An Inertial Measurement Unit-Based Wireless System for Shoulder Motion Assessment in Patients with Cervical Spinal Cord Injury: A Validation Pilot Study in a Clinical Setting
Abstract
1. Introduction
2. Materials and Method
2.1. Shoulder Movements to Evaluate Range of Motion
2.2. Goniometer Measurement Method
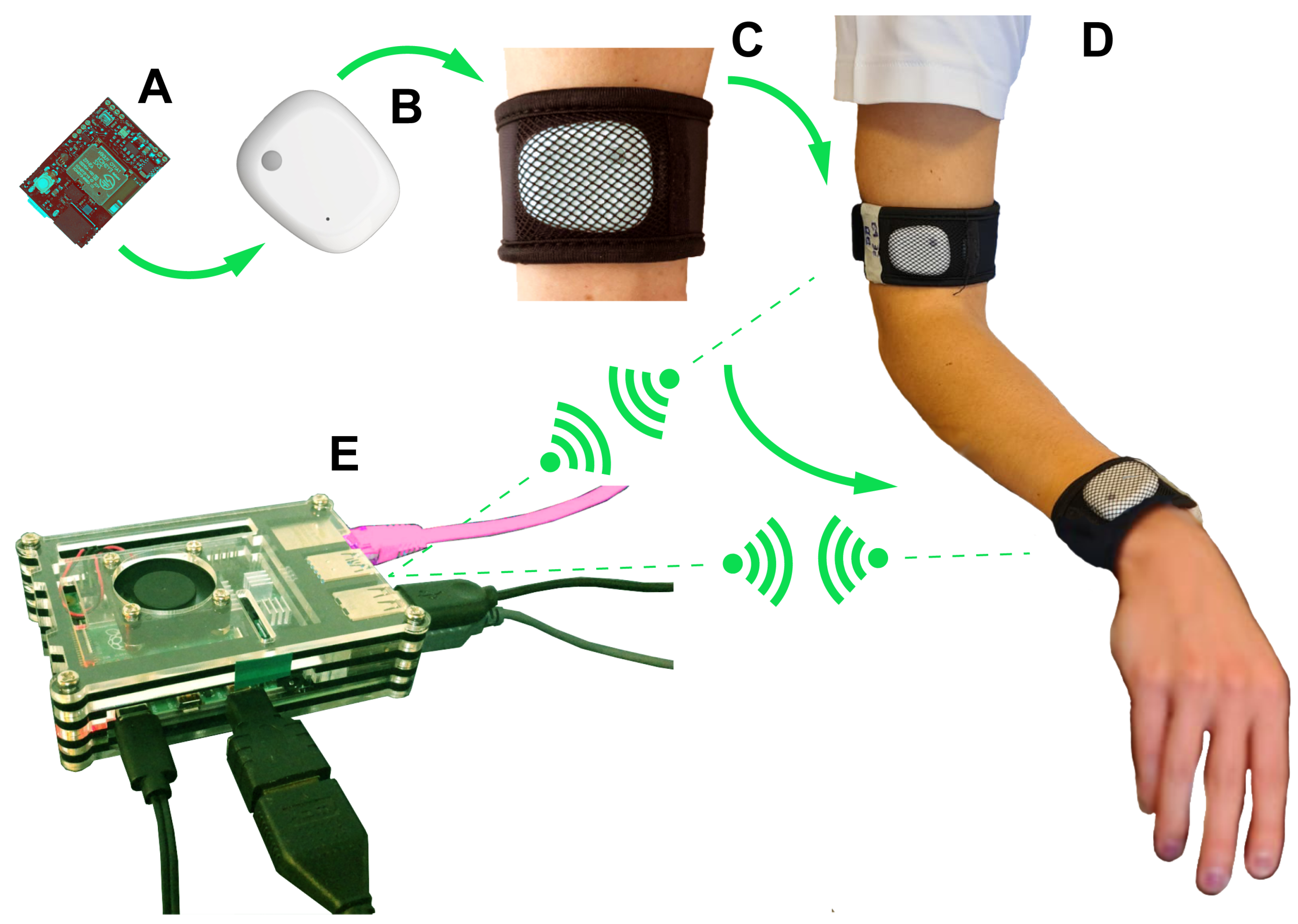
2.3. Wearable Sensors System for Motion Assessment
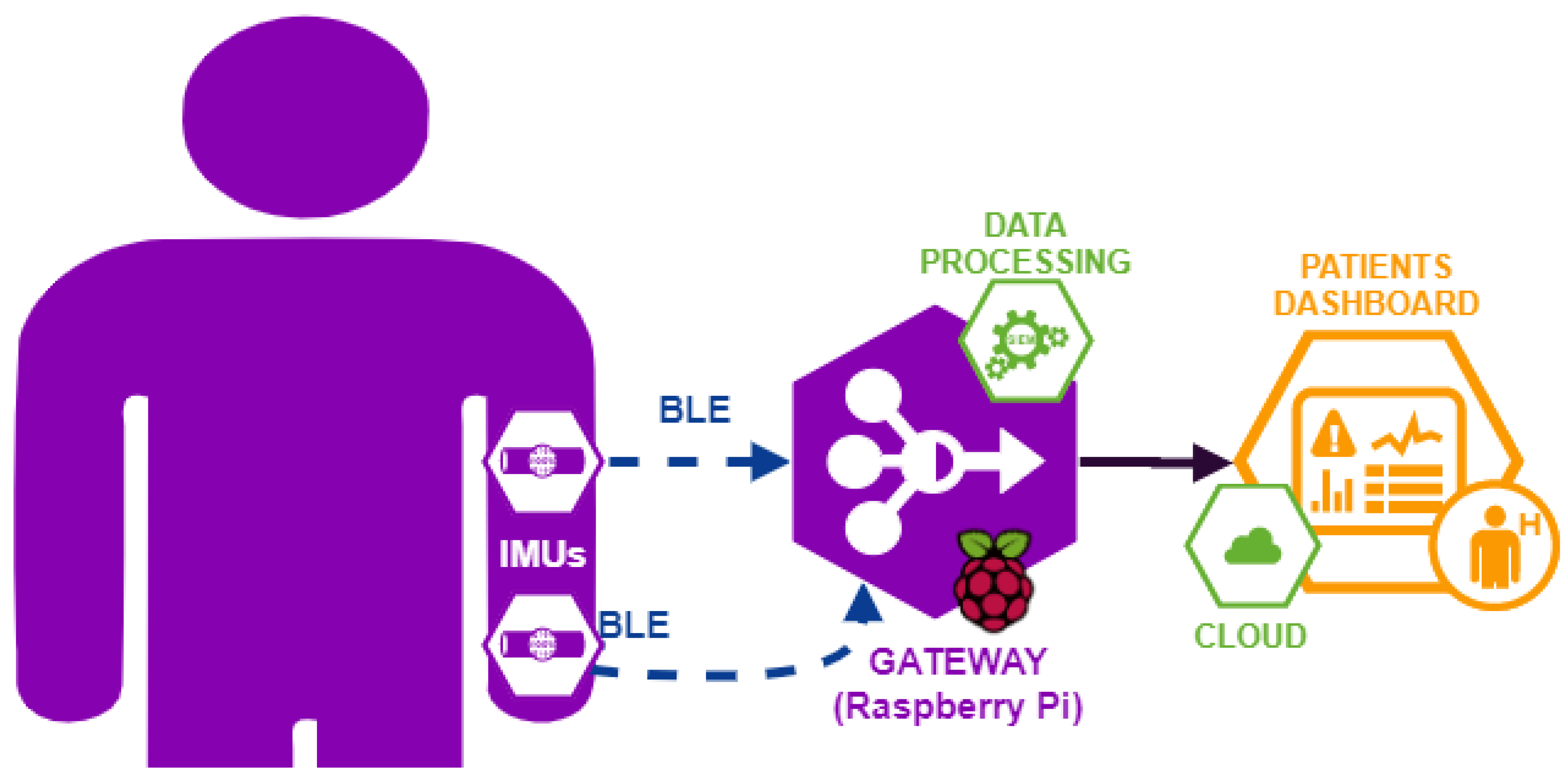
- Hardware module is the part of the system (for complete details see Appendix A.1) composed of IMU sensors and a gateway (Raspberry Pi);
- Software module is the part of the system (for complete details see Appendix A.3) composed of software components, which run on the gateway and provide the following functionalities: IMU sensors synchronization, data collection, and data processing to obtain the kinematics parameters used for medical evaluation of the movement (for complete details see Appendix A.2);
- Data Visualization module is the display part, showing data in real time to clinicians. Since this part is not necessary for the experimental campaign, it will be deployed as future development.
2.4. Experimental Campaign: Setup and Protocols
2.4.1. Participant Recruitment
- subjects over 18 years of age;
- C4–C7 cervical lesion level;
- at least one month post-injury;
- subjects with intact cognitive abilities;
- no joint contracture or severe spasticity in the affected upper limb (modified Ashworth scale greater than 3);
- sufficient Italian language skills.
2.4.2. Ethical Consideration
2.5. Metrics for Statistical Analysis
3. Results
3.1. Accuracy of the IMU-Based System: Laboratory Tests
3.2. Accuracy of IMU-Based and Goniometer Systems: Clinical Tests
- Inter-instrument reliability and accuracy (See Section 3.2.1)
- Inter-tester reliability and accuracy (See Section 3.2.2)
3.2.1. IMU versus Goniometric Measurements
3.2.2. Goniometer vs. Goniometer and IMU vs. IMU Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Technical Information about Wearable Sensors System for Motion Assessment
Appendix A.1. System Hardware Architecture
Appendix A.2. Data Processing and ROM Calculation with IMU-Based System
- is a parameter which weights the accelerometer contribution on the quaternion estimate;
- ⊗ indicates quaternions product;
- indicates an error direction on the solution surface defined by the objective function, (function defined as in (A4)) and its Jacobian;
- indicates a norm of function ;
- is conversion from gyroscope 3D measurements into a quaternion;
- is conversion from gravity 3D vector into a quaternion;
- is conversion from accelerometer 3D measurements into a quaternion;
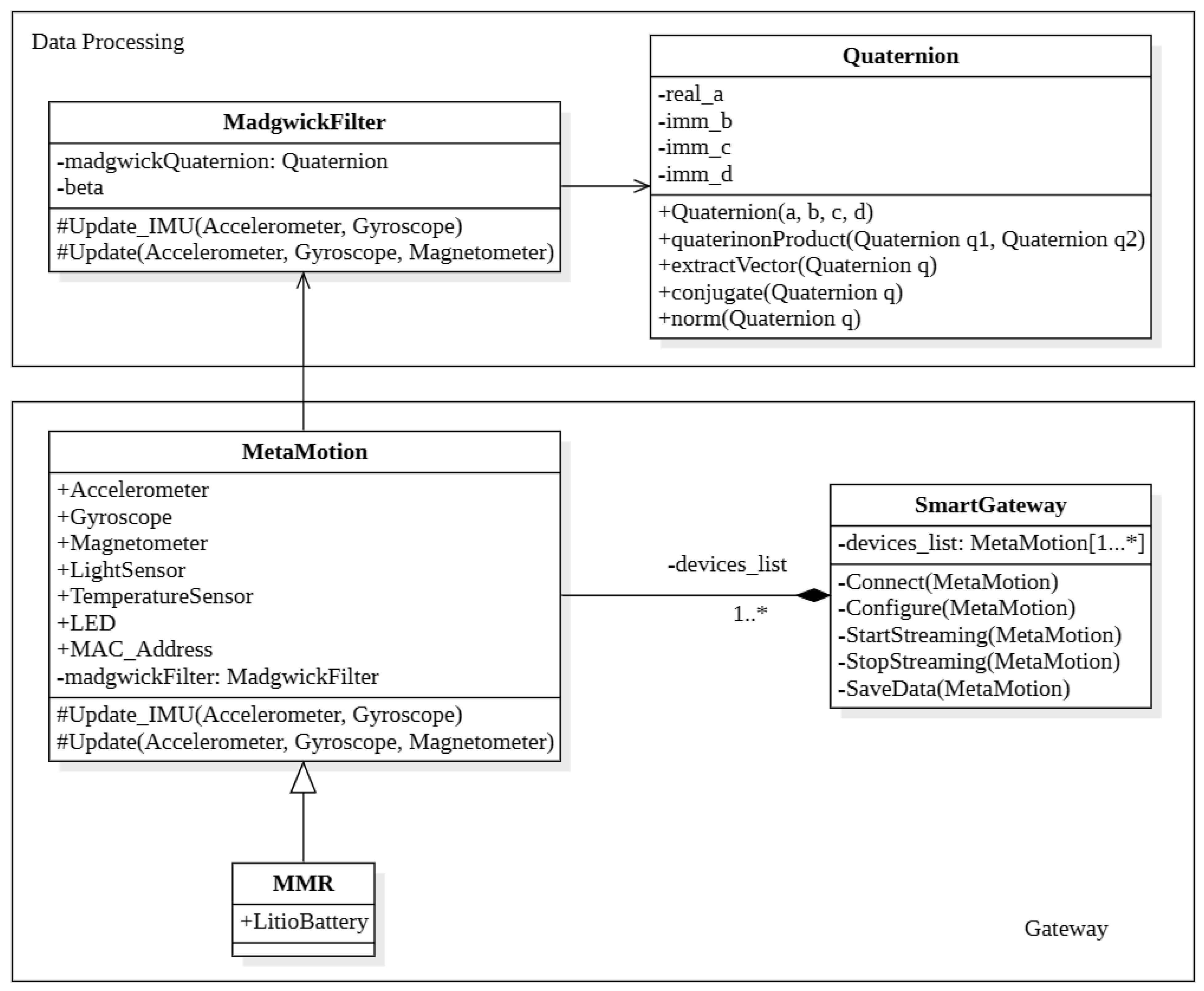
Appendix A.3. System Software Architecture
- MadgwickFilter represents the mathematical tool to update quaternion value from accelerometer and gyroscope measurements. It has two main attributes:
- Quaternion is the implemented mathematical library to help with operations with quaternions, e.g., product between quaternions, conjugate, apply rotation to vector, etc. This mathematical library is been used by MadgwickFilter class to update its quaternion.
- MetaMotion is the main class of the schema in Figure A2, it represents an abstraction of the sensors used for this work. As these devices use the Bluetooth protocol to communicate, they are uniquely identified by their MAC address. The sensor unit has different sensors, in particular accelerometer and gyroscope, used for this work [76].
- SmartGateway (Raspberry) is the back end unit of data (post) processing. This component is in charge of synchronizing the connection with the various devices, which may be of different types. It has to connect with these devices, configure them by setting the date rate parameters, and allow us to broadcast the data stream.The angle of the arm movement has been calculated every display refresh, because the data acquisition rate of these devices is not time-constant. When the movement is finished, this unit has to collect and store all data into his internal storage: this operation has been implemented to analyze and compare data of past movements with the actual.
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Maynard, F.M.; Bracken, M.B.; Creasey, G.; Ditunno, J.F., Jr.; Donovan, W.H.; Ducker, T.B.; Garber, S.L.; Marino, R.J.; Stover, S.L.; Tator, C.H.; et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. Spinal Cord 1997, 35, 266–274. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Wagner, J.P.; Curtin, C.M.; Gater, D.R.; Chung, K.C. Perceptions of People with Tetraplegia Regarding Surgery to Improve Upper-Extremity Function. J. Hand Surg. 2007, 32, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, W.B.; Miller, W.C.; Backman, C.L.; Oliffe, J.L. Association Between Mobility, Participation, and Wheelchair-Related Factors in Long-Term Care Residents Who Use Wheelchairs as Their Primary Means of Mobility. J. Am. Geriatr. Soc. 2012, 60, 1310–1315. [Google Scholar] [CrossRef]
- Eriks-Hoogland, I.; de Groot, S.; Post, M.; van der Woude, L. Correlation of shoulder range of motion limitations at discharge with limitations in activities and participation one year later in persons with spinal cord injury. J. Rehabil. Med. 2011, 43, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Silvestri, J. Current Trends in the Management of the Upper Limb in Spinal Cord Injury. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 178–186. [Google Scholar] [CrossRef]
- Gil-Agudo, Á.; De los Reyes-Guzmán, A.; Dimbwadyo Terrer, I.; Peñasco-Martín, B.; Bernal, A.; López-Monteagudo, P.; Del-Ama, A.; Pons, J. A novel motion tracking system for evaluation of functional rehabilitation of the upper limbs. Neural Regen. Res. 2013, 8, 1773–1782. [Google Scholar] [CrossRef]
- Struyf, F.; Nijs, J.; Mottram, S.; Roussel, N.A.; Cools, A.M.J.; Meeusen, R. Clinical assessment of the scapula: A review of the literature. Br. J. Sports Med. 2014, 48, 883–890. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Ko, H.; Yoon, J.O.; Cho, S.U.; Park, J.H.; Cho, K.H. Determining the Reliability of a New Method for Measuring Joint Range of Motion Through a Randomized Controlled Trial. Ann. Rehabil. Med. 2019, 43, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Rothstein, J.M.; Lamb, R.L. Goniometric Reliability in a Clinical Setting. Phys. Ther. 1987, 67, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.; Gill, S.; Babazadeh, S.; Elsewaisy, O.; Gillies, H.; Nguyen, N.; Pathirana, P.N.; Page, R. Assessment of Shoulder Range of Motion Using a Wireless Inertial Motion Capture Device—A Validation Study. Sensors 2019, 19, 1781. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, M.; Guillot, A.; Sancho, P.O.; Picot, M.; Revol, P.; Rode, G.; Collet, C. Rehabilitation of the Elbow Extension with Motor Imagery in a Patient with Quadriplegia After Tendon Transfer. Arch. Phys. Med. Rehabil. 2010, 91, 1143–1146. [Google Scholar] [CrossRef]
- Grangeon, M.; Revol, P.; Guillot, A.; Rode, G.; Collet, C. Could motor imagery be effective in upper limb rehabilitation of individuals with spinal cord injury? A case study. Spinal Cord 2012, 50, 766–771. [Google Scholar] [CrossRef]
- Chèze, L. The Different Movement Analysis Devices Available on the Market. In Kinematic Analysis of Human Movement; John Wiley & Sons, Inc.: New York, NY, USA, 2014; pp. 17–33. [Google Scholar] [CrossRef]
- Cohen, E.J.; Bravi, R.; Minciacchi, D. 3D reconstruction of human movement in a single projection by dynamic marker scaling. PLoS ONE 2017, 12, e0186443. [Google Scholar] [CrossRef]
- Baskwill, A.J.; Belli, P.; Kelleher, L. Evaluation of a Gait Assessment Module Using 3D Motion Capture Technology. Int. J. Ther. Massage Bodyw. 2017, 10, 3–9. [Google Scholar] [CrossRef]
- Mustapa, A.; Justine, M.; Mustafah, N.M.; Manaf, H. The Effect of Diabetic Peripheral Neuropathy on Ground Reaction Forces during Straight Walking in Stroke Survivors. Rehabil. Res. Pract. 2017, 2017, 5280146. [Google Scholar] [CrossRef] [PubMed]
- Mucchi, L.; Jayousi, S.; Martinelli, A.; Caputo, S.; Marcocci, P. An Overview of Security Threats, Solutions and Challenges in WBANs for Healthcare. In Proceedings of the 2019 13th International Symposium on Medical Information and Communication Technology (ISMICT), Oslo, Norway, 8–10 May 2019. [Google Scholar] [CrossRef]
- Mucchi, L.; Jayousi, S.; Caputo, S.; Paoletti, E.; Zoppi, P.; Geli, S.; Dioniso, P. How 6G Technology Can Change the Future Wireless Healthcare. In Proceedings of the 2020 2nd 6G Wireless Summit (6G SUMMIT), Levi, Finland, 17–20 March 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Tian, Y.; Meng, X.; Tao, D.; Liu, D.; Feng, C. Upper limb motion tracking with the integration of IMU and Kinect. Neurocomputing 2015, 159, 207–218. [Google Scholar] [CrossRef]
- Filippeschi, A.; Schmitz, N.; Miezal, M.; Bleser, G.; Ruffaldi, E.; Stricker, D. Survey of Motion Tracking Methods Based on Inertial Sensors: A Focus on Upper Limb Human Motion. Sensors 2017, 17, 1257. [Google Scholar] [CrossRef]
- Poitras, I.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.S. Validity of Wearable Sensors at the Shoulder Joint: Combining Wireless Electromyography Sensors and Inertial Measurement Units to Perform Physical Workplace Assessments. Sensors 2019, 19, 1885. [Google Scholar] [CrossRef]
- Najafi, B.; Aminian, K.; Paraschiv-Ionescu, A.; Loew, F.; Bula, C.; Robert, P. Ambulatory system for human motion analysis using a kinematic sensor: Monitoring of daily physical activity in the elderly. IEEE Trans. Biomed. Eng. 2003, 50, 711–723. [Google Scholar] [CrossRef]
- Luinge, H.J.; Veltink, P.H. Measuring orientation of human body segments using miniature gyroscopes and accelerometers. Med. Biol. Eng. Comput. 2005, 43, 273–282. [Google Scholar] [CrossRef]
- Sabatini, A.M. Variable-State-Dimension Kalman-Based Filter for Orientation Determination Using Inertial and Magnetic Sensors. Sensors 2012, 12, 8491–8506. [Google Scholar] [CrossRef]
- Ligorio, G.; Sabatini, A. Extended Kalman Filter-Based Methods for Pose Estimation Using Visual, Inertial and Magnetic Sensors: Comparative Analysis and Performance Evaluation. Sensors 2013, 13, 1919–1941. [Google Scholar] [CrossRef] [PubMed]
- Saber-Sheikh, K.; Bryant, E.C.; Glazzard, C.; Hamel, A.; Lee, R.Y. Feasibility of using inertial sensors to assess human movement. Man. Ther. 2010, 15, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Ji, L.Y.; Huang, Z.P.; Wu, J.K. Adaptive Information Fusion for Human Upper Limb Movement Estimation. IEEE Trans. Syst. Man, Cybern. Part A Syst. Hum. 2012, 42, 1100–1108. [Google Scholar] [CrossRef]
- Fasel, B.; Sporri, J.; Chardonnens, J.; Kroll, J.; Muller, E.; Aminian, K. Joint Inertial Sensor Orientation Drift Reduction for Highly Dynamic Movements. IEEE J. Biomed. Health Inform. 2018, 22, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Chan, Y.Y. The Use of Wearable Inertial Motion Sensors in Human Lower Limb Biomechanics Studies: A Systematic Review. Sensors 2010, 10, 11556–11565. [Google Scholar] [CrossRef]
- Giansanti, D.; Maccioni, G.; Benvenuti, F.; Macellari, V. Inertial measurement units furnish accurate trunk trajectory reconstruction of the sit-to-stand manoeuvre in healthy subjects. Med. Biol. Eng. Comput. 2007, 45, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, H.; Tao, Y. Inertial measurements of upper limb motion. Med. Biol. Eng. Comput. 2006, 44, 479–487. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, H. Upper limb motion estimation from inertial measurements. Int. J. Inf. Technol. 2007, 13, 1–14. [Google Scholar]
- Zhou, H.; Stone, T.; Hu, H.; Harris, N. Use of multiple wearable inertial sensors in upper limb motion tracking. Med. Eng. Phys. 2008, 30, 123–133. [Google Scholar] [CrossRef]
- Yoon, T.L. Validity and Reliability of an Inertial Measurement Unit-Based 3D Angular Measurement of Shoulder Joint Motion. J. Korean Phys. Ther. 2017, 29, 145–151. [Google Scholar] [CrossRef]
- Garimella, R.; Peeters, T.; Beyers, K.; Truijen, S.; Huysmans, T.; Verwulgen, S. Capturing Joint Angles of the Off-Site Human Body. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, C.; Chung, S.G.; Kim, H.C.; Kwak, Y.; won Park, H.; Kim, K. Measurement of Shoulder Range of Motion in Patients with Adhesive Capsulitis Using a Kinect. PLoS ONE 2015, 10, e0129398. [Google Scholar] [CrossRef]
- Mateo, S.; Roby-Brami, A.; Reilly, K.T.; Rossetti, Y.; Collet, C.; Rode, G. Upper limb kinematics after cervical spinal cord injury: A review. J. Neuroeng. Rehabil. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Pérez, C.; Garrido-Castro, J.L.; Vidal, F.T.; Alcaraz-Clariana, S.; García-Luque, L.; Alburquerque-Sendín, F.; de Souza, D.P.R. Concurrent Validity and Reliability of an Inertial Measurement Unit for the Assessment of Craniocervical Range of Motion in Subjects with Cerebral Palsy. Diagnostics 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Frye, S.K.; Geigle, P.R.; York, H.S.; Sweatman, W.M. Functional passive range of motion of individuals with chronic cervical spinal cord injury. J. Spinal Cord Med. 2019, 43, 257–263. [Google Scholar] [CrossRef]
- Norkin, C.C.; White, D.J. Measurement of Joint Motion: A Guide to Goniometry, 5th ed.; F.A. Davis: Philadelphia, PA, USA, 2016; pp. 66–114. [Google Scholar]
- Sabari, J.S.; Maltzev, I.; Lubarsky, D.; Liszkay, E.; Homel, P. Goniometric assessment of shoulder range of motion: Comparison of testing in supine and sitting positions. Arch. Phys. Med. Rehabil. 1998, 79, 647–651. [Google Scholar] [CrossRef]
- Hayes, K.; Walton, J.R.; Szomor, Z.L.; Murrell, G.A. Reliability of five methods for assessing shoulder range of motion. Aust. J. Physiother. 2001, 47, 289–294. [Google Scholar] [CrossRef]
- Jain, N.B.; Wilcox, R.B.; Katz, J.N.; Higgins, L.D. Clinical Examination of the Rotator Cuff. PM&R 2013, 5, 45–56. [Google Scholar] [CrossRef]
- Maksimovic, R.; Popovic, M. Classification of tetraplegics through automatic movement evaluation. Med. Eng. Phys. 1999, 21, 313–327. [Google Scholar] [CrossRef]
- Acosta, A.M.; Kirsch, R.F.; van der Helm, F.C.T. Three-dimensional shoulder kinematics in individuals with C5–C6 spinal cord injury. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2001, 215, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kebaetse, M.; McClure, P.; Pratt, N.A. Thoracic position effect on shoulder range of motion, strength, and three-dimensional scapular kinematics. Arch. Phys. Med. Rehabil. 1999, 80, 945–950. [Google Scholar] [CrossRef]
- Powers, C.; Newsam, C.; Gronley, J.; Fontaine, C.; Perry, J. Isometric shoulder torque in subjects with spinal cord injury. Arch. Phys. Med. Rehabil. 1994, 75, 761–765. [Google Scholar] [CrossRef]
- Gronley, J.; Newsam, C.; Mulroy, S.; Rao, S.; Perry, J.; Helm, M. Electromyographic and kinematic analysis of the shoulder during four activities of daily living in men with C6 tetraplegia. J. Rehabil. Res. Dev. 2000, 37, 423–432. [Google Scholar] [PubMed]
- Bravi, R.; Quarta, E.; Tongo, C.D.; Carbonaro, N.; Tognetti, A.; Minciacchi, D. Music, clicks, and their imaginations favor differently the event-based timing component for rhythmic movements. Exp. Brain Res. 2015, 233, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- Bravi, R.; Ioannou, C.I.; Minciacchi, D.; Altenmüller, E. Assessment of the effects of Kinesiotaping on musical motor performance in musicians suffering from focal hand dystonia: A pilot study. Clin. Rehabil. 2019, 33, 1636–1648. [Google Scholar] [CrossRef]
- Bravi, R.; Cohen, E.J.; Martinelli, A.; Gottard, A.; Minciacchi, D. When Non-Dominant Is Better than Dominant: Kinesiotape Modulates Asymmetries in Timed Performance during a Synchronization-Continuation Task. Front. Integr. Neurosci. 2017, 11, 21. [Google Scholar] [CrossRef]
- Cohen, E.J.; Bravi, R.; Minciacchi, D. The effect of fidget spinners on fine motor control. Sci. Rep. 2018, 8, 3144. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P. Quantifying Test-Retest Reliability Using the Intraclass Correlation Coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Nevill, A.M. Statistical Methods For Assessing Measurement Error (Reliability) in Variables Relevant to Sports Medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instrument in Psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Spearman, C. Correlation Calculated From Faulty Data. Br. J. Psychol. 1910, 3, 271–295. [Google Scholar] [CrossRef]
- Brown, W. Some Experimental Results in the Correlation of Mental Abilities. Br. J. Psychol. 1910, 3, 296–322. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical Methods For Assessing Agreement Between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Sedgwick, P. Limits of agreement (Bland-Altman method). Br. Med. J. 2013, 346, f1630. [Google Scholar] [CrossRef] [PubMed]
- Portney, L. Foundations of Clinical Research: Applications to Practice; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2015. [Google Scholar]
- Mullaney, M.J.; McHugh, M.P.; Johnson, C.P.; Tyler, T.F. Reliability of shoulder range of motion comparing a goniometer to a digital level. Physiother. Theory Pract. 2010, 26, 327–333. [Google Scholar] [CrossRef]
- Paulis, W.D.; Horemans, H.L.; Brouwer, B.S.; Stam, H.J. Excellent test–retest and inter-rater reliability for Tardieu Scale measurements with inertial sensors in elbow flexors of stroke patients. Gait Posture 2011, 33, 185–189. [Google Scholar] [CrossRef]
- Roetenberg, D.; Baten, C.; Veltink, P. Estimating Body Segment Orientation by Applying Inertial and Magnetic Sensing Near Ferromagnetic Materials. IEEE Trans. Neural Syst. Rehabil. 2007, 15, 469–471. [Google Scholar] [CrossRef]
- de Vries, W.; Veeger, H.; Baten, C.; van der Helm, F. Magnetic distortion in motion labs, implications for validating inertial magnetic sensors. Gait Posture 2009, 29, 535–541. [Google Scholar] [CrossRef]
- Kendell, C.; Lemaire, E.D. Effect of mobility devices on orientation sensors that contain magnetometers. J. Rehabil. Res. Dev. 2009, 46, 957. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.; Rossi, S.; Patanè, F.; Cappa, P. Experimental evaluation of indoor magnetic distortion effects on gait analysis performed with wearable inertial sensors. Physiol. Meas. 2014, 35, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Haering, D.; Raison, M.; Begon, M. Measurement and Description of Three-Dimensional Shoulder Range of Motion with Degrees of Freedom Interactions. J. Biomech. Eng. 2014, 136. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Hefner, J.E.; Kodish-Wachs, J.E.; Iaccarino, M.A.; Paganoni, S. Telehealth in Physical Medicine and Rehabilitation: A Narrative Review. PM&R 2017, 9, S51–S58. [Google Scholar] [CrossRef]
- Martinez, R.N.; Hogan, T.P.; Balbale, S.; Lones, K.; Goldstein, B.; Woo, C.; Smith, B.M. Sociotechnical Perspective on Implementing Clinical Video Telehealth for Veterans with Spinal Cord Injuries and Disorders. Telemed. e-Health 2017, 23, 567–576. [Google Scholar] [CrossRef]
- Cardinale, A.M. The Opportunity for Telehealth to Support Neurological Health Care. Telemed. e-Health 2018, 24, 969–978. [Google Scholar] [CrossRef]
- MbientLab. Metamotion R Datasheet. Available online: https://mbientlab.com/metamotionr (accessed on 3 February 2021).
- Madgwick, S.O.H.; Harrison, A.J.L.; Vaidyanathan, R. Estimation of IMU and MARG orientation using a gradient descent algorithm. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar] [CrossRef]



| Shoulder Movement | Goniometer Landmarks | ||
|---|---|---|---|
| Center Fulcrum | Stationary Arm | Moving Arm | |
| Flexion | Lateral aspect of the glenohumeral joint | Parallel to the midline of the trunk | Lateral epicondyle of the humerus |
| Abduction | Posterior aspect of the glenohumeral joint | Laterally along the trunk, parallel to the spine. | Lateral epicondyle of the humerus |
| External Rotation at 90° abduction | Olecranon process ulna | Parallel to the floor | Ulna styloid process |
| Internal Rotation at 90° abduction | Olecranon process ulna | Parallel to the floor | Ulna styloid process |
| Patients | Sex | Age (Years) | Dominant Arm | Lesion Level | AIS Grade | Etiology | Severity of Lesion | Time since Injury (Years) | Shoulder Intervention |
|---|---|---|---|---|---|---|---|---|---|
| P1 | Male | 56 | R | C4 | D | T | Incomplete | 1 | / |
| P2 | Male | 63 | R | C6–C7 | C | T | Incomplete | 2 | / |
| P3 | Male | 63 | R | C5–C6 | B | T | Incomplete | 5 | SURG |
| P4 | Male | 32 | R | C4 | D | T | Incomplete | 1 | BTOX |
| P5 | Male | 52 | L | C6 | D | T | Incomplete | 2 | / |
| P6 | Male | 43 | R | C6–C7 | A | T | Complete | 21 | BTOX |
| P7 | Male | 59 | R | C4 | D | T | Incomplete | 5 | SURG |
| P8 | Female | 34 | R | C4–C5 | D | T | Incomplete | 3 | / |
| HC1 | Male | 65 | R | ||||||
| HC2 | Female | 27 | R | ||||||
| HC3 | Male | 27 | R | ||||||
| HC4 | Male | 34 | L | ||||||
| HC5 | Male | 49 | R | ||||||
| HC6 | Male | 36 | R | ||||||
| HC7 | Male | 37 | R | ||||||
| HC8 | Male | 76 | R |
| Reliability | ICC(2, 1) | ICC(2, m) | |
|---|---|---|---|
| m = 2 | m = 4 | ||
| Poor | <0.4 | <0.57 | <0.73 |
| Fair | 0.4–0.6 | 0.57–0.75 | 0.73–0.86 |
| Good | 0.6–0.75 | 0.75–0.85 | 0.86–0.92 |
| Excellent | >0.75 | >0.85 | >0.92 |
| Goniometer | IMUs Average () | Difference | ICC (95% CI) | LOA |
|---|---|---|---|---|
| 0° | 1.45° (0.77°) | 1.45° | 0.9996 (0.9994; 0.9997) | −3.19°; 4.92° |
| 15° | 15.19° (0.96°) | 0.19° | ||
| 30° | 30.90° (0.94°) | 0.90° | ||
| 45° | 45.23° (1.19°) | 0.23° | ||
| 60° | 61.51° (1.64°) | 1.51° | ||
| 75° | 76.11° (0.82°) | 1.11° | ||
| 90° | 92.06° (1.16°) | 2.06° | ||
| 120° | 122.74° (1.42°) | 2.74° | ||
| 150° | 151.40° (2.56°) | 1.40° | ||
| 180° | 177.02° (2.00°) | 2.98° |
| Whole Group n = 48 | CSCI Group n = 24 | Healthy Group n = 24 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ICC(2, m) | LB | UB | ICC(2, m) | LB | UB | ICC(2, m) | LB | UB | |
| Flexion | 0.94 | 0.86 | 0.97 | 0.94 | 0.82 | 0.98 | 0.84 | 0.65 | 0.93 |
| Abduction | 0.97 | 0.96 | 0.98 | 0.97 | 0.95 | 0.99 | 0.94 | 0.88 | 0.97 |
| External Rotation | 0.97 | 0.95 | 0.98 | 0.95 | 0.91 | 0.98 | 0.97 | 0.94 | 0.98 |
| Internal Rotation | 0.95 | 0.91 | 0.97 | 0.96 | 0.90 | 0.98 | 0.94 | 0.86 | 0.97 |
| Goniometer Average () | IMUs Average () | Difference () | ICC (95% CI) | LOA | |
|---|---|---|---|---|---|
| Whole group (n = 96) | |||||
| Flexion | 140° (18°) | 134° (20°) | 6° (12°) | 0.86 (0.75; 0.92) | −19°; 30° |
| Abduction | 146° (20°) | 149° (21°) | −3° (8°) | 0.95 (0.91; 0.97) | −19°; 13° |
| External Rotation | 79° (16°) | 78° (18°) | 1° (8°) | 0.94 (0.91; 0.96) | −15°; 16° |
| Internal Rotation | 53° (14°) | 56° (13°) | −3° (8°) | 0.90 (0.81; 0.94) | −19°; 12° |
| CSCI group (n = 48) | |||||
| Flexion | 131° (20°) | 124° (20°) | 7° (13°) | 0.86 (0.65; 0.93) | −17°; 32° |
| Abduction | 135° (20°) | 136° (19°) | −1° (9°) | 0.95 (0.91; 0.97) | −18°; 15° |
| External Rotation | 71° (13°) | 70° (18°) | 1° (10°) | 0.88 (0.78; 0.93) | −19°; 20° |
| Internal Rotation | 51° (16°) | 58° (16°) | −7° (8°) | 0.90 (0.55; 0.96) | −22°; 8° |
| Healthy group (n = 48) | |||||
| Flexion | 149° (9°) | 145° (14°) | 4° (12°) | 0.61 (0.31; 0.78) | −20°; 28° |
| Abduction | 156° (13°) | 162° (13°) | −6° (7°) | 0.87 (0.53; 0.95) | −20°; 9° |
| External Rotation | 87° (14°) | 86° (14°) | 1° (5°) | 0.97 (0.94; 0.98) | −9°; 11° |
| Internal Rotation | 55° (12°) | 55° (10°) | 0° (7°) | 0.89 (0.81; 0.94) | −14°; 13° |
| Goniometer Average () RATER 1 | IMUs Average () IMU 1 | Difference () | ICC (95% CI) | LOA | |
|---|---|---|---|---|---|
| Whole group (n = 48) | |||||
| Flexion | 134° (16°) | 135° (21°) | 0° (11°) | 0.90 (0.83; 0.95) | −22°; 21° |
| Abduction | 144° (18°) | 148° (21°) | −5° (8°) | 0.94 (0.86; 0.97) | −20°; 12° |
| External Rotation | 77° (13°) | 78° (17°) | −2° (10°) | 0.89 (0.80; 0.94) | −21°; 17° |
| Internal Rotation | 56° (13°) | 57° (13°) | −1° (9°) | 0.89 (0.80; 0.94) | −18°; 15° |
| CSCI group (n = 24) | |||||
| Flexion | 124° (18°) | 124° (20°) | 1° (11°) | 0.92 (0.81; 0.96) | −20°; 22° |
| Abduction | 133° (17°) | 136° (19°) | −3° (8°) | 0.94 (0.85; 0.97) | −20°; 14° |
| External Rotation | 68° (11°) | 71° (18°) | −2° (13°) | 0.77 (0.49; 0.90) | −27°; 22° |
| Internal Rotation | 53° (15°) | 58° (16°) | −5° (8°) | 0.91 (0.72; 0.96) | −21°; 11° |
| Healthy group (n = 24) | |||||
| Flexion | 144° (7°) | 146° (14°) | −2° (12°) | 0.62 (0.10; 0.83) | −24°; 21° |
| Abduction | 156° (11°) | 161° (13°) | −6° (8°) | 0.81 (0.33; 0.93) | 3°; 22° |
| External Rotation | 85° (9°) | 86° (13°) | −1° (5°) | 0.94 (0.86; 0.97) | −12°; 9° |
| Internal Rotation | 58° (11°) | 56° (10°) | 2° (7°) | 0.85 (0.66; 0.94) | −12°; 17° |
| Goniometer Average () RATER 2 | IMUs Average () IMU 2 | Difference () | ICC (95% CI) | LOA | |
|---|---|---|---|---|---|
| Whole group (n = 48) | |||||
| Flexion | 146° (18°) | 134° (20°) | 12° (11°) | 0.83 (0.09; 0.94) | −10°; 33° |
| Abduction | 147° (21°) | 149° (21°) | −2° (8°) | 0.96 (0.93; 0.98) | −17°; 14° |
| External Rotation | 81° (18°) | 78° (19°) | 3° (5°) | 0.97 (0.92; 0.99) | −6°; 13° |
| Internal Rotation | 50° (15°) | 56° (14°) | −6° (6°) | 0.91 (0.56; 0.97) | −18°; 7° |
| CSCI group (n = 24) | |||||
| Flexion | 137° (21°) | 123° (20°) | 14° (11°) | 0.82 (−0.15; 0.95) | −7°; 35° |
| Abduction | 137° (22°) | 136° (20°) | 1° (8°) | 0.96 (0.91; 0.98) | −15°; 17° |
| External Rotation | 73° (14°) | 69° (18°) | 4° (6°) | 0.96 (0.85; 0.98) | −8°; 15° |
| Internal Rotation | 49° (18°) | 58° (16°) | −9° (6°) | 0.91 (0.08; 0.98) | −21°; 4° |
| Healthy group (n = 24) | |||||
| Flexion | 154° (8°) | 145° (14°) | 9° (11°) | 0.62 (−0.12; 0.85) | −12°; 30° |
| Abduction | 157° (15°) | 162° (13°) | −5° (7°) | 0.91 (0.64; 0.97) | −18°; 8° |
| External Rotation | 89° (17°) | 87° (15°) | 3° (4°) | 0.98 (0.92; 0.99) | −5°; 10° |
| Internal Rotation | 51° (11°) | 54° (10°) | −3° (5°) | 0.93 (0.79; 0.97) | −13°; 7° |
| Goniometer Average () RATER 1 | Goniometer Average () RATER 2 | Difference () | ICC (95% CI) | LOA | |
|---|---|---|---|---|---|
| Whole group (n = 48) | |||||
| Flexion | 134° (16°) | 146° (18°) | −12° (8°) | 0.85 (−0.15; 0.96) | −26°; 3° |
| Abduction | 144° (18°) | 147° (21°) | −3° (10°) | 0.93 (0.88; 0.96) | −22°; 16° |
| External Rotation | 77° (13°) | 81° (18°) | −5° (9°) | 0.89 (0.75; 0.94) | −23°; 13° |
| Internal Rotation | 56° (13°) | 50° (15°) | 5° (8°) | 0.89 (0.62; 0.95) | −9°; 20° |
| CSCI group (n = 24) | |||||
| Flexion | 124° (18°) | 137° (21°) | −13° (8°) | 0.85 (−0.16; 0.96) | −29°; 3° |
| Abduction | 133° (17°) | 137° (22°) | −4° (11°) | 0.90 (0.77; 0.96) | −27°; 18° |
| External Rotation | 68° (11°) | 73° (14°) | −5° (9°) | 0.83 (0.55; 0.93) | −22°; 12° |
| Internal Rotation | 53° (15°) | 49° (18°) | 4° (9°) | 0.91 (0.78; 0.97) | −13°; 21° |
| Healthy group (n = 24) | |||||
| Flexion | 144° (7°) | 154° (8°) | −10° (6°) | 0.47 (−0.30; 0.81) | −23°; 2° |
| Abduction | 156° (11°) | 157° (15°) | −1° (8°) | 0.90 (0.77; 0.96) | −16°; 14° |
| External Rotation | 85° (9°) | 89° (17°) | −4° (10°) | 0.84 (0.61; 0.93) | −24°; 15° |
| Internal Rotation | 58° (11°) | 51° (11°) | 7° (6°) | 0.84 (0.04; 0.95) | −5°; 19° |
| IMUs Average () IMU 1 | IMUs Average () IMU 2 | Difference () | ICC (95% CI) | LOA | |
|---|---|---|---|---|---|
| Whole group (n = 48) | |||||
| Flexion | 135° (21°) | 134° (20°) | 0° (2°) | 0.997 (0.995; 0.998) | −4°; 5° |
| Abduction | 149° (21°) | 149° (21°) | 0° (2°) | 0.999 (0.998; 0.999) | −3°; 3° |
| External Rotation | 78° (17°) | 78° (19°) | 0° (4°) | 0.988 (0.978; 0.993) | −7°; 8° |
| Internal Rotation | 57° (13°) | 56° (14°) | 1° (2°) | 0.993 (0.983; 0.996) | −3°; 5° |
| CSCI group (n = 24) | |||||
| Flexion | 124° (20°) | 123° (20°) | 1° (2°) | 0.997 (0.993; 0.999) | −4°; 5° |
| Abduction | 136° (19°) | 136° (19°) | −1° (2°) | 0.998 (0.994; 0.999) | −4°; 3° |
| External Rotation | 71° (18°) | 69° (18°) | 1° (4°) | 0.988 (0.972; 0.995) | −6°; 9° |
| Internal Rotation | 58° (16°) | 58° (16°) | 0° (2°) | 0.996 (0.990; 0.998) | −4°; 5° |
| Healthy group (n = 24) | |||||
| Flexion | 146° (14°) | 145° (14°) | 0° (2°) | 0.995 (0.988; 0.998) | −4°; 5° |
| Abduction | 162° (13°) | 162° (13°) | 0° (1°) | 0.998 (0.995; 0.999) | −2°; 2° |
| External Rotation | 86° (13°) | 87° (15°) | −1° (4°) | 0.979 (0.952; 0.991) | −8°; 7° |
| Internal Rotation | 56° (10°) | 54° (10°) | 2° (2°) | 0.985 (0.852; 0.996) | −2°; 5° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravi, R.; Caputo, S.; Jayousi, S.; Martinelli, A.; Biotti, L.; Nannini, I.; Cohen, E.J.; Quarta, E.; Grasso, S.; Lucchesi, G.; et al. An Inertial Measurement Unit-Based Wireless System for Shoulder Motion Assessment in Patients with Cervical Spinal Cord Injury: A Validation Pilot Study in a Clinical Setting. Sensors 2021, 21, 1057. https://doi.org/10.3390/s21041057
Bravi R, Caputo S, Jayousi S, Martinelli A, Biotti L, Nannini I, Cohen EJ, Quarta E, Grasso S, Lucchesi G, et al. An Inertial Measurement Unit-Based Wireless System for Shoulder Motion Assessment in Patients with Cervical Spinal Cord Injury: A Validation Pilot Study in a Clinical Setting. Sensors. 2021; 21(4):1057. https://doi.org/10.3390/s21041057
Chicago/Turabian StyleBravi, Riccardo, Stefano Caputo, Sara Jayousi, Alessio Martinelli, Lorenzo Biotti, Ilaria Nannini, Erez James Cohen, Eros Quarta, Stefano Grasso, Giacomo Lucchesi, and et al. 2021. "An Inertial Measurement Unit-Based Wireless System for Shoulder Motion Assessment in Patients with Cervical Spinal Cord Injury: A Validation Pilot Study in a Clinical Setting" Sensors 21, no. 4: 1057. https://doi.org/10.3390/s21041057
APA StyleBravi, R., Caputo, S., Jayousi, S., Martinelli, A., Biotti, L., Nannini, I., Cohen, E. J., Quarta, E., Grasso, S., Lucchesi, G., Righi, G., Del Popolo, G., Mucchi, L., & Minciacchi, D. (2021). An Inertial Measurement Unit-Based Wireless System for Shoulder Motion Assessment in Patients with Cervical Spinal Cord Injury: A Validation Pilot Study in a Clinical Setting. Sensors, 21(4), 1057. https://doi.org/10.3390/s21041057













