Enhancement of Photoacoustic Signal Strength with Continuous Wave Optical Pre-Illumination: A Non-Invasive Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theory
2.2. Enhancement of PA-Signal Strength
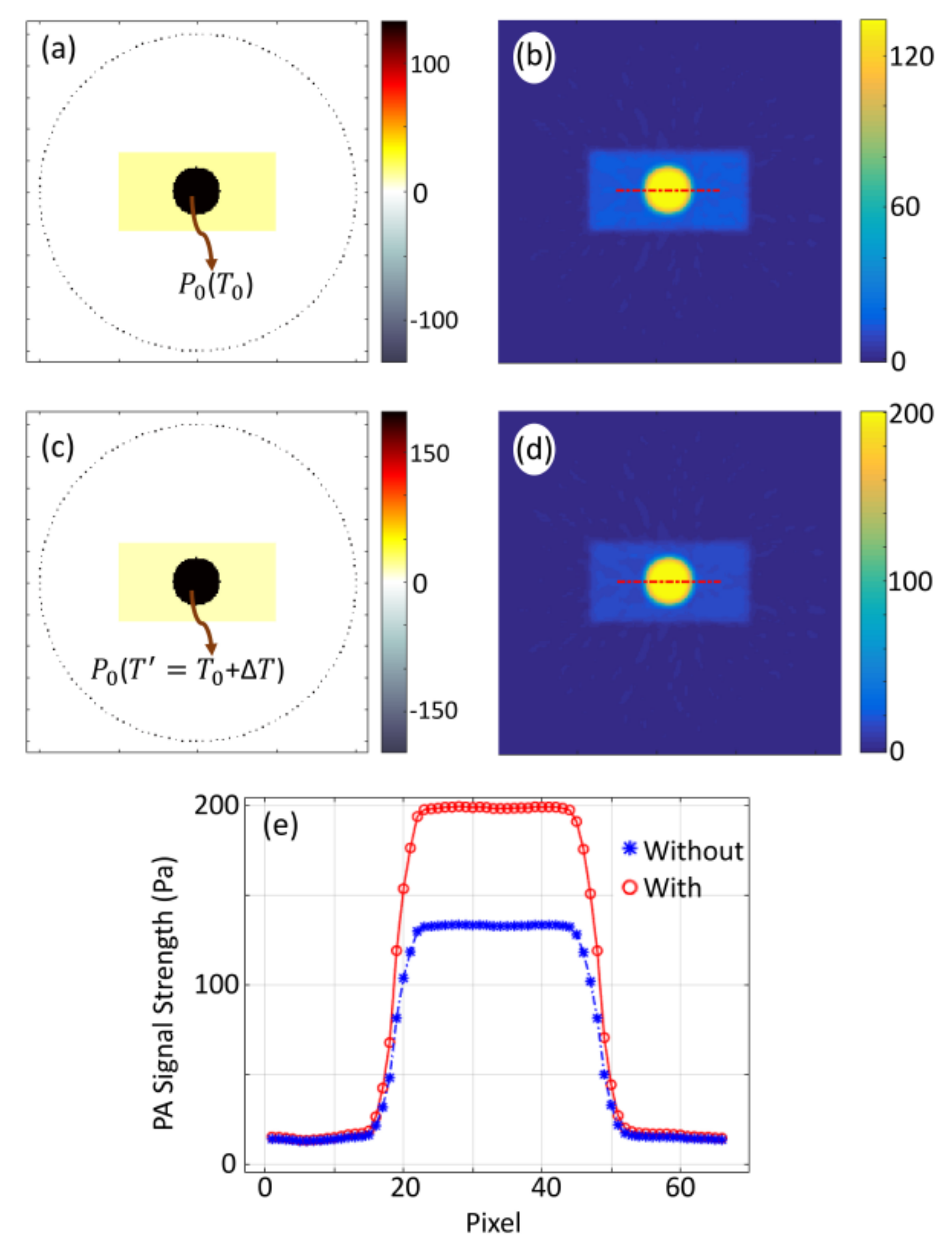
2.3. Numerical Simulation
2.4. Experimental Set-Up
3. Experimental Results and Discussion
3.1. Enhancement of PA-Signal from Methylene Blue
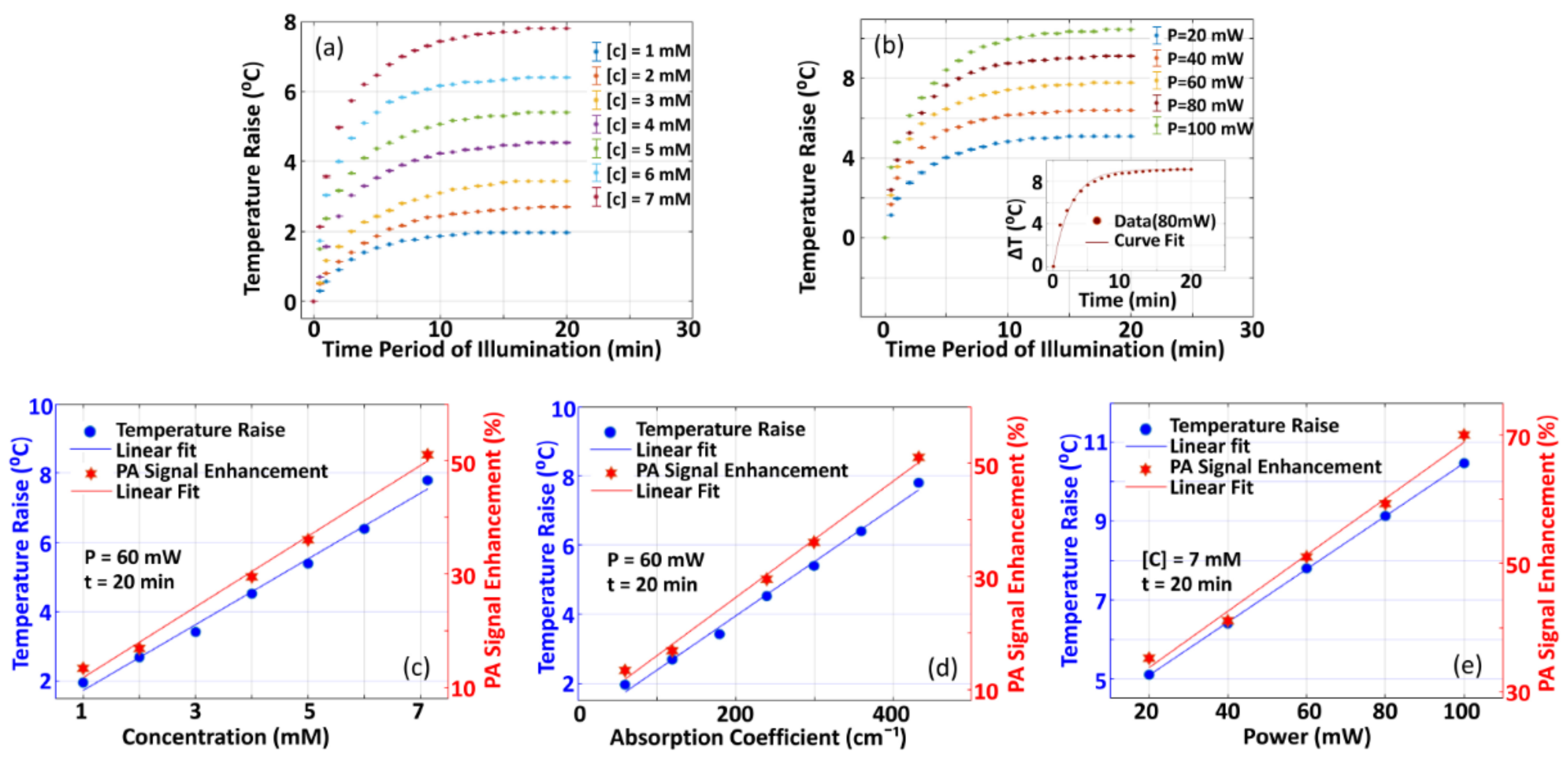
3.1.1. Variation of PA-Signal Enhancement with Variation in Different Parameters
3.1.2. Variation of Temperature Raise with Variation in Different Parameters
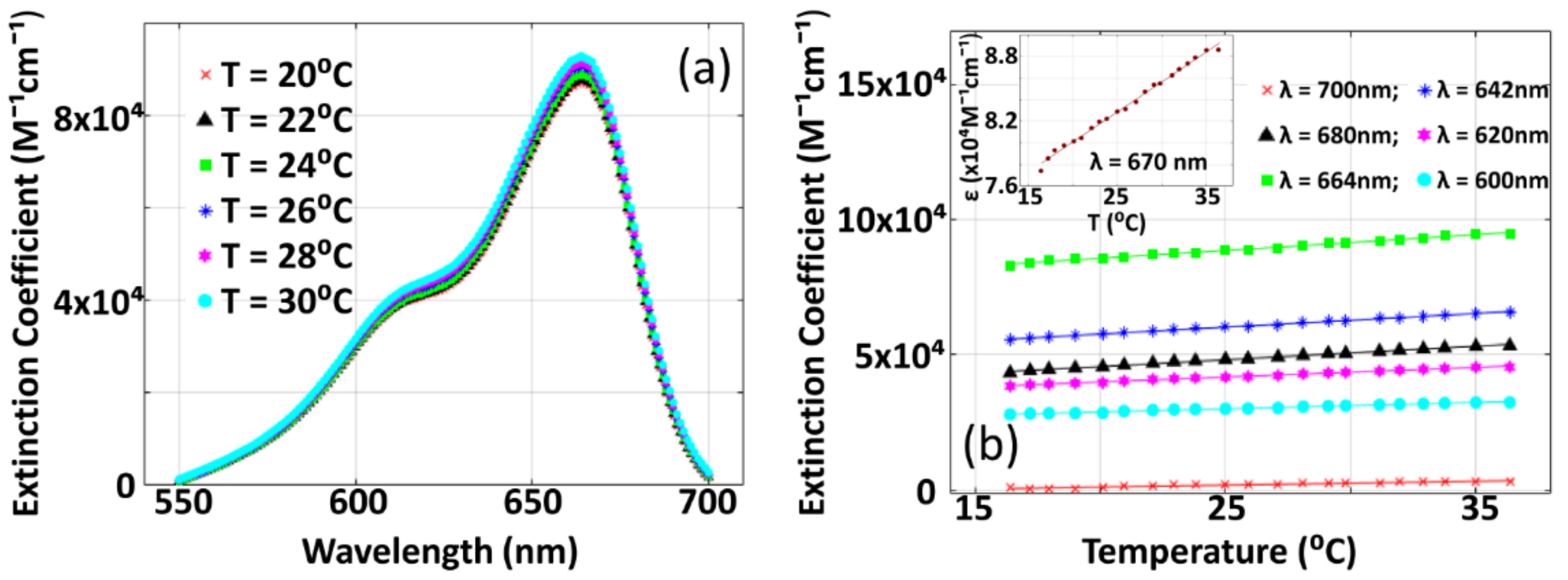
3.2. Variation of Optical Extinction Coefficient of Methylene Blue with Temperature
3.3. Comparison of Numerical Simulation and Experimental Results, and Validation of Theoretical Hypothesis
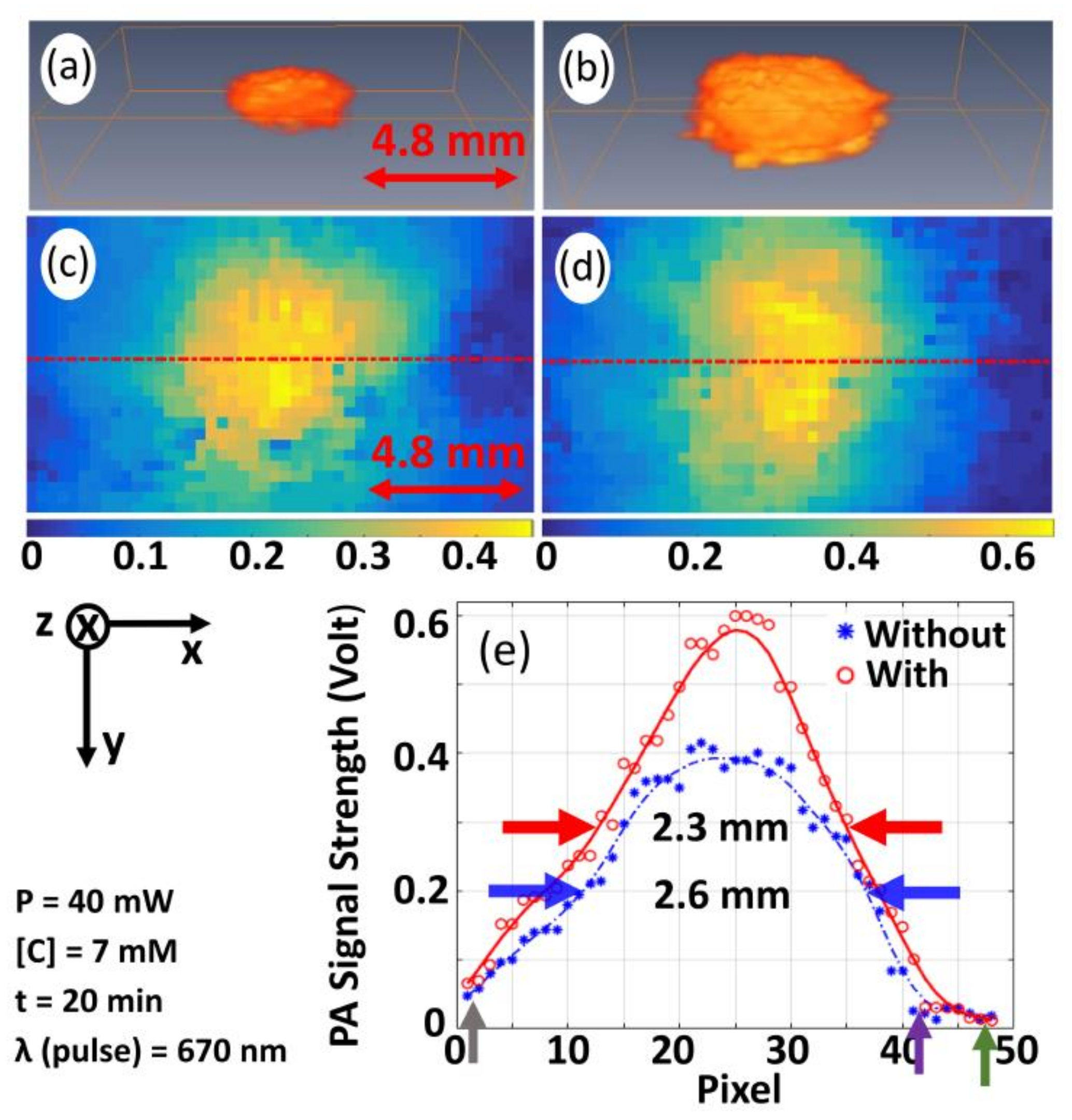
3.4. Validation of the Proposed Technique for Pre-Clinical/Clinical Studies
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Dependence of Initial Pressure Waves on Thermodynamic Parameters for Low Concentration Materials
References
- Strohm, E.M.; Moore, M.J.; Kolios, M.C. Single cell photoacoustic microscopy: A review. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 6801215. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.S.; Jiang, H. Elastic property attributes to photoacoustic signals: An experimental phantom study. Opt. Lett. 2014, 39, 3970–3973. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Jiang, H. Ultrasound (US) transducer of higher operating frequency detects photoacoustic (PA) signals due to the contrast in elastic property. AIP Adv. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Singh, M.S.; Jiang, H. Estimating both direction and magnitude of flow velocity using photoacoustic microscopy. Appl. Phys. Lett. 2014, 104, 1–5. [Google Scholar] [CrossRef]
- Jiang, H.; Yuan, Z.; Gu, X. Spatially varying optical and acoustic property reconstruction using finite element-based photoacoustic tomography. J. Opt. Soc. 2006, 23, 878–888. [Google Scholar] [CrossRef]
- Dean-Ben, X.L.; Fehm, T.F.; Ford, S.J.; Gottschalk, S.; Razansky, D. Spiral volumetric optoacoustic tomography visualizes multi-scale dynamics in mice. Light Sci. Appl. 2017, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K.A.; Agano, T.; Sato, N.; Shigeta, Y.; Uemura, T. Real-time in vivo imaging of human lymphatic system using an LED-based photoacoustic/ultrasound imaging system. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2018, San Francisco, CA, USA, 19 February 2018. [Google Scholar]
- Zhu, Y.; Feng, T.; Cheng, Q.; Wang, X.; Du, S.; Sato, N.; Yuan, J.; Singh, M.K.A. Towards clinical translation of LED-based Photoacoustic imaging: A review. Sensors 2020, 20, 2484. [Google Scholar] [CrossRef]
- Singh, M.K.A. LED-Based Photoacoustic Imaging from Bench to Bedside; Springer nature Singapore Pte Ltd.: Singapore, 2020. [Google Scholar]
- Zhong, H.; Duan, T.; Lan, H.; Zhou, M.; Gao, F. Review of low-cost photoacoustic sensing and imaging based on laser diode and light-emitting diode. Sensors 2018, 18, 2264. [Google Scholar] [CrossRef] [Green Version]
- Larina, I.V.; Larin, K.V.; Esenaliev, R.O. Real-time optoacostic monitoring of temperature in tissues. J. Phys. D 2005, 38, 2633–2639. [Google Scholar] [CrossRef]
- Petrova, E.; Anton, L.; Alexander, A.O.; Sergey, A.E. Temperature-dependent optoacoustic response and transient through zero Grüneisen parameter in optically contrasted media. Photoacoustics 2017, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, S.M.; Khokhlova, T.D.; Pelivanov, I.M. In-vitro study of the temperature dependence of the optoacoustic conversion efficiency in biological tissues. IEEE J. Quantum Electron. 2012, 46, 269–276. [Google Scholar] [CrossRef]
- Nikitin, S.M.; Khokhlova, T.D.; Pelivanov, I.M. Temperature dependence of the optoacoustic transformation efficiency in ex-vivo tissues for application in monitoring thermal therapies. J. Biomed. Opt. 2012, 46, 061214. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Larina, I.V.; Esenaliev, R.O. Monitoring of tissue coagulation during thermotherapy using optoacoustic technique. J. Phys. D Appl. Phys. 2005, 38, 2645–2653. [Google Scholar] [CrossRef]
- Petrova, E.; Ermilov, S.; Su, R.; Nadvoretskiy, V.; Conjusteau, A.; Oraevsky, A. Using optoacoustic imaging for measuring the temperature dependence of Grüeisenparameter in optically absorbing solutions. Opt. Exp. 2013, 21, 195733. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zang, C.; Wang, L.V. Grueneisen relaxation photoacoustic microscopy. Phys. Rev. Lett. 2014, 113, 174301. [Google Scholar] [CrossRef]
- Tian, C.; Xie, Z.; Fabiilli, M.L.; Liu, S.; Wang, C.; Cheng, Q.; Wang, X. Dual-pulse nonlinear photoacoustic technique: A practical investigation. Biomed. Opt. Exp. 2015, 6, 241695. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodkalayeh, S.; Jooya, H.Z.; Hariri, A.; Zhou, Y.; Xu, Q.; Ansari, M.A.; Avanaki, M.R.N. Low temperature-mediated enhancement of photoacoustic imaging depth. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Olivier, S.; Amaury, P.; Jerome, G.; Bossy, E. Influence of nanoscale temperature rises on photoacoustic generation: Discrimination between optical absorbers based on thermal nonlinearity at high frequency. Photoaocustics 2015, 3, 20–25. [Google Scholar]
- Zharov Vladimir, P. Ultrasharp nonlinear photothermal and photoacoustic resonances and holes beyond the spectral limit. Nat. Photonics 2011, 5, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Esenaliev, R.O. Optoacoustic monitoring of physiologic variables. Front. Physiol. 2017, 8, 1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast agents for photoacoustic and thermoacoustic imaging: A review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef] [PubMed]
- Dean-Ben, X.L.; Fehm, T.F.; Ford, S.J.; Gottschalk, S.; Razansky, D. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar]
- Amaury, P.; Florian, P.; Emmanuel, B. Photoacoustic generation by a gold nanosphere: From the linear to the nonlinear thermoelastic regime. Phys. Rev. B 2015, 92, 115450. [Google Scholar]
- Irio, G.C.; Craig, W.; Diebold, G.J. Photoacoustic point source. Phys. Rev. Lett. 2001, 86, 3550–3553. [Google Scholar]
- Shah, J.; Park, S.; Aglyamov, S.; Larson, T.; Ma, L.; Sokolov, K.; Johnston, K.; Milner, T.; Emelianov, S.Y. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J. Biomed. Opt. 2008, 13, 034024. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Larina, I.V.; Motamedi, M.; Esenaliev, R.O. Optoacoustic laser monitoring of cooling and freezing of tissuess. IEEE J. Quantum Electron. 2002, 32, 953–958. [Google Scholar] [CrossRef]
- Esenaliev, R.O.; Petrov, Y.Y.; Cicenaite, I.; Chumakova, O.V.; Petrova, I.Y.; Patrikeev, I.; Liopo, A. Real-time noninvasive optoacoustic monitoring of nanoparticle-mediated photothermal therapy of tumors. In Photons Plus Ultrasound: Imaging and Sensing 2007: The Eighth Conference on Biomedical Thermoacoustics, Optoacoustics, and Acousto-Optics; International Society for Optics and Photonics: San Jose, CA, USA, 19 March 2007; Volume 6437, p. 64370Q-1. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Wang, X.; Ku, G.; Gill, K.L.; O’Neal, D.P.; Stoica, G.; Wang, L.V. Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Lett. 2004, 4, 1689–1692. [Google Scholar] [CrossRef]
- Copland, J.A.; Eghtedari, M.; Popov, V.L.; Kotov, N.; Mamedova, N.; Motamedi, M.; Oraevsky, A.A. Bioconjugated gold nanoparticles as a molecular based contrast agent: Implications for imaging of deep tumors using optoacoustic tomography. Mol. Imaging Biol. 2004, 6, 341–349. [Google Scholar] [CrossRef]
- Mallidi, S.; Larson, T.; Aaron, J.; Sokolov, K.; Emelianov, S. Molecular specific optoacoustic imaging with plasmonic nanoparticles. Opt. Exp. 2007, 15, 6583–6588. [Google Scholar] [CrossRef]
- Pramanik, M.; Wang, L.V. Thermoacoustic and photoacoustic sensing of temperature. J. Biomed. Opt. 2009, 14, 054024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, B.T.; Laufer, J.; Kostli, K.; Beard, P. Experimental validation of photoacoustic k-space propagation models. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing, International Society for Optics and Photonics, San Jose, CA, USA, 25–26 January 2004; Volume 5320, pp. 238–248. [Google Scholar]
- Treeby, B.E.; Cox, B.T. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J. Biomed. Opt. 2010, 15, 021314. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interf. Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Paul, S.; Thomas, A. Fundamentals of photoacoustic imaging: A theoretical tutorial. In LED-Based Photoacoustic Imaging; Progress in Optical Science and, Photonics; Kuniyil Ajith Singh, M., Ed.; Springer: Singapore, 2020; pp. 3–21. [Google Scholar]
- Chen, Y.S.; Frey, W.; Aglyamov, S.; Emelianov, S. Environment-dependent generation of photoacoustic waves from plasmonic nanoparticles. Small 2012, 8, 47–52. [Google Scholar] [CrossRef]
- Zemansky, M.W.; Dittman, R.H. Heat and Thermodynamics, 7th ed.; McGraw-Hill Companies Inc.: New York, NY, USA, 1997; pp. 154–196. [Google Scholar]
- Hunter, S.D.; Jones, W.V.; Malbrough, D.J.; Van Buren, A.L.; Liboff, A.; Bowen, T.; Jones, J.J.; Learned, J.G.; Bradner, H.; Pfeffer, L.; et al. Acoustic-signals of nonthermal origin from high-energy protons in water. J. Acoust. Soc. Am. 1981, 69, 1557–1562. [Google Scholar] [CrossRef]
- Gusev, V.E.; Karabutov, A.A. Laser Optoacoustics; AIP: New York, NY, USA, 1993. [Google Scholar]
- Haemmerich, D.; Dos Santos, I.; Schutt, D.J.; Webster, J.G.; Mahvi, D.M. In vitro measurements of temperature-dependent specific heat of liver tissue. Communication 2006, 28, 194–197. [Google Scholar]
- Burmistrova, L.V.; Karabutov, A.A.; Rudenko, O.V.; Cherepetskaya, E.B. Influence of thermal nonlinearity on the thermooptical generation of sound. Sov. Phys. Acoust. USSR 1979, 25, 348–350. [Google Scholar]
- Singh, M.S.; Yalavarthy, P.K. Born-ratio type data normalization improves quantitation in photoacoustic tomography. In Proceedings of the Medical Imaging 2014: Ultrasonic Imaging and Tomography, International Society for Optics and Photonics, San Diego, CA, USA, 20 March 2014; Volume 9040. [Google Scholar] [CrossRef]
- Ultrasonic Transducers Technical Notes. Available online: https://ctac.mbi.ufl.edu/files/2017/02/ultrasound-basics.pdf (accessed on 22 November 2020).
- Wang, L.V. Tutorial on Photoacoustic Microscopy and Computed Tomography. IEEE J. Sel. Top. Quant. Elect. 2008, 11, 171–179. [Google Scholar] [CrossRef] [Green Version]
- American National Standard for Safe Use of Lasers; ANSI Z136.1-2007; Laser Institute of America: Orlando, FL, USA, 2007; pp. 76–77. Available online: https://webstore.ansi.org/standards/lia/ansiz1362007 (accessed on 27 January 2021).
- Ramachandran, T.; Sreenivasan, K.; Sivakumar, R. Water vaporization from heated tissue: An in vitro study by differential scanning calorime-try. Lasers Surg. Med. 1996, 19, 413–415. [Google Scholar] [CrossRef]
- Durduran, T.; Choe, R.; Culver, J.P.; Zubkov, L.; Holboke, M.J.; Giammarco, J.; Chance, B.; Yodh, A.G. Bulk optical properties of healthy female breast tissue. Phys. Med. Biol. 2002, 47, 2847–2861. [Google Scholar] [CrossRef] [Green Version]
- Rinaldo, C.; Antonio, P.; Paola, T.; Alessandro, T.; Gianluca, V. A solid tissue phantom for photon migration studies. Phys. Med. Biol. 1997, 42, 197–199. [Google Scholar]





| Temperature (°C or K) | β(water) (10−6 K−1) | κ(water) (10−10 Pa−1) | ⍴(water) (kg m−3) | CP(water) (kJ kg K−1) | CV(water) (kJ kg K−1) |
|---|---|---|---|---|---|
| 20 or 293 | 206.80 | 4.5891 | 998.21 | 4.1818 | 4.1545 |
| 30 or 303 | 303.23 | 4.4770 | 995.65 | 4.1784 | 4.1159 |
| 40 or 113 | 385.30 | 4.4240 | 992.22 | 4.1785 | 4.0826 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, A.; Paul, S.; Mitra, J.; Singh, M.S. Enhancement of Photoacoustic Signal Strength with Continuous Wave Optical Pre-Illumination: A Non-Invasive Technique. Sensors 2021, 21, 1190. https://doi.org/10.3390/s21041190
Thomas A, Paul S, Mitra J, Singh MS. Enhancement of Photoacoustic Signal Strength with Continuous Wave Optical Pre-Illumination: A Non-Invasive Technique. Sensors. 2021; 21(4):1190. https://doi.org/10.3390/s21041190
Chicago/Turabian StyleThomas, Anjali, Souradip Paul, Joy Mitra, and Mayanglambam Suheshkumar Singh. 2021. "Enhancement of Photoacoustic Signal Strength with Continuous Wave Optical Pre-Illumination: A Non-Invasive Technique" Sensors 21, no. 4: 1190. https://doi.org/10.3390/s21041190
APA StyleThomas, A., Paul, S., Mitra, J., & Singh, M. S. (2021). Enhancement of Photoacoustic Signal Strength with Continuous Wave Optical Pre-Illumination: A Non-Invasive Technique. Sensors, 21(4), 1190. https://doi.org/10.3390/s21041190






