Quantifying Circadian Aspects of Mobility-Related Behavior in Older Adults by Body-Worn Sensors—An “Active Period Analysis”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Instruments
2.4. Data Collection
2.5. Data Processing and Statistical Analysis
3. Results
3.1. Participants
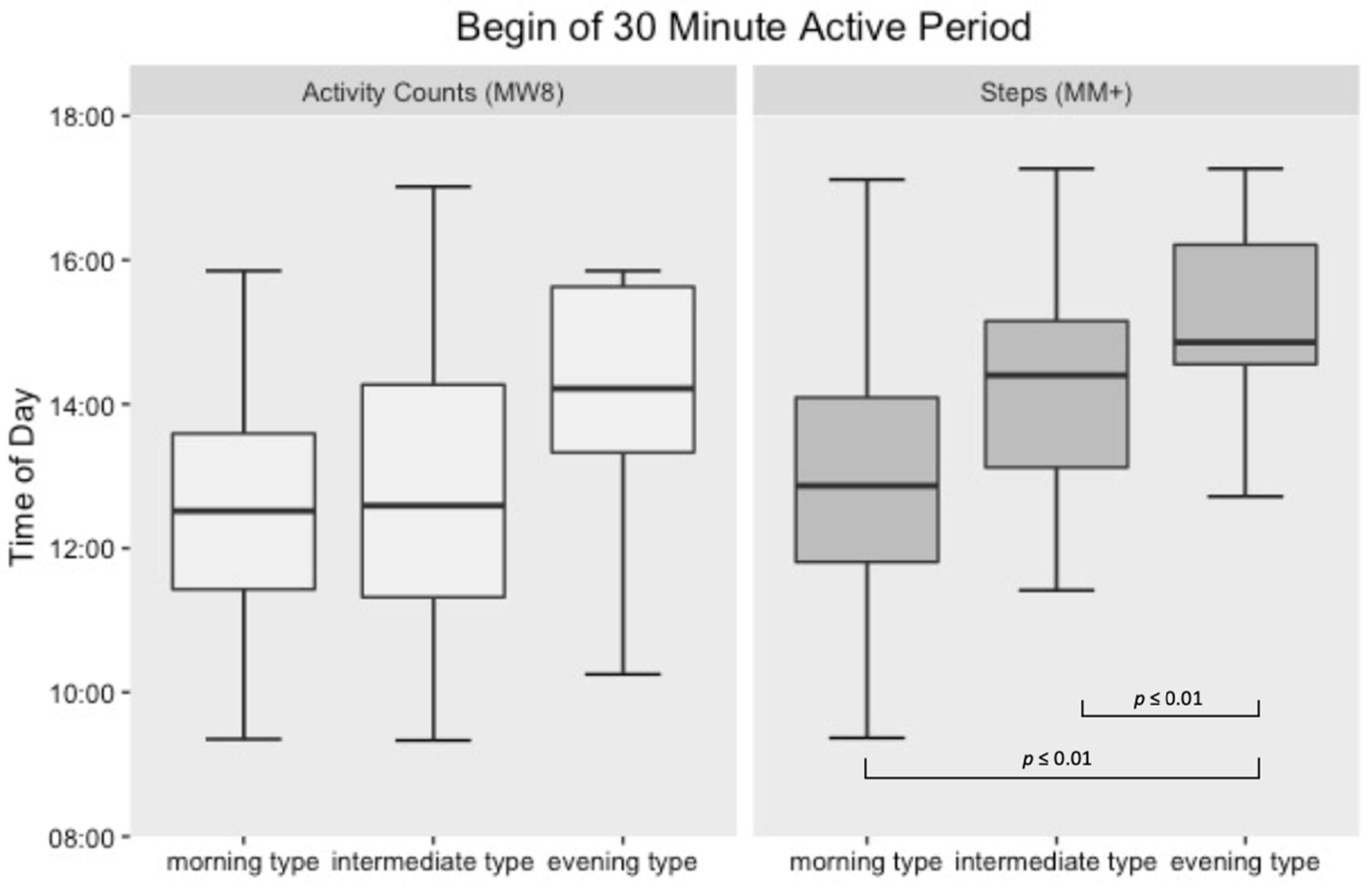
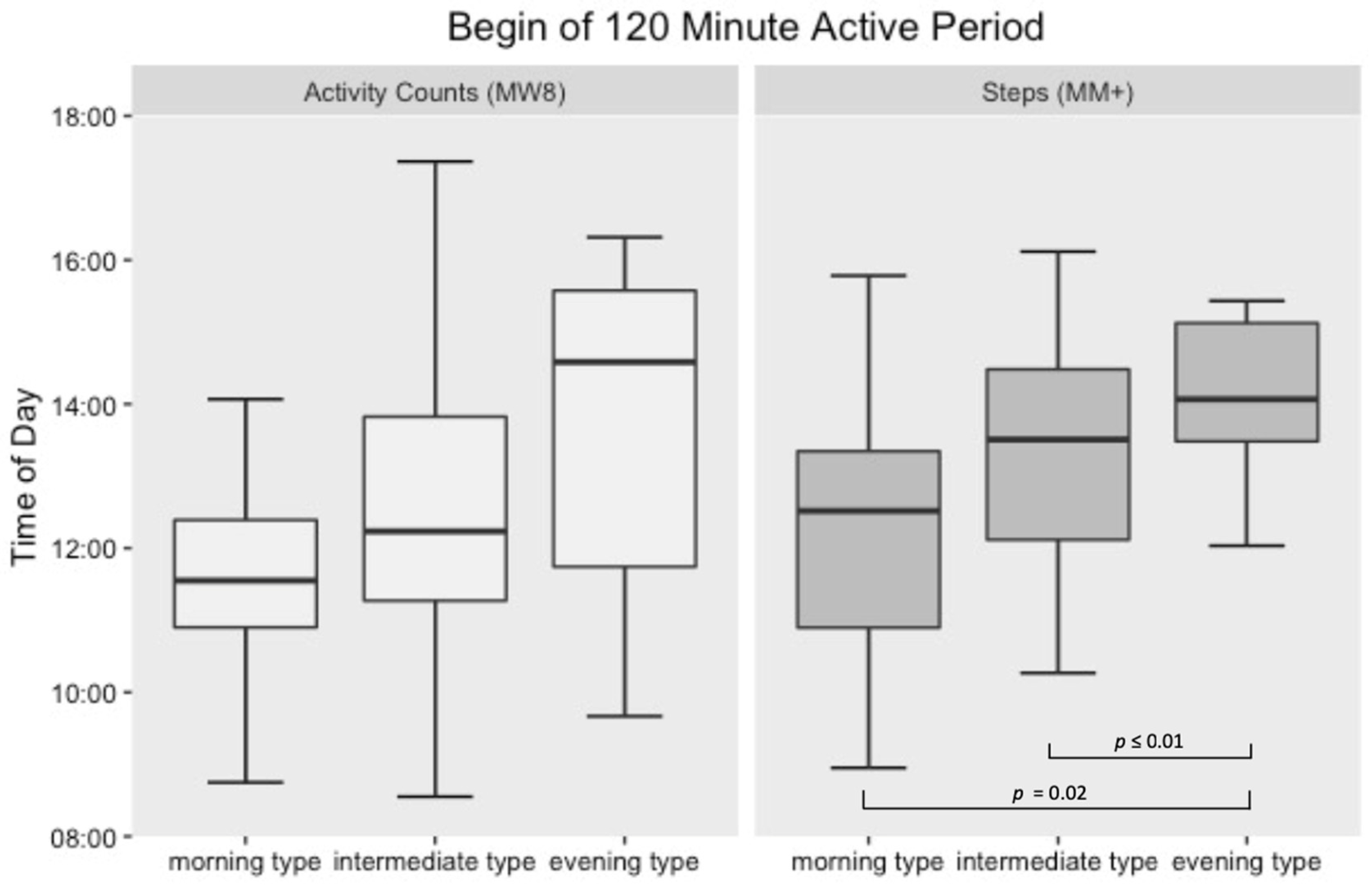
3.2. Active Period Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colwell, C.S. Circadian Medicine; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Van Someren, E.J.W.; Riemersma-Van Der Lek, R.F. Live to the Rhythm, Slave to the Rhythm. Sleep Med. Rev. 2007, 11, 465–484. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Circadian and Sleep Disturbances in the Elderly. Exp. Gerontol. 2000, 35, 1229–1237. [Google Scholar] [CrossRef]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Liu, R.-Y.; Wang, Q.-S.; Van Someren, E.J.; Xu, H.; Zhou, J.-N. Age-Associated Difference in Circadian Sleep–Wake and Rest–Activity Rhythms. Physiol. Behav. 2002, 76, 597–603. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Circadian Rhythms and Sleep in Human Aging. Chronobiol. Int. 2000, 17, 233–243. [Google Scholar] [CrossRef]
- Harper, D.G. Sleep and Circadian Rhythm Disturbances in Alzheimer’s Disease. In Principles and Practice of Geriatric Sleep Medicine; Pandi-Perumal, S.R., Monti, J.M., Monjan, A.A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 214–226. [Google Scholar]
- Coogan, A.N.; Schutová, B.; Husung, S.; Furczyk, K.; Baune, B.T.; Kropp, P.; Häßler, F.; Thome, J. The Circadian System in Alzheimer’s Disease: Disturbances, Mechanisms, and Opportunities. Biol. Psychiatry 2013, 74, 333–339. [Google Scholar] [CrossRef]
- Boronat, A.C.; Ferreira-Maia, A.P.; Wang, Y.P. Sundown Syndrome in Older Persons: A Scoping Review. J. Am. Med. Dir. Assoc. 2019, 664–671.e5. [Google Scholar] [CrossRef] [PubMed]
- Landry, G.J.; Liu-Ambrose, T. Buying Time: A Rationale for Examining the Use of Circadian Rhythm and Sleep Interventions to Delay Progression of Mild Cognitive Impairment to Alzheimer’s Disease. Front. Aging Neurosci. 2014, 6, 325. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, A.; Scuratti, A.; Zanetti, O.; Binetti, G.; Frisoni, G.B.; Magni, E.; Trabucchi, M. Predictors of Mortality and Institutionalization in Alzheimer Disease Patients 1 Year after Discharge from an Alzheimer Dementia Unit. Dement. Geriatr. Cogn. Disord. 1995, 6, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Di Milia, L.; Adan, A.; Natale, V.; Randler, C. Reviewing the Psychometric Properties of Contemporary Circadian Typology Measures. Chronobiol. Int. 2013, 30, 1261–1271. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive Assessment of Psychopathology in Dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef] [Green Version]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.A.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenthaler, T.; Alessi, C.; Friedman, L.; Owens, J.; Kapur, V.; Boehlecke, B.; Brown, T.; Chesson, A.; Coleman, J.; Lee-Chiong, T.; et al. Practice Parameters for the Use of Actigraphy in the Assessment of Sleep and Sleep Disorders: An Update for 2007. Sleep 2007, 30, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Van Someren, E.J.W. Actigraphic Monitoring of Sleep and Circadian Rhythms. In Handbook of Clinical Neurology; Montagna, P., Chokroverty, S., Eds.; Elsevier: Amsterdam, Netherlands, 2011; Volume 98, pp. 55–63. [Google Scholar] [CrossRef]
- Witting, W.; Kwa, I.H.; Eikelenboom, P.; Mirmiran, M.; Swaab, D.F. Alterations in the Circadian Rest-Activity Rhythm in Aging and Alzheimer’s Disease. Biol. Psychiatry 1990, 27, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.E.R.; Dodds, R.; Bacon, D.; Sayer, A.A.; Roberts, H.C. Physical Activity among Hospitalised Older People: Insights from Upper and Lower Limb Accelerometry. Aging Clin. Exp. Res. 2018, 30, 1363–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trumpf, R.; Zijlstra, W.; Haussermann, P.; Fleiner, T. Quantifying Habitual Physical Activity and Sedentariness in Older Adults—Different Outcomes of Two Simultaneously Body-Worn Motion Sensor Approaches and a Self-Estimation. Sensors 2020, 20, 1877. [Google Scholar] [CrossRef] [Green Version]
- Baldacchino, F.V.; Pedrinolla, A.; Venturelli, M. Exercise-Induced Adaptations in Patients with Alzheimer’s Disease: The Role of Circadian Scheduling. Sport Sci. Health 2018, 14, 227–234. [Google Scholar] [CrossRef]
- Fleiner, T.; Zijlstra, W.; Dauth, H.; Haussermann, P. Evaluation of a Hospital-Based Day-Structuring Exercise Programme on Exacerbated Behavioural and Psychological Symptoms in Dementia—The Exercise Carrousel: Study Protocol for a Randomised Controlled Trial. Trials 2015, 16, 228. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, W.; Becker, C.; Pfeiffer, K. Wearable Systems for Monitoring Mobility Related Activities. In E-Health, Assistive Technologies and Applications for Assisted Living; Röcker, C., Ziefle, M., Eds.; IGI Global: Hershey, PA, USA, 2011; pp. 244–267. [Google Scholar] [CrossRef]
- Klenk, J.; Peter, R.S.; Rapp, K.; Dallmeier, D.; Rothenbacher, D.; Denkinger, M.; Büchele, G.; Group, S.; Becker, T.; Böhm, B.; et al. Lazy Sundays: Role of Day of the Week and Reactivity on Objectively Measured Physical Activity in Older People. Eur. Rev. Aging Phys. Act. 2019, 1, 16–19. [Google Scholar]
- Abel, B.; Pomiersky, R.; Werner, C.; Lacroix, A.; Schäufele, M.; Hauer, K. Day-to-Day Variability of Multiple Sensor-Based Physical Activity Parameters in Older Persons with Dementia. Arch. Gerontol. Geriatr. 2019, 85, 103911. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv-Ionescu, A.; Mellone, S.; Colpo, M.; Bourke, A.; Ihlen, E.A.F.; el Achkar, C.M.; Chiari, L.; Becker, C.; Aminian, K. Patterns of Human Activity Behavior. In Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing Adjunct—UbiComp ’16; Lukowicz, P., Krüger, A., Bulling, A., Lim, Y.-K., Patel, S.N., Eds.; ACM Press: New York, NY, USA, 2016; pp. 841–845. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.E.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the Detection of Dementia in Clinically Unevaluated People Aged 65 and over in Community and Primary Care Populations. Cochrane Database Syst. Rev. 2016, 2016, CD011145. [Google Scholar] [CrossRef] [Green Version]
- Groll, D.; To, T.; Bombardier, C.; Wright, J. The Development of a Comorbidity Index with Physical Function as the Outcome. J. Clin. Epidemiol. 2005, 58, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Ostberg, O. A Self-Assessment Questionnaire to Determine Morningness-Eveningness in Human Circadian Rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Hoffmann, R.M.; Müller, T.; Hajak, G.; Cassel, W. Abend-Morgenprotokolle in Schlafforschung Und Schlafmedizin—Ein Standardinstrument Für Den Deutschsprachigen Raum. Somnologie 1997, 1, 103–109. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and Physical Activity for Older Adults. Med. Sci. Sport. Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Venturelli, M.; Sollima, A.; Cè, E.; Limonta, E.; Bisconti, A.V.; Brasioli, A.; Muti, E.; Esposito, F. Effectiveness of Exercise- and Cognitive-Based Treatments on Salivary Cortisol Levels and Sundowning Syndrome Symptoms in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 53, 1631–1640. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How Many Steps/Day Are Enough? For Older Adults and Special Populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Landry, G.J.; Falck, R.S.; Beets, M.W.; Liu-Ambrose, T. Measuring Physical Activity in Older Adults: Calibrating Cut-Points for the MotionWatch 8©. Front. Aging Neurosci. 2015, 7, 165. [Google Scholar] [CrossRef]
- Larsen, R.T.; Wagner, V.; Keller, C.; Juhl, C.B.; Langberg, H.; Christensen, J. Physical Activity Monitors to Enhance Amount of Physical Activity in Older Adults—A Systematic Review and Meta-Analysis. Eur. Rev. Aging Phys. Act. 2019, 16, 53. [Google Scholar] [CrossRef]

| N (%) | Mean | SD | Min | Max | |||
|---|---|---|---|---|---|---|---|
| Sample | 81 | ||||||
| Female | 40 (49.4) | ||||||
| Age (years) | 71.5 | 5.0 | 65 | 84 | |||
| Mass (kg) | 76.9 | 15.4 | 54 | 119 | |||
| Height (cm) | 170.3 | 8.3 | 154 | 188 | |||
| MEQ score | 57.7 | 9.9 | 31 | 77 | |||
| Number of diseases | 2.0 | 1.4 | 0 | 7 | |||
| Duration of wakefulness (h) | 15.9 | 0.8 | 13.5 | 18.1 | |||
| Move monitor+ | 75 | ||||||
| Activity/posture [hh:mm]/24 h | |||||||
| lying | 09:01 | 01:38 | 06:18 | 14:29 | |||
| sitting | 09:13 | 01:53 | 05:08 | 14:13 | |||
| standing | 03:02 | 00:48 | 01:02 | 04:48 | |||
| shuffling | 00:29 | 00:10 | 00:11 | 01:11 | |||
| walking | 01:57 | 00:36 | 00:44 | 03:51 | |||
| other activities * | 00:05 | 00:11 | 00:00 | 00:59 | |||
| not worn | 00:13 | 00:25 | 00:00 | 03:07 | |||
| steps/24 h | 9860.1 | 3279.9 | 3278.0 | 17,319.2 | |||
| MotionWatch 8 | 66 | ||||||
| counts/min [24 h] | 317.9 | 87.3 | 121.6 | 556.9 | |||
| Morning Type | Intermediate Type | Evening Type | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | M | SD | N (%) | M | SD | N (%) | M | SD | |||
| Sample | 40 (49.4) | 35 (43.2) | 6 (7.4) | ||||||||
| female | 15 (37.5) | 22 (62.9) | 3 (50.0) | ||||||||
| Age (years) | 72.1 | 5.2 | 71.4 | 5.1 | 67.8 | 3.1 | 0.10 | ||||
| Wakefulness (h/day) | 16.1 | 0.6 | 15.8 | 0.8 | 15.9 | 0.8 | 0.55 | ||||
| MM+ Steps (number/24 h) | 37 | 9791.2 | 3157.1 | 32 | 9823.9 | 3101.1 | 6 | 10,478.4 | 4310.3 | 0.98 | |
| MW8 Counts/min (24 h) | 30 | 316.8 | 73.9 | 30 | 319.1 | 103.2 | 6 | 317.9 | 40.0 | 0.98 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleiner, T.; Trumpf, R.; Hollinger, A.; Haussermann, P.; Zijlstra, W. Quantifying Circadian Aspects of Mobility-Related Behavior in Older Adults by Body-Worn Sensors—An “Active Period Analysis”. Sensors 2021, 21, 2121. https://doi.org/10.3390/s21062121
Fleiner T, Trumpf R, Hollinger A, Haussermann P, Zijlstra W. Quantifying Circadian Aspects of Mobility-Related Behavior in Older Adults by Body-Worn Sensors—An “Active Period Analysis”. Sensors. 2021; 21(6):2121. https://doi.org/10.3390/s21062121
Chicago/Turabian StyleFleiner, Tim, Rieke Trumpf, Anna Hollinger, Peter Haussermann, and Wiebren Zijlstra. 2021. "Quantifying Circadian Aspects of Mobility-Related Behavior in Older Adults by Body-Worn Sensors—An “Active Period Analysis”" Sensors 21, no. 6: 2121. https://doi.org/10.3390/s21062121






