 670–678. [Google Scholar]
- Zhou, Q.; Su, R.; Bai, Y.; Zhang, C.; Shi, X. Characterization of chromophoric dissolved organic matter (CDOM) in Zhoushan Fishery Using Excitation-Emission Matrix Spectroscopy (EEMs) and Parallel Factor Analysis (PARAFAC). Environ. Sci. 2015, 36, 163–171. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, D.; Bai, Y.; Wu, K.; Chen, C.A.; Sun, J.; Zhang, L. The satellite reversion of dissolved organic carbon (DOC) based on the analysis of the mixing behavior of DOC and colored dissolved organic matter: The East China Sea as an example. Acta Oceanol. Sin. 2013, 32, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, X.; Deng, H.; Du, Y.; Jin, H.Y. Absorption features of chromophoric dissolved organic matter (CDOM) and tracing implication for dissolved organic carbon (DOC) in Changjiang Estuary, China. Biogeosci. Discuss. 2013, 101, 2217–12250. [Google Scholar] [CrossRef]
- Yu, X.L.; Shen, F.; Liu, Y.Y. Light absorption properties of CDOM in the Changjiang (Yangtze) estuarine and coastal waters: An alternative approach for DOC estimation. Estuar. Coast. Shelf Sci. 2016, 181, 302–311. [Google Scholar] [CrossRef]
- Bodineau, L.; Thournelin, G.; Beghin, V.; Wartel, M. Tidal Time-scale Changes in the Composition of Particulate Organic Matter within the Estuarine Turbidity Maximum Zone in the Macrotidal Seine Estuary, France: The Use of Fatty Acid and Sterol Biomarkers. Estuar. Coast. Shelf Sci. 1998, 47, 37–49. [Google Scholar] [CrossRef]
- Yang, L.Y.; Chen, W.; Zhuang, W.E.; Cheng, Q.; Li, W.X.; Wang, H.; Guo, W.D.; Chen, C.T.A.; Liu, M.H. Characterization and bioavailability of rainwater dissolved organic matter at the southeast coast of China using absorption spectroscopy and fluorescence EEM-PARAFAC. Coast. Shelf Sci. 2019, 217, 45–55. [Google Scholar] [CrossRef]
- William, L.M.; Mary, A.M. Interaction of photochemical and microbial processes in the degradation of refractory dissolved organic matter from a coastal marine environment. Limnol. Oceanogr. 1997, 42, 1317–1324. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A.; Michaels, A.F. Seasonal dynamics of colored dissolved material in the Sargasso Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 1998, 45, 931–957. [Google Scholar] [CrossRef]
- Siegel, D.A.; Maritorena, S.; Nelson, N.B.; Behrenfeld, M.J. Independence and interdependencies among global ocean color properties: Reassessing the bio-optical assumption. J. Geophys. Res. Ocean. 2005, 110, 1–14. [Google Scholar] [CrossRef]
- Yang, L.Y.; Guo, W.D.; Hong, H.S.; Wang, G.Z. Non-conservative behaviors of chromophoric dissolved organic matter in a turbid estuary: Roles of multiple biogeochemical processes. Estuar. Coast. Shelf Sci. 2013, 133, 285–292. [Google Scholar] [CrossRef]
- Dai, Z.; Fagherazzi, S.; Mei, X. Decline in suspended sediment concentration delivered by the Changjiang (Yangtze) River into the East China Sea between 1956 and 2013. Geomorphology 2016, 268, 123–132. [Google Scholar] [CrossRef]
- Zhao, B.R.; Limeburner, R.; Hu, D.X.; Cui, M.C. Oceanographic charactreistics of the southern Yellow sea and the northern east China sea in summer. Oceanol. Limnol. Sin. 1991, 22, 132–139. [Google Scholar] [CrossRef]
- Zhu, J.R.; Shen, H.T.; Zhou, J. Numerical Simulation of the Impact of the Subei Coastal Current on the Expansion of the Changjiang River Diluted Water in Summer. Oceanol. Limnol. Sin. 1997, 2, 62–67. [Google Scholar] [CrossRef]
- Lin, C.; Ning, X.; Su, J.; Lin, Y.; Xu, B. Environmental changes and the responses of the ecosystems of the Yellow Sea during 1976–2000. J. Mar. Syst. 2004, 55, 223–234. [Google Scholar] [CrossRef]
- Sun, X. Chinese Offshore Marine Area (Chinese Edition); Ocean Press: Beijing, China, 2006; ISBN 9787502766108. [Google Scholar]
- Hu, D.X. Some Striking Features of Circulation in Huanghai Sea and East China Sea. In Oceanology of China Seas; Springer: Dordrecht, The Netherlands, 1994; pp. 27–38. ISBN 978–0-7923-2616-8. [Google Scholar]
- Wei, Q.S.; Yu, Z.G.; Wang, B.D.; Fu, M.Z.; Xia, C.S.; Liu, L.; Ge, R.F.; Wang, H.W.; Zhan, R. Coupling of the spatial–temporal distributions of nutrients and physical conditions in the southern Yellow Sea. J. Mar. Syst. 2016, 156, 30–45. [Google Scholar] [CrossRef]
- Alan, L.S.; Jonathan, D.T. Marine snow: Microscale nutrient patched. Limnol. Oceanogr. 1979, 24, 850–854. [Google Scholar] [CrossRef]
- Fu, M.; Wang, Z.; Li, Y.; Li, R.; Sun, P.; Wei, X.; Lin, X.; Guo, J. Phytoplankton biomass size structure and its regulation in the Southern Yellow Sea (China): Seasonal variability. Cont. Shelf Res. 2009, 29, 2178–2194. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Duan, H.; Barnes, B.; Ma, R. An EOF-Based Algorithm to Estimate Chlorophyll a Concentrations in Taihu Lake from MODIS Land-Band Measurements: Implications for Near Real-Time Applications and Forecasting Models. Remote Sens. 2014, 6, 10694–10715. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, J.; Wu, Y. Recent sediment accumulation and carbon burial in the East China Sea. Glob. Biogeochem. Cycles 2006, 20, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zang, J.Y.; Xing, M.A.Y.; Ling, L.L.; Liu, W.; Zhang, B.T.; Ran, X.B. Variability and influence of dissolved silica in the Qiangtangjiang Estuary. Mar. Sci. 2015, 8, 51–57. [Google Scholar]
- Kang, L.K.; Lu, H.M.; Sung, P.T.; Chan, Y.F.; Lin, Y.C.; Gong, G.C.; Chiang, K.P. The summer distribution of coccolithophores and its relationship to water masses in the East China Sea. J. Oceanogr. 2016, 72, 883–893. [Google Scholar] [CrossRef]
- Han, B.; Zhang, X.; Yang, J.; Pei, J. Multiple-height analytical continuation in processing the gravity anomaly data from the East China Sea and adjacent regions. Chin. High Technol. Lett. 2007, 17, 1272–1277. [Google Scholar] [CrossRef]
- Ogawa, H.; Tanoue, E. Dissolved Organic Matter in Oceanic Waters. J. Oceanogr. 2003, 59, 129–147. [Google Scholar] [CrossRef]
- Aoyama, M.; Hirose, K. Radiometric determination of anthropogenic radionuclides in seawater. Radioact. Environ. 2008, 11, 137–162. [Google Scholar] [CrossRef]
- Wang, S.Y.; Liu, J.P. Delving into the relationship between autumn Arctic sea ice and central–eastern Eurasian winter climate. Atmos. Ocean Sci. Lett. Engl. Version 2016, 9, 366–374. [Google Scholar] [CrossRef]
- Chen, C.C.; Gong, G.C.; Shiah, F.K. Hypoxia in the East China Sea: One of the largest coastal low-oxygen areas in the world. Mar. Environ. Res. 2007, 64, 399–408. [Google Scholar] [CrossRef]
- Wang, B.; Hu, J.; Li, S.; Liu, D. Anumerical analysis of biogeochemical controls with physical modulation on hypoxia during summer in the Pearl River estuary. Biogeoences 2017, 14, 1–31. [Google Scholar] [CrossRef]
- Murphy, K.R.; Dunsmuir, W.T.; Waite, T.D.; Ruiz, G.M.; Coble, P.G. Chromophoric dissolved organic matter (CDOM): Fluorescence losses in frozen samples. In Proceeding of the American Geophysical Union Fall Meeting, San Francisco, CA, USA, 11–15 December 2005. [Google Scholar]
- Griffin, C.G.; McClelland, J.W.; Vonk, J.E.; Holmes, R.M.; Frey, K.E. Chromophoric dissolved organic matter during the Mackenzie River spring freshet: Observations and freeze-thaw experiments. In Proceedings of the American Geophysical Union Fall Meeting, Vienna, Austria, 22–27 April 2012. [Google Scholar]
- Zhang, J.B.; Song, C.C.; Yang, W.Y. Seasonal dynamics of dissolved organic carbon and its impact factors in the Doyeuxia augustifolia marsh soil. J. Environ. Sci. 2005, 25, 1397–1402. [Google Scholar] [CrossRef]
- Su, J. China Offshore Hydrological (Chinese Edition); Ocean Press: Beijing, China, 2005; ISBN 9787502762926. [Google Scholar]
- Robyn, N.C.; Paula, G.C.; Robert, F.C.; Gardner, B.G. Optical properties of colored dissolved organic matter in the Northern Gulf of Mexico. Mar. Chem. 2004, 89, 127–144. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Kaas, H. Optical Properties and Signatures of Chromophoric Dissolved Organic Matter (CDOM) in Danish Coastal Waters. Estuar. Coast. Shelf Sci. 2000, 51, 267–278. [Google Scholar] [CrossRef]
- Colin, A.S.; Stiig, M.; Rasmus, B. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Mark, A.V.D.; Liu, M.L.; Zhu, G.W.; Qin, B.Q. The contribution of phytoplankton degradation to chromophoric dissolved organic matter (CDOM) in eutrophic shallow lakes: Field and experimental evidence. Water Res. 2009, 43, 4685–4697. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, E.L.; Yin, Y.; Mark, A.V.D.; Feng, L.Q.; Shi, Z.Q.; Liu, M.L.; Qin, B.Q. Characteristics and sources of chromophoric dissolved organic matter in lakes of the Yungui Plateau, China, differing in trophic state and altitude. Limnol. Oceanogr. 2010, 55, 2645–2659. [Google Scholar] [CrossRef]
- Colin, A.S.; Rasmus, B. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Lin, J.R.; Li, X.L.; Chen, W.F. Sampling and determination of marine dissolved organic carbon. Oceanol. Limnol. Sin. 2008, 39, 604–611. [Google Scholar] [CrossRef]
- National Marine Standardization Technical Committee. The Specification for Marine Monitoring GB 17378.4-2007. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=9FB14D0EE23D77A96D54A9BDAAF6EA07 (accessed on 7 November 2008).
- Zhang, J.; Wu, Y.; Jennerjahn, T.C.; Ittekkot, V.; He, Q. Distribution of organic matter in the Changjiang (Yangtze River) Estuary and their stable carbon and nitrogen isotopic ratios: Implications for source discrimination and sedimentary dynamics. Mar. Chem. 2007, 106, 111–126. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Li, Y.; Pan, J.M. Distributions of colored dissolved organic matter and dissolved organic carbon in the Pearl River Estuary, China. Cont. Shelf Res. 2004, 24, 1845–1856. [Google Scholar] [CrossRef]
- Guo, W.; Yang, L.; Zhai, W. Runoff-mediated seasonal oscillation in the dynamics of dissolved organic matter in different branches of a large bifurcated estuary-The Changjiang Estuary. J. Geophys. Res. Biogeosci. 2014, 119, 776–793. [Google Scholar] [CrossRef]
- Blough, N.V.; Oliver, Z.; Bonilla, J.J. Optical absorption spectra of waters from the Orinoco River outflow: Terrestrial input of colored organic matter to the Caribbean. J. Geophys. Res. Atmos. 1993, 98, 2271–2278. [Google Scholar] [CrossRef]
- Ferrari, G.M.; Dowell, M.D. CDOM Absorption Characteristics with Relation to Fluorescence and Salinity in Coastal Areas of the Southern Baltic Sea. Estuar. Coast. Shelf Sci. 1998, 47, 91–105. [Google Scholar] [CrossRef]
- Guo, L.; Santschi, P.H.; Bianchi, T.S. Dissolved organic matter in estuaries of the Gulf of Mexico. Biogeochem. Gulf Coast Estuaries 1999, 269–299. [Google Scholar] [CrossRef]
- Guo, W.D.; Colin, A.S.; Han, Y.C.; Wu, F.; Yu, X.X.; Hu, M.H. The conservative and non-conservative behavior of chromophoric dissolved organic matter in Chinese estuarine waters. Mar. Chem. 2007, 107, 357–366. [Google Scholar] [CrossRef]
- Kong, D.X.; Yang, H.; Wu, J.H. Optical absorption properties of chromophoric dissolvable organic matter in Changjiang Estuary. Mar. Environ. Sci. 2008, 27, 629–631. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Wu, X.L.; Kong, D.X. Optical absorption properties of chromophoric dissolved organic matter in the seawater of outer Yangtze Estuary. J. Oceanogr. Taiwan Strait 2010, 29, 518–524. [Google Scholar] [CrossRef]
- Hong, H.S.; Wu, J.Y.; Shang, S.L.; Hu, C.M. Absorption and fluorescence of chromophoric dissolved organic matter in the Pearl River Estuary, South China. Mar. Chem. 2005, 97, 78–89. [Google Scholar] [CrossRef]
- Rosa, A.; Véronique, R.; Christiane, L. Coloured dissolved organic matter (CDOM) in Southern North Sea waters: Optical characterization and possible origin. Estuar. Coast. Shelf Sci. 2009, 85, 633–640. [Google Scholar] [CrossRef]
- Rochelle-Newall, E.J.; Fisher, T.R. Chromophoric dissolved organic matter and dissolved organic carbon in Chesapeake Bay. Mar. Chem. 2002, 77, 23–41. [Google Scholar] [CrossRef]
- Sarah, A.G.; Neil, V.B. Optical Absorption and Fluorescence Properties of Chromophoric Dissolved Organic Matter in Natural Waters. Limnol. Oceanogr. 1994, 39, 1903–1916. [Google Scholar] [CrossRef]
- Hoge, F.E.; Vodacek, A.; Swift, R.N.; Yungel, J.K.; Blough, N.V. Inherent optical properties of the ocean: Retrieval of the absorption coefficient of chromophoric dissolved organic matter from airborne laser spectral fluorescence measurements. Appl. Opt. 1995, 34, 1394–1402. [Google Scholar] [CrossRef]
- Fichot, C.G.; Benner, R. The spectral slope coefficient of chromophoric dissolved organic matter (S275–295) as a tracer of terrigenous dissolved organic carbon in river-influenced ocean margins. Limnol. Oceanogr. 2012, 57, 1453–1466. [Google Scholar] [CrossRef]
- Matsuoka, A.; Bricaud, A.; Benner, R.; Para, J.; Sempéré, R.; Prieur, L.; Bélanger, S.; Babin, M. Tracing the transport of colored dissolved organic matter in water masses of the Southern Beaufort Sea: Relationship with hydrographic characteristics. Biogeosciences 2012, 9, 925–940. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, B.H.; Song, Y.H.; Qin, Y.W. Correlation between molecular absorption spectral slope ratios and fluorescence humification indices in characterizing CDOM. Aquat. Sci. 2011, 73, 103–112. [Google Scholar] [CrossRef]
- Mannino, A.; Russ, M.E.; Hooker, S.B. Algorithm development and validation for satellite-derived distributions of DOC and CDOM in the U.S. Middle Atlantic Bight. J. Geophys. Res. Ocean. 2008, 113, 827–830. [Google Scholar] [CrossRef]
- Blough, N.; del Vecchio, R. Chromophoric DOM in the Coastal Environment. Biogeochem. Mar. Dissolved Org. Matter. 2002, 509–546. [Google Scholar] [CrossRef]
- Piotr, K.; Michael, J.D.; Heather, Y.; Amanda, E.K.; William, J.C.; Michael, G. Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: Interannual variability. Mar. Chem. 2009, 113, 182–196. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Qin, B.Q.; Yang, L.Y. Spatial distribution and absorption characteristics with relation to fluorescence of chromophoric dissolved organic matter (CDOM) in Meiliang Bay of Lake Taihu. Lake Sci. 2006, 18, 319–326. [Google Scholar] [CrossRef][Green Version]
- Huang, W.; Zhou, L.; Zheng, X. Seasonal Variation of Colloidal Organic Carbon in Changjiang (Yangtze) River Estuary. Oceanol. Limnol. 2013, 44, 1194–1199. [Google Scholar] [CrossRef]
- Colin, A.S.; Stiig, M. Resolving the Variability in Dissolved Organic Matter Fluorescence in a Temperate Estuary and Its Catchment Using PARAFAC Analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Stiig, M.; Warwick, F.V. Spectral Light Attenuation and the Absorption of UV and Blue Light in Natural Waters. Limnol. Oceanogr. 2000, 45, 642–650. [Google Scholar] [CrossRef]
- Youhei, Y.; Rudolf, J.; Nagamitsu, M.; Eiichiro, T. Assessing the Dynamics of Dissolved Organic Matter (DOM) in Coastal Environments by Excitation Emission Matrix Fluorescence and Parallel Factor Analysis (EEM-PARAFAC). Limnol. Oceanogr. 2008, 53, 1900–1908. [Google Scholar] [CrossRef]
- Paula, G.C.; Carlos, E.D.C.; Bernard, A. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep-Sea Res. Part II 1998, 45, 2195–2223. [Google Scholar] [CrossRef]
- Paula, G.C. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruiz, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Guo, W.D.; Yang, L.Y.; Hong, H.S.; Colin, A.S.; Wang, F.L.; Xu, J.; Xie, Y.Y. Assessing the dynamics of chromophoric dissolved organic matter in a subtropical estuary using parallel factor analysis. Mar. Chem. 2011, 124, 125–133. [Google Scholar] [CrossRef]
- Jason, C.N.; Gregory, P.A. Dissolved Organic Carbon in Terrestrial Ecosystems: Synthesis and a Model. Ecosystems 2001, 4, 29–48. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, J.; Jiang, Y.; Li, J. Variability of terrigenous dissolved organic matter as a response to coastal plume in Zhoushan area of the East China Sea. Acta Oceanol. Sin. 2011, 33, 66–73. [Google Scholar] [CrossRef]
- Piotr, K.; William, J.C.; Michael, J.D.; Amanda, E.K.; Michael, G.; Heather, Y. Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: Relationships between fluorescence and its components, absorption coefficients and organic carbon concentrations. Mar. Chem. 2009, 118, 22–36. [Google Scholar] [CrossRef]
- Peter, J.H.; Ronald, B. Photochemical and microbial degradation of dissolved lignin phenols: Implications for the fate of terrigenous dissolved organic matter in marine environments. J. Geophys. Res. Ocean. 2003, 108, 351–378. [Google Scholar] [CrossRef]
- John, R.H.; Aron, S.; Jason, D.R.; Elizabeth, C.M.; David, J.K.; Kenneth, M. Absorption Spectral Slopes and Slope Ratios as Indicators of Molecular Weight, Source, and Photobleaching of Chromophoric Dissolved Organic Matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Yan, B.; Pan, D.L.; Cai, W.J.; He, X.Q.; Wang, D.F.; Bang, Y.T.; Zhu, Q.K. Remote sensing of salinity from satellite-derived CDOM in the Changjiang River dominated East China Sea. J. Geophys. Res. Ocean. 2013, 118, 227–243. [Google Scholar] [CrossRef]
- Yan, L.H.; Chen, X.J.; Su, R.G.; Han, X.R.; Zhang, C.S.; Shi, X.Y. Resolving Characteristic of CDOM by Excitation-Emission Matrix Spectroscopy Combined with Parallel Factor Analysis in the Seawater of Outer Yangtze Estuary in Autumn in 2010. Environ. Sci. 2013, 34, 51–60. [Google Scholar]
- Baker, A. Fluorescence Excitation-Emission Matrix Characterization of Some Sewage-Impacted Rivers. Environ. Sci. Technol. 2001, 35, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.D.; Huang, J.P.; Hong, H.S.; Xu, J.; Deng, X. Resolving excitation emission matrix spectroscopy of estuarine CDOM with parallel factor analysis and its application in organic pollution monitoring. Environ. Sci. 2010, 31, 1419–1427. [Google Scholar] [CrossRef]
- Hong, H.S.; Yang, L.Y.; Guo, W.D.; Wang, F.L.; Yu, X.X. Characterization of dissolved organic matter under contrasting hydrologic regimes in a subtropical watershed using PARAFAC model. Biogeochemistry 2012, 109, 163–174. [Google Scholar] [CrossRef]
- Wen, F.; Sun, X.X.; Zhang, S.; Luo, X.; Feng, Q.; Sun, S. Spatial and seasonal variations of chlorophyll-a and primary productivity in spring and summer in the Yellow Sea and East China Sea. Oceanol. Limnol. Sin. 2012, 43, 438–444. [Google Scholar]
- Zhang, X.Y.; Zhang, F.Y.; Gao, A.G.; Zhang, W.Y. Source tracing implication of Pt and Pd fractionation in the surface sediment of continental shelf around Changjiang Estuary. Earth Sci. J. China Univ. Geosci. 2009, 34, 604–612. [Google Scholar] [CrossRef]
- Lawrence, M.M.; Linda, L.S.; Theodore, C.L. Dissolved protein fluorescence in two Maine estuaries. Mar. Chem. 1999, 64, 171–179. [Google Scholar] [CrossRef]
- Vignudelli, S.; Santinelli, C.; Murru, E.; Nannicini, L.; Seritti, A. Distributions of dissolved organic carbon (DOC) and chromophoric dissolved organic matter (CDOM) in coastal waters of the northern Tyrrhenian Sea (Italy). Estuar. Coast. Shelf Sci. 2003, 60, 133–149. [Google Scholar] [CrossRef]




| Research Areas | Salinity (‰) | References | |||
|---|---|---|---|---|---|
| Changjiang Estuary (summer) | 0.1–3.2 | 0.017–0.020 (300–650 nm) | 0–32.0 | [52] | |
| Changjiang Estuary (spring) | 1.152–8.715 | 0.0034–0.014 (380–800 nm) | / | [53] | |
| Changjiang Estuary (summer) | 0.20–0.73 | / | 0.2–25.3 | [54] | |
| Changjiang Estuary (summer) | 0.20–0.77 | / | 0.3–29.5 | ||
| Changjiang Estuary (spring) | 0.10–2.82 | 0.017–0.020 (300–500 nm) | 0.12–29.4 | [3] | |
| Changjiang Estuary (winter) | 0.11–1.20 | 0.008–0.018 (275–295 nm) | 18.7–34.9 | [8] | |
| Changjiang Estuary (summer) | 0.23–1.91 | 0.012–0.025 (275–295 nm) | 4.0–33.6 | ||
| Pearl River Estuary (November) | 0.24–1.93 | 0.0138–0.018 (300–500 nm) | 0–32.49 | [55] | |
| Pearl River Estuary (June) | 0.34–1.40 | / | 0–34.96 | [47] | |
| South of the North Sea (February) | Scheldt Estuary | 0.97–4.30 | 0.0167–0.019 (350–500 nm) | 0.7–29.6 | [56] |
| Belgium coastal sea | 0.20–1.31 | 0.0110–0.020 (350–500 nm) | 29.8–33.6 | ||
| Chesapeake Bay | River end member | 2.2–4.1 | 0.0163–0.019 (280–650 nm) | 0–35.0 | [57] |
| coast | 0.4–1.1 | 0.0178–0.022 (280–650 nm) | |||
| Mississippi Estuary (summer) | 1.2–4.2 | / | / | [49] | |
| Amazon Estuary (winter) | 0.14–3.12 | / | / | [58] | |
| Georgia coast | 0.06–1.20 | / | / | [59] | |
| Northern Gulf of Mexico | 3.96–17.52 | / | 0–37.0 | [60] | |
| Southern Beaufort Sea | 0.018–1.08 | 0.015–0.023 (350–500 nm) | 0–35.0 | [61] | |
| Section XM (August 2011) | 2.476–3.742 | 0.0176–0.018 | 0.18–0.20 | This study | |
| Section PN (March 2011) | 0.046–0.207 | 0.0143–0.023 | 31.31–34.5 | ||
| Section F (March 2011) | 0.115–0.253 | 0.025–0.0318 | 30.72–33.9 | ||
| Ex/Em (nm) | Coble [71,72] (Ex/Em (nm)) | References (Ex/Em (nm)) | |
|---|---|---|---|
| C1 | 240/456 | peak A: 230–260/380–460; humic-like component | C3: 270 (360)/478 [69] |
| C4: 250 (360)/440 [67] | |||
| C8: 250 (380)/416 [72] | |||
| C1: 270 (365)/453 [40] | |||
| C1: ≤250 (335)/428 [73] | |||
| C2 | 280/328 | peak T: 270–280/320–350; protein-like component | C5: 280(240)/368 [69] |
| C7: 280/344 [67] | |||
| C7: 240 (300)/338 [72] | |||
| C4: 280/318 [68] | |||
| C6: 250(290)/356 [74] | |||
| C4: 275/328 [73] | |||
| C3 | 230/366 | combination of peak N (280/370) and peak T (270–280/320–350); protein-like component | C5:280(<240)/368 [69] |
| C4: 250 (320)/370 [72] | |||
| C5: 285/362 [68] | |||
| C2: ≤250(300)/368 [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Du, Y.; Mao, Z.; Bi, L.; Chen, J.; Jin, H.; Ma, S. Dissolved Organic Carbon Source Attribution in the Changjiang Outflow Region of the East China Sea. Sensors 2021, 21, 8450. https://doi.org/10.3390/s21248450
Zhang X, Du Y, Mao Z, Bi L, Chen J, Jin H, Ma S. Dissolved Organic Carbon Source Attribution in the Changjiang Outflow Region of the East China Sea. Sensors. 2021; 21(24):8450. https://doi.org/10.3390/s21248450
Chicago/Turabian StyleZhang, Xiaoyu, Yong Du, Zhihua Mao, Lei Bi, Jianyu Chen, Haiyan Jin, and Shuchang Ma. 2021. "Dissolved Organic Carbon Source Attribution in the Changjiang Outflow Region of the East China Sea" Sensors 21, no. 24: 8450. https://doi.org/10.3390/s21248450
APA StyleZhang, X., Du, Y., Mao, Z., Bi, L., Chen, J., Jin, H., & Ma, S. (2021). Dissolved Organic Carbon Source Attribution in the Changjiang Outflow Region of the East China Sea. Sensors, 21(24), 8450. https://doi.org/10.3390/s21248450






