Intertwined Carbon Nanotubes and Ag Nanowires Constructed by Simple Solution Blending as Sensitive and Stable Chloramphenicol Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
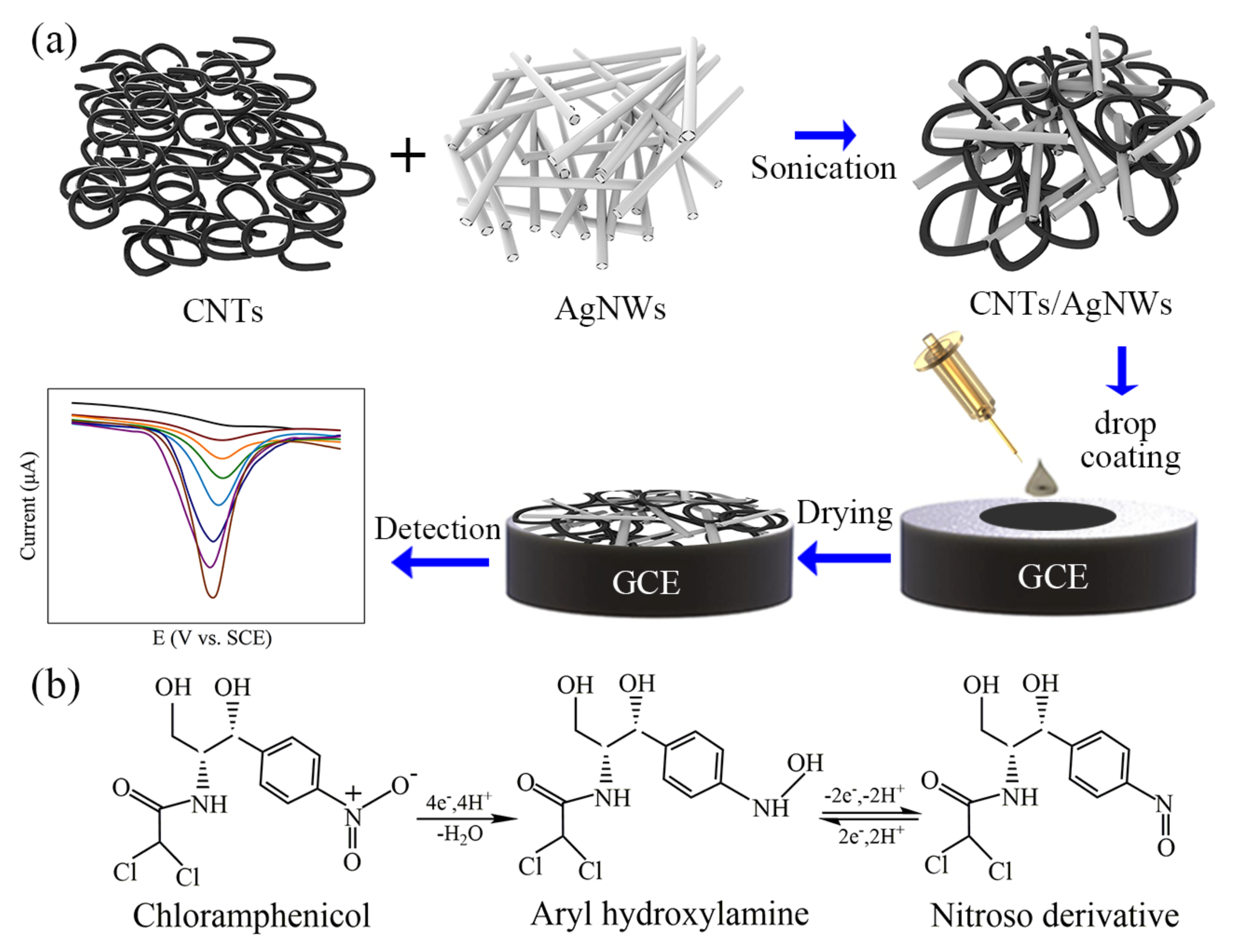
2.2. Fabrication of CNTs/AgNWs Electrodes
2.3. Characterizations
2.4. Electrochemical Tests
3. Results and Discussion
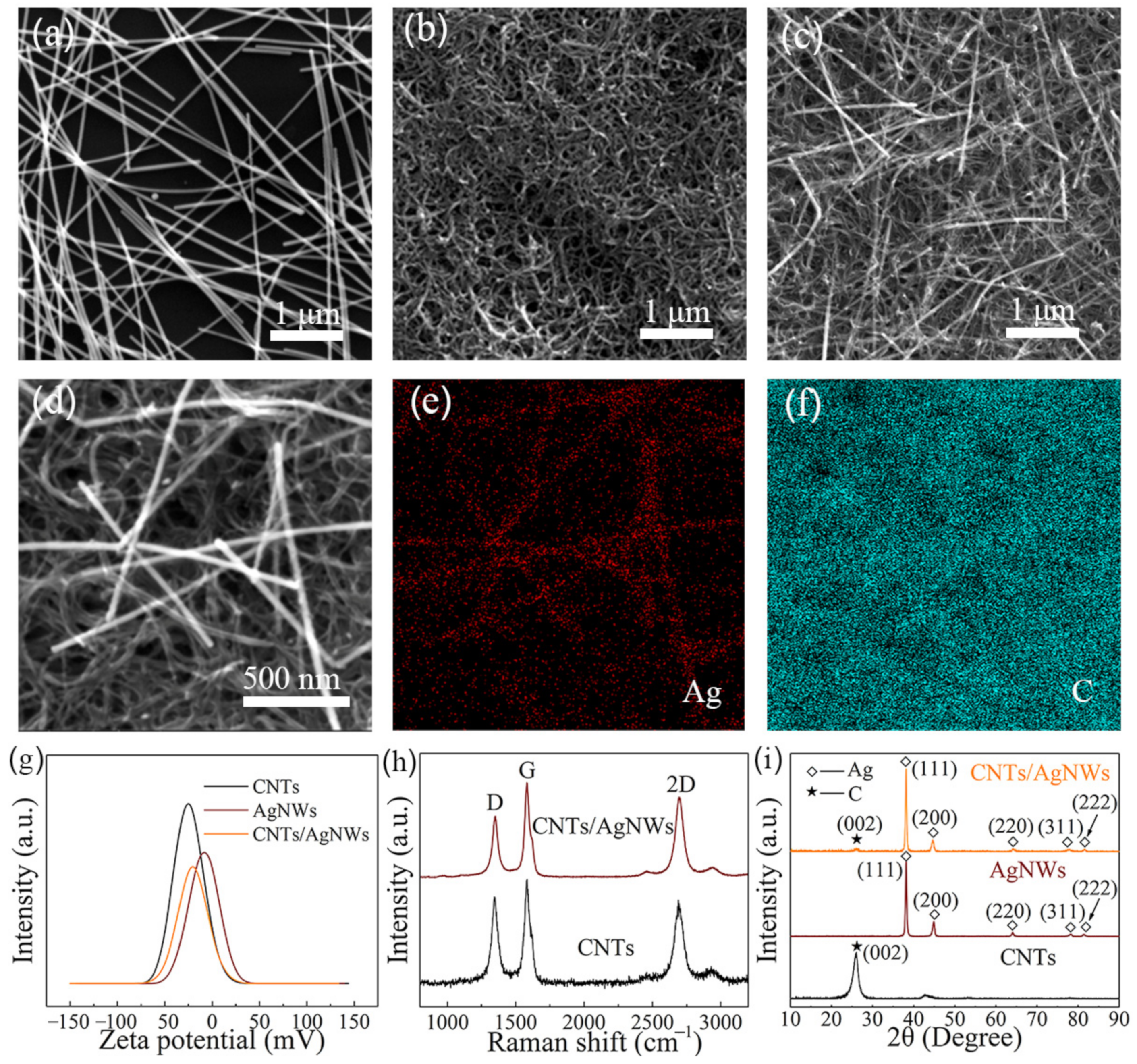
3.1. Characterization of CNTs/AgNWs Nanocomposite
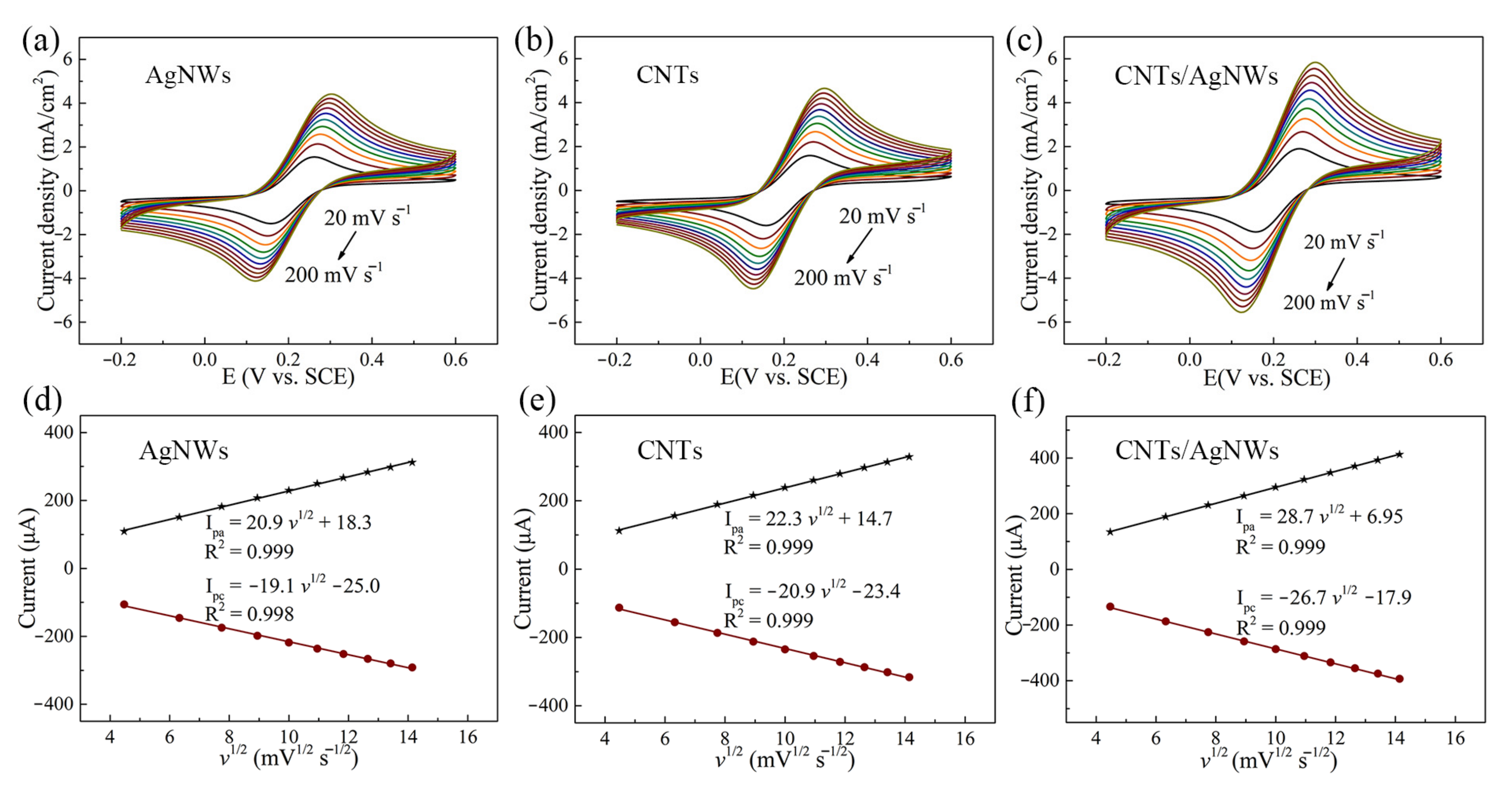
3.2. Electrochemical Behavior and Performance Optimization of CAP
3.3. Electrochemical Determination of CAP with Different Concentrations
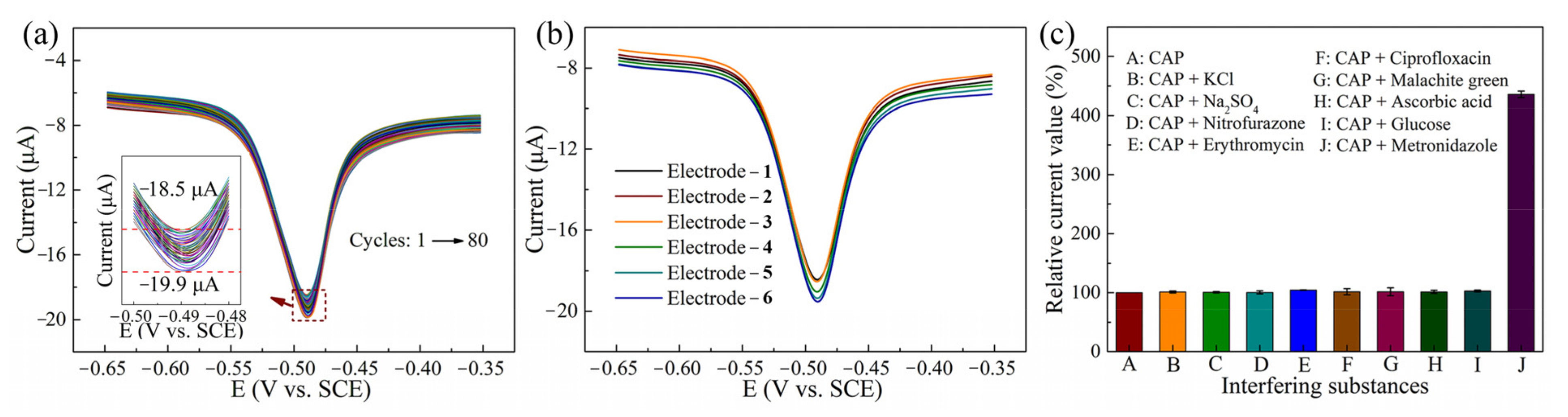
3.4. Repeatability, Reproducibility, Interference and Real Sample Analysis of CAP Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, G.M.; Zhao, F.Q. Electrochemical sensor for chloramphenicol based on novel multiwalled carbon nanotubes@ molecularly imprinted polymer. Biosens. Bioelectron. 2015, 64, 416–422. [Google Scholar] [CrossRef]
- Peng, X.Z.; Tan, J.H.; Tang, C.M.; Yu, Y.Y.; Wang, Z.D. Multiresidue determination of fluoroquinolone, sulfonamide, trimethoprim, and chloramphenicol antibiotics in urban waters in China. Environ. Toxicol. Chem. 2008, 27, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Ren, L.S.; Yu, X.; Hu, J.; Chen, Y.; He, G.S.; Jiang, Q.W. Antibiotic residues in meat, milk and aquatic products in Shanghai and human exposure assessment. Food Control 2017, 80, 217–225. [Google Scholar] [CrossRef]
- Roushani, M.; Rahmatia, Z.; Farokhia, S.; Hoseini, S.J.; Fath, R.H. The development of an electrochemical nanoaptasensor to sensing chloramphenicol using a nanocomposite consisting of graphene oxide functionalized with (3-Aminopropyl) triethoxysilane and silver nanoparticles. Mat. Sci. Eng. C 2020, 108, 110388. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Govindasamy, M.; Chen, S.M.; Mani, V.; Lou, B.S.; Devasenathipathy, R.; Hou, Y.S.; Elangovan, A. Green synthesized gold nanoparticles decorated graphene oxide for sensitive determination of chloramphenicol in milk, powdered milk, honey and eye drops. J. Colloid. Interf. Sci. 2016, 475, 46–56. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Verdian, A.; Housaindokht, M.R.; Izadyar, M.; Rouhbakhsh, Z. Aptasensors as the future of antibiotics test kits-a case study of the aptamer application in the chloramphenicol detection. Biosens. Bioelectron. 2018, 122, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar, S.; Mehta, J.; Dardenne, F.; Robbens, J.; Blust, R.; Wael, K.D. Aptasensing of chloramphenicol in the presence of its analogues: Reaching the maximum residue limit. Anal. Chem. 2012, 84, 6753–6758. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhang, Z.Y.; Li, P.; Zang, Y.G.; Liu, X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotox. Environ. Safe 2020, 199, 110668. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.; Nafady, A.; Talpur, F.N.; Guo, X.S.; Cai, Q.; Zhu, S.M. Rapid detection of chloramphenicol residues in aquatic products using colloidal gold immunochromatographic assay. Sensor. Actuators B 2015, 211, 359–369. [Google Scholar] [CrossRef]
- Tang, C.M.; Huang, Q.X.; Yu, Y.Y.; Peng, X.Z. Multiresidue determination of sulfonamides, macrolides, trimethoprim, and chloramphenicol in sewage sludge and sediment using ultrasonic extraction coupled with solid phase extraction and liquid chromatography-tandem mass spectrometry. Chin. J. Anal. Chem. 2009, 37, 1119–1124. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bečanová, J.; Scheringer, M.; Sharma, A.; Bharat, G.K.; Whitehead, P.G.; Klánová, J.; Nizzetto, L. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin. India. Sci. Total Environ. 2019, 646, 1459–1467. [Google Scholar] [CrossRef]
- Falagas, M.E.; Grammatikos, A.P.; Michalopoulos, A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert. Rev. Anti-Infect. Ther. 2008, 6, 593–600. [Google Scholar] [CrossRef]
- Ji, W.; Yao, W.R. Rapid surface enhanced Raman scattering detection method for chloramphenicol residues. Spectrochim. Acta. A 2015, 144, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; Vogels, J.; Kemmerling, J.; Fehlert, E.; Rühl-Fehlert, C.; Vohr, H.W.; Krul, C. Integrated analysis of toxicity data of two pharmaceutical immunosuppressants and two environmental pollutants with immunomodulating properties to improve the understanding of side effects-a toxicopathologist’s view. Eur. J. Pharmacol. 2015, 759, 343–355. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.; Fan, J.; Zhu, L.; Zhang, Y.; Jin, J.; Huang, B. A new sensitive method for the detection of chloramphenicol in food using time-resolved fluoroimmunoassay. Eur. Food Res. Technol. 2015, 240, 619–625. [Google Scholar] [CrossRef]
- Shen, H.Y.; Jiang, H.L. Screening, determination and confirmation of chloramphenicol in sea food, meat and honey using ELISA, HPLC-UVD, GC-ECD, GC-MS-EI-SIM and GCMS-NCI-SIM methods. Anal. Chim. Acta 2005, 535, 33–41. [Google Scholar] [CrossRef]
- Sniegocki, T.; Posyniak, A.; Gbylik-Sikorska, M.; Zmudzki, J. Determination of chloramphenicol in milk using a QuEChERS-based on liquid chromatography tandem mass spectrometry method. Anal. Lett. 2014, 47, 568–578. [Google Scholar] [CrossRef]
- Azzouz, A.; Ballesteros, E. Multiresidue method for the determination of pharmacologically active substances in egg and honey using a continuous solid-phase extraction system and gas chromatography–mass spectrometry. Food. Chem. 2015, 178, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Y.; Zhang, H.Y.; Ye, C.; Yuan, Q.L.; Chee, K.W.A.; Su, W.T.; Yu, A.M.; Yu, J.H.; Lin, C.-T.; Dai, D.; et al. Electrochemical enantiomer recognition based on sp3-to-sp2 converted regenerative graphene/diamond electrode. Nanomaterials 2018, 8, 1050. [Google Scholar] [CrossRef]
- Gao, J.Y.; Yuan, Q.L.; Ye, C.; Guo, P.; Du, S.Y.; Lai, G.S.; Yu, A.M.; Jiang, N.; Fu, L.; Lin, C.-T.; et al. Label-free electro chemical detection of vanillin through low-defect graphene electrodes modified with Au nanoparticles. Materials 2018, 11, 489. [Google Scholar] [CrossRef]
- Chen, F.Y.; Fan, Z.Q.; Zhu, Y.G.; Sun, H.F.; Yu, J.H.; Jiang, N.; Zhao, S.C.; Lai, G.S.; Yu, A.M.; Lin, C.-T.; et al. β-Cy clodextrin-immobilized Ni/graphene electrode for electrochemical enantiorecognition of phenylalanine. Materials 2020, 13, 777. [Google Scholar] [CrossRef]
- Shaikh, T.; Nafady, A.; Talpur, F.N.; Agheem, M.H.; Shah, M.R.; Sherazi, S.T.H.; Soomro, R.A.; Siddiqui, S. Tranexamic acid derived gold nanoparticles modified glassy carbon electrode as sensitive sensor for determination of nalbuphine. Sens. Actuators B 2015, 211, 359–369. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Srivastava, A.K. Simultaneous voltammetric determination of acetaminophen and tramadol using Dowex50wx2 and gold nanoparticles modified glassy carbon paste electrode. Anal. Chim. Acta 2011, 706, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Selvi, S.V.; Nataraj, N.; Chen, S.M. The electro-catalytic activity of nanosphere strontium doped zinc oxide with rGO layers screen-printed carbon electrode for the sensing of chloramphenicol. Microchem. J. 2020, 159, 105580. [Google Scholar] [CrossRef]
- Peng, B.; Lu, Y.; Luo, J.; Zhang, Z.L.; Zhu, X.; Tang, L.; Wang, L.L.; Deng, Y.C.; Ouyang, X.L.; Tan, J.S.; et al. Visible light-activated self-powered photoelectrochemical aptasensor for ultrasensitive chloramphenicol detection based on DFT-proved Z-schemeAg2CrO4/g-C3N4/graphene oxide. J. Hazard. Mater. 2021, 401, 123395. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Kamalzadeh, Z.; Hamzehloei, A. Electrochemical determination of Clozapine on MWCNTs/New Coccine doped PPY modified GCE: An experimental design approach. Bioelectrochemistry 2013, 90, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Tahir, M.A.; Shaheen, A.; Lieberzeit, P.A.; Khan, W.S.; Bajwa, S.Z. Investigating nanohybrid material based on 3D CNTs @ Cu nanoparticle composite and imprinted polymer for highly selective detection of chloramphenicol. J. Hazard. Mater. 2018, 342, 96–106. [Google Scholar] [CrossRef]
- Zhao, H.M.; Chen, Y.Q.; Tian, J.P.; Yu, H.T.; Quan, X. Selectively electrochemical determination of chloramphenicol in aqueous solution using molecularly imprinted polymer-carbon nanotubes-gold nanoparticles modified electrode. J. Electrochem. Soc. 2012, 159, J231–J236. [Google Scholar] [CrossRef]
- Loan, P.T.K.; Zhang, W.j.; Lin, C.-T.; Wei, K.-H.; Li, L.-J.; Chen, C.-H. Graphene/MoS2 heterostructures for ultrasensitive detection of DNA hybridisation. Adv. Mater. 2014, 26, 4838–4844. [Google Scholar] [CrossRef]
- Li, L.; Li, X.M.; Du, M.D.; Guo, Y.C.; Li, Y.C.; Li, H.B.; Yang, Y.; Alam, F.E.; Lin, C.-T.; Fang, Y. Solid-phase coalescence of electrochemically exfoliated graphene flakes into a continuous film on copper. Chem. Mater. 2016, 28, 3360–3366. [Google Scholar] [CrossRef]
- Yuan, Q.L.; Wu, S.D.; Ye, C.; Liu, X.Q.; Gao, J.Y.; Cui, N.Y.; Guo, P.; Lai, G.S.; Wei, Q.P.; Yang, M.H.; et al. Sensitivity enhancement of potassium ion (K+) detection based on graphene field-effect transistors with surface plasma pretreatment. Sens. Actuators B 2019, 285, 333–340. [Google Scholar] [CrossRef]
- Zhai, H.Y.; Liang, Z.X.; Chen, Z.G.; Wang, H.H.; Liu, Z.P.; Su, Z.H.; Zhou, Q. Simultaneous detection of metronidazole and chloramphenicol by differential pulse stripping voltammetry using a silver nanoparticles/sulfonate functionalized graphene modified glassy carbon electrode. Electrochim. Acta 2015, 171, 105–113. [Google Scholar] [CrossRef]
- Borowiec, J.; Wang, R.; Zhu, L.; Zhang, J.D. Synthesis of nitrogen-doped graphene nanosheets decorated with gold nanoparticles as an improved sensor for electrochemical determination of chloramphenicol. Electrochim. Acta 2013, 99, 138–144. [Google Scholar] [CrossRef]
- Zhang, C.; Govindaraju, S.; Giribabu, K.; Huh, Y.S.; Yun, K. AgNWs-PANI nanocomposite based electrochemical sensor for detection of 4-nitrophenol. Sens. Actuators B 2017, 252, 616–623. [Google Scholar] [CrossRef]
- Schoen, D.T.; Schoen, A.P.; Hu, L.B.; Kim, H.S.; Heilshorn, S.C.; Cui, Y. High Speed Water Sterilization Using One-Dimensional Nanostructures. Nano Lett. 2010, 10, 3628–3632. [Google Scholar] [CrossRef]
- Yuan, Q.L.; Liu, Y.; Ye, C.; Sun, H.Y.; Dai, D.; Wei, Q.P.; Lai, G.S.; Wu, T.Z.; Yu, A.M.; Fu, L.; et al. Highly stable and regenerative graphene-diamond hybrid electrochemical biosensor for fouling target dopamine detection. Biosens. Bioelectron. 2018, 111, 117–123. [Google Scholar] [CrossRef]
- Yia, W.W.; Lia, Z.Q.; Dong, C.; Li, H.W.; Li, J.F. Electrochemical detection of chloramphenicol using palladium nanoparticles decorated reduced graphene oxide. Microchem. J. 2019, 148, 774–783. [Google Scholar] [CrossRef]
- Yadav, M.; Ganesan, V.; Gupta, R.; Yadav, D.K.; Sonkar, P.K. Cobalt oxide nanocrystals anchored on graphene sheets for electrochemical determination of chloramphenicol. Microchem. J. 2019, 146, 881–887. [Google Scholar] [CrossRef]
- Viliana, A.T.E.; Ohb, S.Y.; Rethinasabapathy, M.; Umapathi, R.; Hwang, S.K.; Oh, C.W.; Park, B.; Huh, Y.S.; Han, Y.K. Improved conductivity of flower-like MnWO4 on defect engineered graphitic carbon nitride as an efficient electrocatalyst for ultrasensitive sensing of chloramphenicol. J. Hazard. Mater. 2020, 399, 122868. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidou, K.; Brasinika, D.; Trompeta, A.F.A.; Bergamaschi, E.; Karoussis, I.K.; Charitidis, C.A. In vitro cytotoxicity assessment of pristine and carboxyl-functionalized MWCNTs. Food Chem. Toxicol. 2020, 141, 111374. [Google Scholar] [CrossRef] [PubMed]
- Naseri, A.; Hormozi-Nezhad, M.R.; Shahrokhian, S.; Asadian, E. Silver nanowires immobilized on gold-modified glassy carbon electrode for electrochemical quantification of atorvastatin. J. Electroanal. Chem. 2020, 876, 114540. [Google Scholar] [CrossRef]
- Salam, M.A.; Burk, R. Synthesis and characterization of multi-walled carbon nanotubes modified with octadecylamine and polyethylene glycol. Arab. J. Chem. 2017, 10, S921–S927. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, F.; Tu, X.L.; Ma, X.; Xu, Q.; Dai, R.Y.; Huang, X.G.; Yu, Y.F.; Lu, L.M. Facile synthesis of MXene/electrochemically reduced graphene oxide composites and their application for electrochemical sensing of carbendazim. J. Electrochem. Soc. 2019, 166, B1673–B1680. [Google Scholar] [CrossRef]
- Mani, V.; Dinesh, B.; Chen, S.M.; Saraswathi, R. Direct electrochemistry of myoglobin at reduced graphene oxide-multiwalled carbon nanotubes-platinum nanoparticles nanocomposite and biosensing towards hydrogen peroxide and nitrite. Biosens. Bioelectron. 2014, 53, 420–427. [Google Scholar] [CrossRef]
- Baikeli, Y.; Mamat, X.; He, F.; Xin, X.L.; Li, Y.T.; Aisa, H.A.; Hu, G.Z. Electrochemical determination of chloramphenicol and metronidazole by using a glassy carbon electrode modified with iron, nitrogen co-doped nanoporous carbon derived from a metal-organic framework (type Fe/ZIF-8). Ecotox. Environ. Safe 2020, 204, 111066. [Google Scholar] [CrossRef]
- Meenakshi, S.; Sophia, S.J.; Pandian, K. High surface graphene nanoflakes as sensitive sensing platform for simultaneous electrochemical detection of metronidazole and chloramphenicol. Mat. Sci. Eng. C 2018, 90, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Luo, Y.; Zhang, J.W.; Wang, J.Y.; Li, W.W.; Wang, W. Facile synthesis of reduced graphene oxide supported Pt-Pd nanocubes with enhanced electrocatalytic activity for chloramphenicol determination. J. Electroanal. Chem. 2016, 781, 389–394. [Google Scholar] [CrossRef]
- Kaewnu, K.; Promsuwan, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. A simple and sensitive electrochemical sensor for chloramphenicol detection in pharmaceutical samples. J. Electrochem. Soc. 2020, 167, 087506. [Google Scholar] [CrossRef]
- Xiao, L.L.; Xu, R.Y.; Yuan, Q.H.; Wang, F. Highly sensitive electrochemical sensor for chloramphenicol based on MOF derived exfoliated porous carbon. Talanta 2017, 167, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, M.; Angeloni, R.; Martini, E.; Castrucci, M.; Campanella, L. Enzymatic DMFC device used for direct analysis of chloramphenicol and a comparison with the competitive immunosensor method. Sens. Actuators B 2018, 255, 1545–1552. [Google Scholar] [CrossRef]


| Modified Electrodes | Measurements | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| Chemical synthesis | ||||
| CNTs/CuNPs | CV | 10–500 | 10 | [27] |
| N-doped Graphene/AuNPs | LSV a | 2.0–80 | 0.59 | [33] |
| rGO/ Co3O4 | CV | 1.0–2000 | 0.55 | [36] |
| rGO/ ZnO | LSV a | 0.19–2847 | 0.13 | [24] |
| rGO/ Pt-Pd nanocubes | LSV a | 0.20–30 | 0.10 | [47] |
| rGO/PdNPs | DPV | 0.05–1.0 | 0.05 | [35] |
| Graphene/AgNPs | DPV | 0.02–20 | 0.01 | [32] |
| Poly (Eriochrome black T) | SWV b | 0.01–4.0 | 0.003 | [48] |
| Exfoliated porous carbon | SWV b | 0.01–1.0 | 0.003 | [49] |
| CNTs/Molecularly imprinted polymer | DPV | 0.005–4.0 | 0.0001 | [1] |
| Enzymatic method | ||||
| Alcohol dehydrogenase | Amperometry | 3–5000 | 1 | [50] |
| Physical blending | ||||
| AuNPs/GO | Amperometry | 1.5–2.95 | 0.25 | [5] |
| CNTs/AgNWs | DPV | 0.1–100 | 0.08 | This work |
| Samples | Added (μM) | Founded (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Tap water | 0.100 | 0.109 | 1.48 | 109 |
| 1.00 | 0.983 | 4.55 | 98.3 | |
| River water | 0.100 | 0.111 | 3.03 | 111 |
| 1.00 | 1.08 | 4.88 | 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Li, X.; Xu, Y.; Wu, L.; Yu, A.; Lai, G.; Wei, Q.; Chi, H.; Jiang, N.; Fu, L.; et al. Intertwined Carbon Nanotubes and Ag Nanowires Constructed by Simple Solution Blending as Sensitive and Stable Chloramphenicol Sensors. Sensors 2021, 21, 1220. https://doi.org/10.3390/s21041220
Zhu Y, Li X, Xu Y, Wu L, Yu A, Lai G, Wei Q, Chi H, Jiang N, Fu L, et al. Intertwined Carbon Nanotubes and Ag Nanowires Constructed by Simple Solution Blending as Sensitive and Stable Chloramphenicol Sensors. Sensors. 2021; 21(4):1220. https://doi.org/10.3390/s21041220
Chicago/Turabian StyleZhu, Yangguang, Xiufen Li, Yuting Xu, Lidong Wu, Aimin Yu, Guosong Lai, Qiuping Wei, Hai Chi, Nan Jiang, Li Fu, and et al. 2021. "Intertwined Carbon Nanotubes and Ag Nanowires Constructed by Simple Solution Blending as Sensitive and Stable Chloramphenicol Sensors" Sensors 21, no. 4: 1220. https://doi.org/10.3390/s21041220
APA StyleZhu, Y., Li, X., Xu, Y., Wu, L., Yu, A., Lai, G., Wei, Q., Chi, H., Jiang, N., Fu, L., Ye, C., & Lin, C.-T. (2021). Intertwined Carbon Nanotubes and Ag Nanowires Constructed by Simple Solution Blending as Sensitive and Stable Chloramphenicol Sensors. Sensors, 21(4), 1220. https://doi.org/10.3390/s21041220










