Association between Oxygen Consumption and Surface Electromyographic Amplitude and Its Variation within Individual Calf Muscles during Walking at Various Speeds
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Design
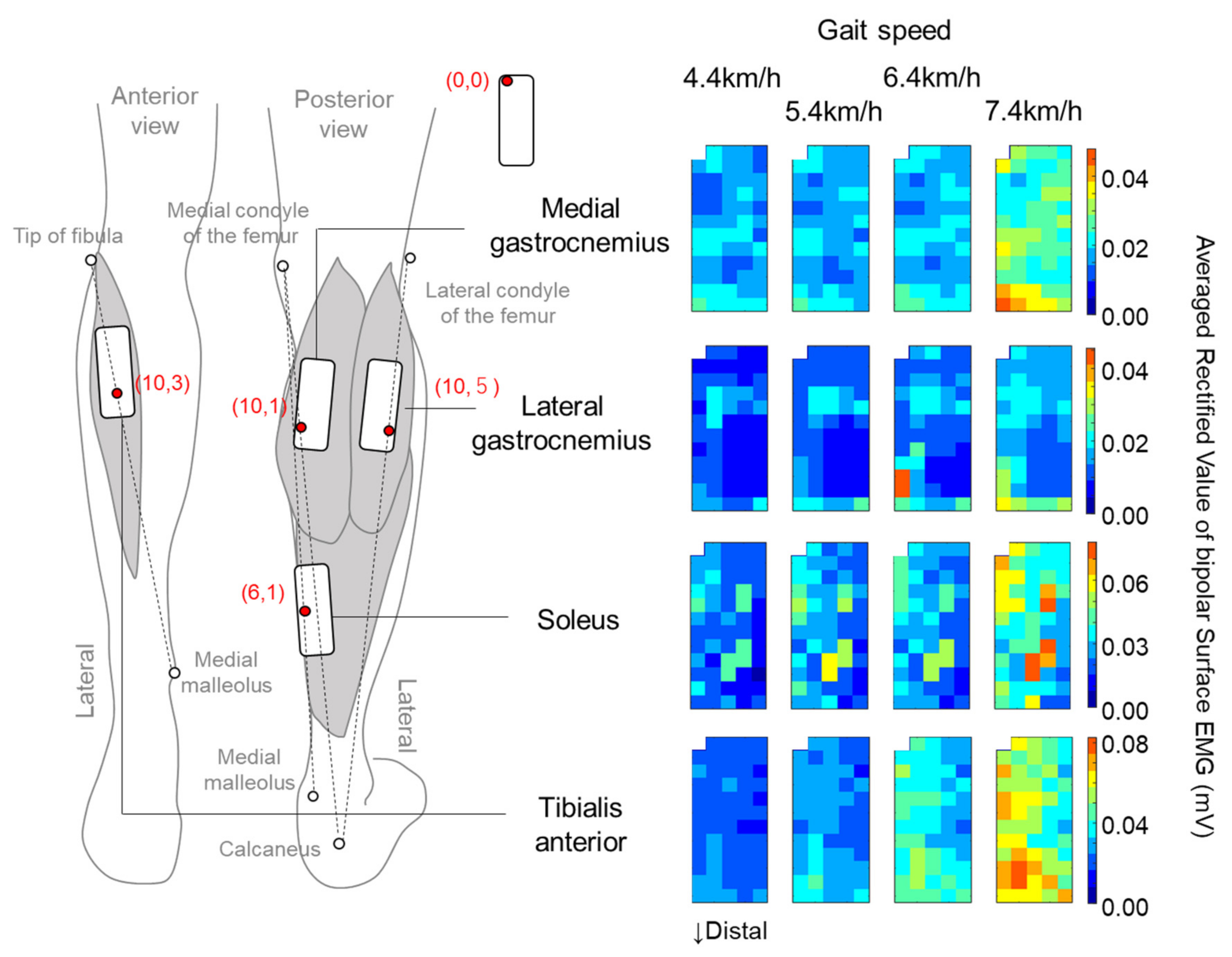
2.3. Surface EMG Recording
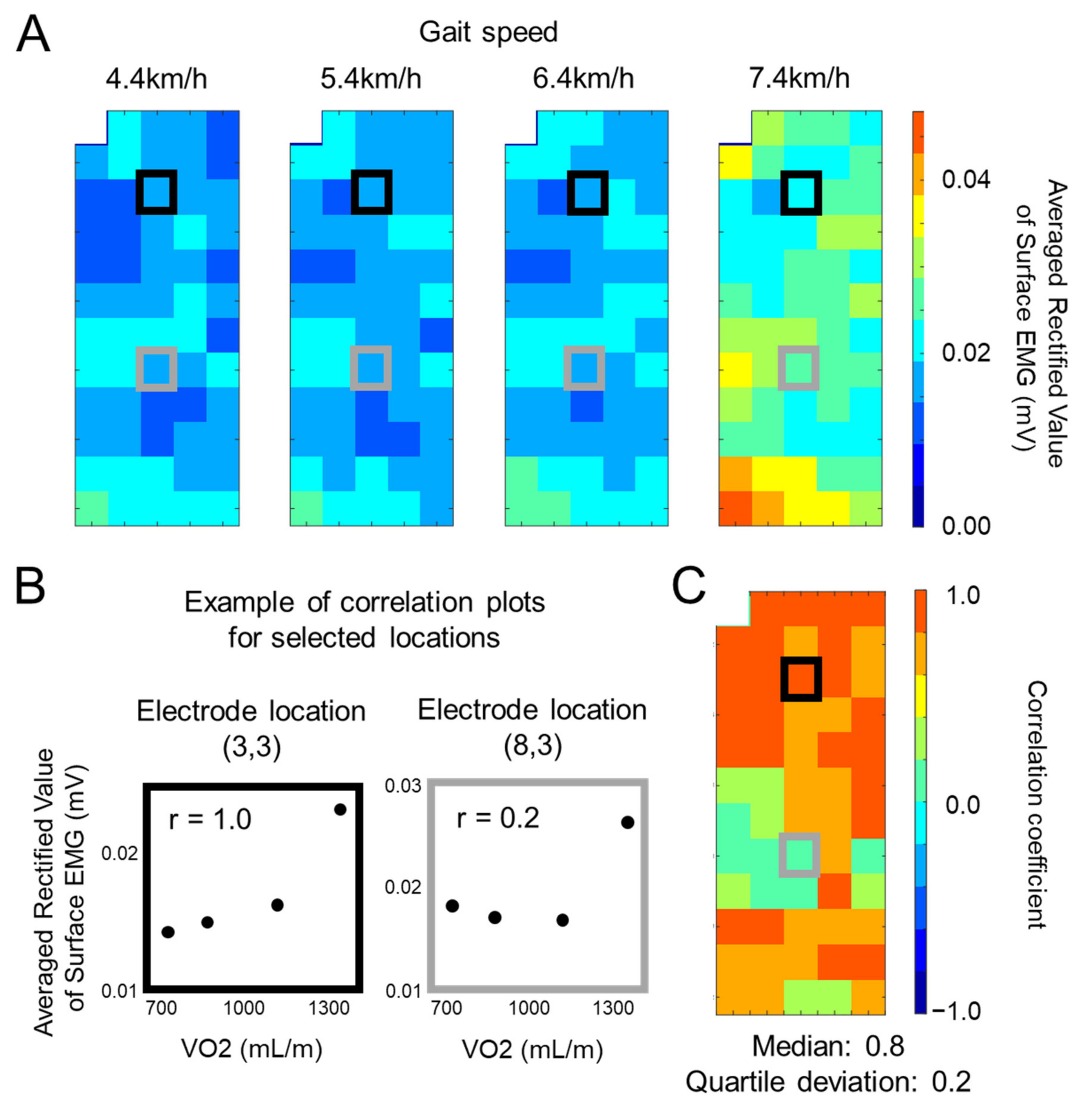
2.4. Data Analysis and Statistics
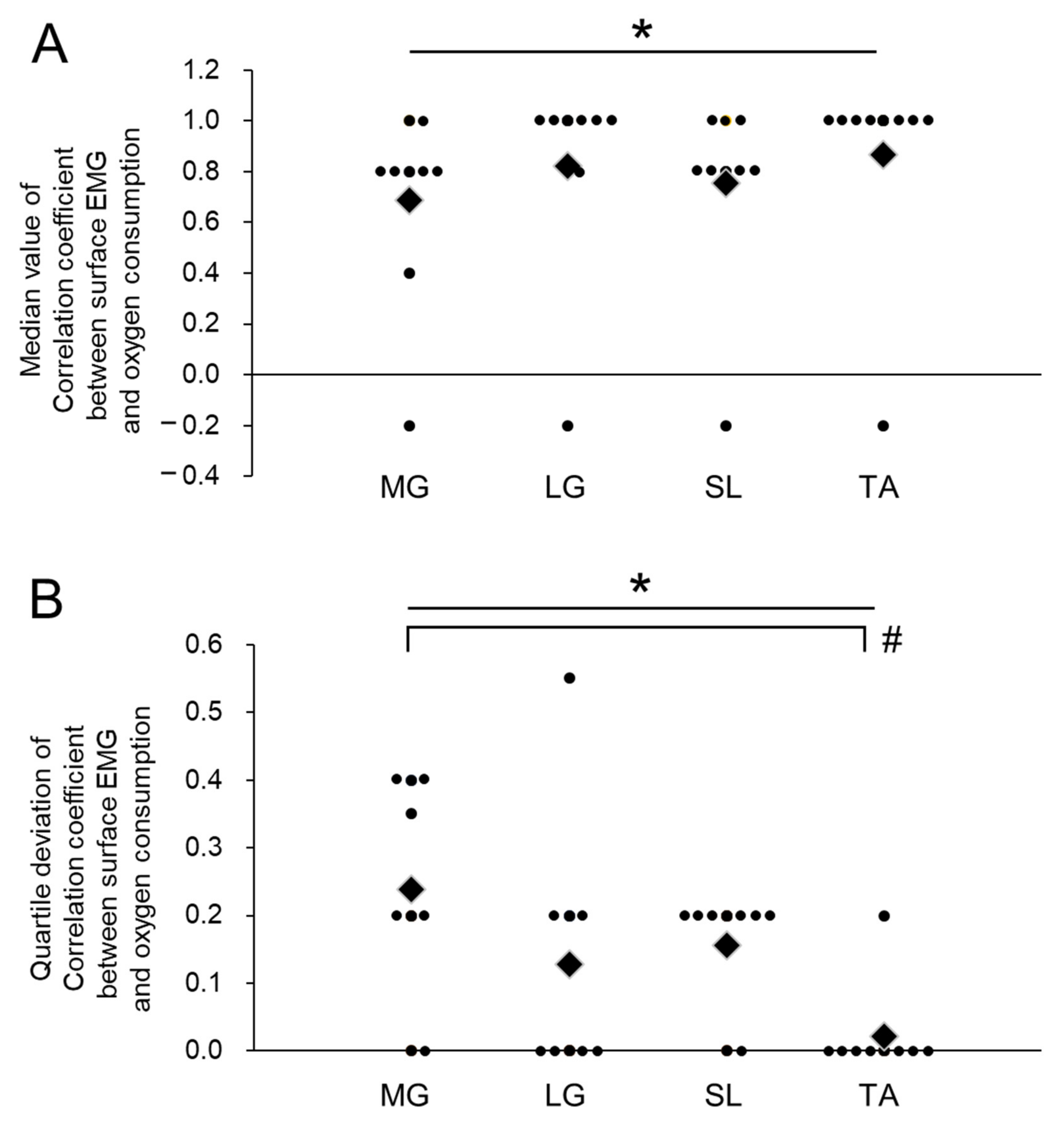
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARV | Averaged rectified value |
| EMG | Electromyography |
| LG | Lateral gastrocnemius |
| MG | Medial gastrocnemius |
| SL | Soleus |
| TA | Tibialis anterior |
| O2 | Oxygen consumption |
References
- Benson, L.C.; Clermont, C.A.; Bosnjak, E.; Ferber, R. The use of wearable devices for walking and running gait analysis outside of the lab: A systematic review. Gait Posture 2018, 63, 124–138. [Google Scholar] [CrossRef]
- Chehab, E.F.; Andriacchi, T.P.; Favre, J. Speed, age, sex, and body mass index provide a rigorous basis for comparing the kinematic and kinetic profiles of the lower extremity during walking. J. Biomech. 2017, 58, 11–20. [Google Scholar] [CrossRef]
- Colberg, S.R.; Albright, A.L.; Blissmer, B.J.; Braun, B.; Chasan-Taber, L.; Fernhall, B.; Regensteiner, J.G.; Rubin, R.R.; Sigal, R.J. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: Joint position statement. Exercise and type 2 diabetes. Med. Sci. Sports Exerc. 2010, 42, 2282–2303. [Google Scholar] [CrossRef]
- DeVita, P.; Hortobagyi, T. Age causes a redistribution of joint torques and powers during gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef]
- Farina, D.; Cescon, C.; Merletti, R. Influence of anatomical, physical, and detection-system parameters on surface EMG. Biol. Cybern. 2002, 86, 445–456. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry Electrodes for Human Bioelectrical Signal Monitoring. Sensors (Basel) 2020, 20, 3651. [Google Scholar] [CrossRef]
- Gallina, A.; Merletti, R.; Vieira, T.M. Are the myoelectric manifestations of fatigue distributed regionally in the human medial gastrocnemius muscle? J. Electromyogr. Kinesiol. 2011, 21, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, J.S.; Kram, R. Energy cost and muscular activity required for propulsion during walking. J. Appl. Physiol. 2003, 94, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Freriks, B.; Hermens, H. European Recommendations for Surface ElectroMyoGraphy, Results of the SENIAM Project; (CD-ROM); Roessingh Research and Development: Enschede, The Netherlands, 2000. [Google Scholar]
- Hodson-Tole, E.F.; Loram, I.D.; Vieira, T.M. Myoelectric activity along human gastrocnemius medialis: Different spatial distributions of postural and electrically elicited surface potentials. J. Electromyogr. Kinesiol. 2013, 23, 43–50. [Google Scholar] [CrossRef]
- Hof, A.L.; Elzinga, H.; Grimmius, W.; Halbertsma, J.P. Speed dependence of averaged EMG profiles in walking. Gait Posture 2002, 16, 78–86. [Google Scholar] [CrossRef]
- Hulley, S.; Cummings, S.; Browner, W.; Grady, D.; Newman, T. Designing Clinical Research; Wolter Kluwer: Philadelphia, PA, USA, 2013. [Google Scholar]
- Ingraham, K.A.; Ferris, D.P.; Remy, C.D. Evaluating physiological signal salience for estimating metabolic energy cost from wearable sensors. J. Appl. Physiol. 2019, 126, 717–729. [Google Scholar] [CrossRef]
- McGibbon, C.A. Toward a better understanding of gait changes with age and disablement: Neuromuscular adaptation. Exerc. Sport Sci. Rev. 2003, 31, 102–108. [Google Scholar] [CrossRef]
- Merletti, R.; Botter, A.; Troiano, A.; Merlo, E.; Minetto, M.A. Technology and instrumentation for detection and conditioning of the surface electromyographic signal: State of the art. Clin. Biomech. (BristolAvon) 2009, 24, 122–134. [Google Scholar] [CrossRef]
- Mesin, L.; Merletti, R.; Vieira, T.M. Insights gained into the interpretation of surface electromyograms from the gastrocnemius muscles: A simulation study. J. Biomech. 2011, 44, 1096–1103. [Google Scholar] [CrossRef]
- Moore, I.S.; Willy, R.W. Use of Wearables: Tracking and Retraining in Endurance Runners. Curr. Sports Med. Rep. 2019, 18, 437–444. [Google Scholar] [CrossRef]
- Neptune, R.R.; Kautz, S.A.; Zajac, F.E. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J. Biomech. 2001, 34, 1387–1398. [Google Scholar] [CrossRef]
- Nose, H.; Morikawa, M.; Yamazaki, T.; Nemoto, K.; Okazaki, K.; Masuki, S.; Kamijo, Y.; Gen-No, H. Beyond epidemiology: Field studies and the physiology laboratory as the whole world. J. Physiol. 2009, 587, 5569–5575. [Google Scholar] [CrossRef]
- Oi, N.; Iwaya, T.; Itoh, M.; Yamaguchi, K.; Tobimatsu, Y.; Fujimoto, T. FDG-PET imaging of lower extremity muscular activity during level walking. J. Orthop. Sci. 2003, 8, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.O.; Della Croce, U.; Kerrigan, D.C. Propulsive adaptation to changing gait speed. J. Biomech. 2001, 34, 197–202. [Google Scholar] [CrossRef]
- Schmitz, A.; Silder, A.; Heiderscheit, B.; Mahoney, J.; Thelen, D.G. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J. Electromyogr. Kinesiol. 2009, 19, 1085–1091. [Google Scholar] [CrossRef]
- Silder, A.; Heiderscheit, B.; Thelen, D.G. Active and passive contributions to joint kinetics during walking in older adults. J. Biomech. 2008, 41, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Staudenmann, D.; Kingma, I.; Daffertshofer, A.; Stegeman, D.F.; van Dieen, J.H. Heterogeneity of muscle activation in relation to force direction: A multi-channel surface electromyography study on the triceps surae muscle. J. Electromyogr. Kinesiol. 2009, 19, 882–895. [Google Scholar] [CrossRef]
- Sutherland, D.H. The evolution of clinical gait analysis part l: Kinesiological EMG. Gait Posture 2001, 14, 61–70. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 1, 19–25. [Google Scholar]
- van Hedel, H.J.; Tomatis, L.; Muller, R. Modulation of leg muscle activity and gait kinematics by walking speed and bodyweight unloading. Gait Posture 2006, 24, 35–45. [Google Scholar] [CrossRef]
- Vieira, T.M.; Botter, A. The Accurate Assessment of Muscle Excitation Requires the Detection of Multiple Surface Electromyograms. Exerc. Sport Sci. Rev. 2021, 49. [Google Scholar] [CrossRef]
- Vieira, T.M.; Botter, A.; Minetto, M.A.; Hodson-Tole, E.F. Spatial variation of compound muscle action potentials across human gastrocnemius medialis. J. Neurophysiol. 2015, 114, 1617–1627. [Google Scholar] [CrossRef]
- Vieira, T.M.; Loram, I.D.; Muceli, S.; Merletti, R.; Farina, D. Postural activation of the human medial gastrocnemius muscle: Are the muscle units spatially localised? J. Physiol. (Lond.) 2011, 589, 431–443. [Google Scholar] [CrossRef]
- Vieira, T.M.; Merletti, R.; Mesin, L. Automatic segmentation of surface EMG images: Improving the estimation of neuromuscular activity. J. Biomech. 2010, 43, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Taniguchi, Y.; Moritani, T. Metabolic and cardiovascular responses during voluntary pedaling exercise with electrical muscle stimulation. Eur. J. Appl. Physiol. 2014, 114, 1801–1807. [Google Scholar] [CrossRef]
- Winter, D.A.; Yack, H.J. EMG profiles during normal human walking: Stride-to-stride and inter-subject variability. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Yang, J.F.; Winter, D.A. Surface EMG profiles during different walking cadences in humans. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 485–491. [Google Scholar] [CrossRef]
- Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Biomechanics and muscle coordination of human walking. Part I: Introduction to concepts, power transfer, dynamics and simulations. Gait Posture 2002, 16, 215–232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, K.; Narouei, S. Association between Oxygen Consumption and Surface Electromyographic Amplitude and Its Variation within Individual Calf Muscles during Walking at Various Speeds. Sensors 2021, 21, 1748. https://doi.org/10.3390/s21051748
Watanabe K, Narouei S. Association between Oxygen Consumption and Surface Electromyographic Amplitude and Its Variation within Individual Calf Muscles during Walking at Various Speeds. Sensors. 2021; 21(5):1748. https://doi.org/10.3390/s21051748
Chicago/Turabian StyleWatanabe, Kohei, and Shideh Narouei. 2021. "Association between Oxygen Consumption and Surface Electromyographic Amplitude and Its Variation within Individual Calf Muscles during Walking at Various Speeds" Sensors 21, no. 5: 1748. https://doi.org/10.3390/s21051748
APA StyleWatanabe, K., & Narouei, S. (2021). Association between Oxygen Consumption and Surface Electromyographic Amplitude and Its Variation within Individual Calf Muscles during Walking at Various Speeds. Sensors, 21(5), 1748. https://doi.org/10.3390/s21051748






