Characterization of Pelvic Floor Activity in Healthy Subjects and with Chronic Pelvic Pain: Diagnostic Potential of Surface Electromyography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database Composition
2.2. sEMG Signal Recording
2.3. sEMG Signal Conditioning
- (1)
- 5 segments of 5 s of single contractions, one for each maximum voluntary contraction performed by the PFM.
- (2)
- 1 segment of 10 s of basal activity before the first contraction, when the PFM shows a maximum state of relaxation [35].
- (3)
- 1 segment of 67 s from 1 s before the first contraction to 1 s after the last contraction, including the 5 contractions and the 4 resting periods.
2.4. sEMG Signal Parametrization
2.5. Statistical Analysis
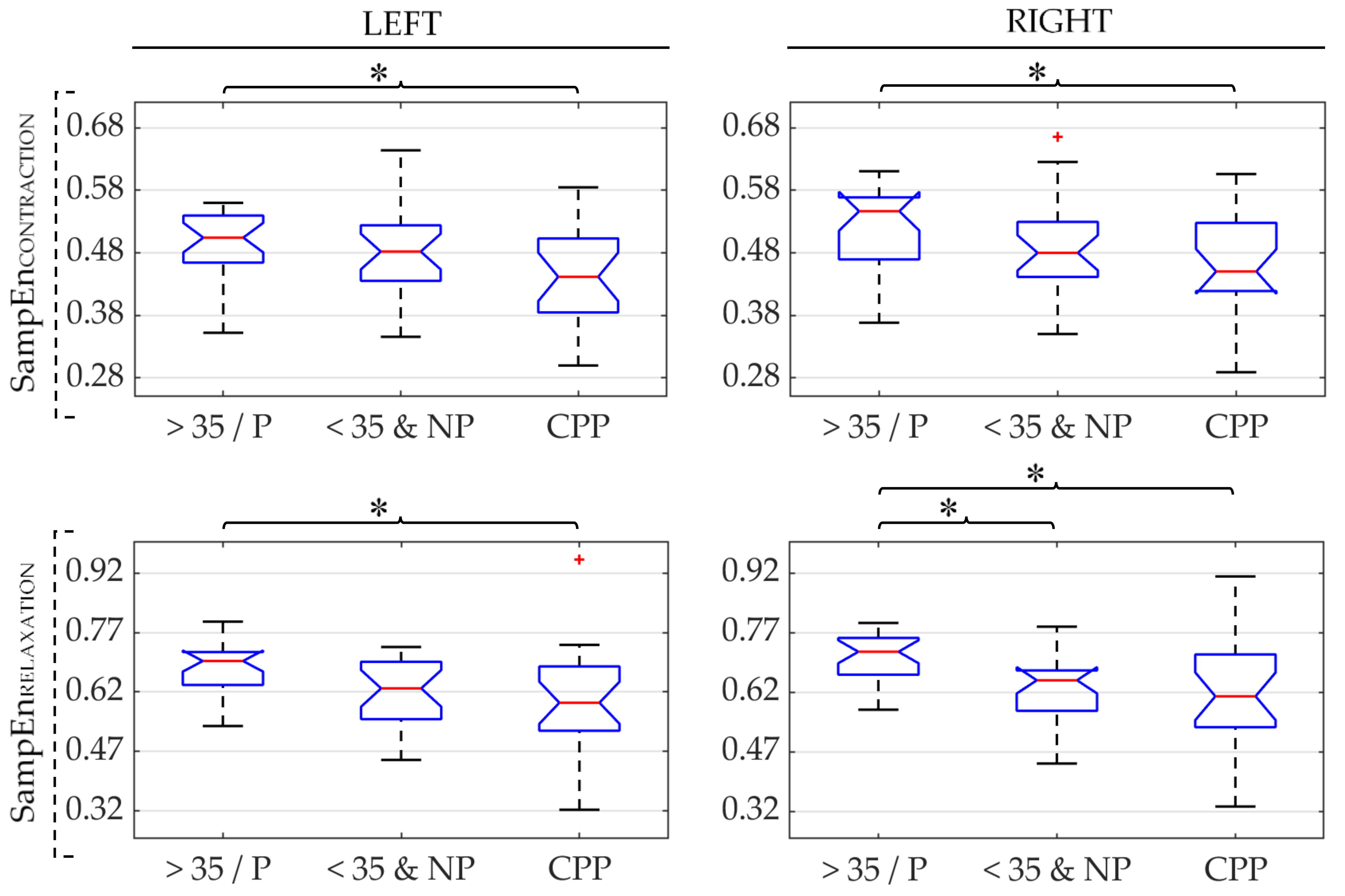
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fall, M.; Baranowski, A.P.; Elneil, S.; Engeler, D.; Hughes, J.; Messelink, E.J.; Oberpenning, F.; de Williams, A.C. EAU guidelines on chronic pelvic pain. Eur. Urol. 2010, 57, 35–48. [Google Scholar] [CrossRef]
- Hatzimouratidis, K.; Hatzichristou, D. Sexual dysfunctions: Classifications and definitions. J. Sex. Med. 2007, 4, 241–250. [Google Scholar] [CrossRef]
- Orr, N.; Wahl, K.; Joannou, A.; Hartmann, D.; Valle, L.; Yong, P.; Babb, C.; Kramer, C.W.; Kellogg-Spadt, S.; Renzelli-Cain, R.I. Deep Dyspareunia: Review of Pathophysiology and Proposed Future Research Priorities. Sex. Med. Rev. 2020, 8, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Latthe, P.; Latthe, M.; Say, L.; Gülmezoglu, M.; Khan, K.S. WHO systematic review of prevalence of chronic pelvic pain: A neglected reproductive health morbidity. BMC Public Health 2006, 6, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, K.R.; Geary, R.; Graham, C.A.; Datta, J.; Wellings, K.; Sonnenberg, P.; Field, N.; Nunns, D.; Bancroft, J.; Jones, K.G.; et al. Painful sex (dyspareunia) in women: Prevalence and associated factors in a British population probability survey. BJOG 2017, 124, 1689–1697. [Google Scholar] [CrossRef] [Green Version]
- West, S.L.; Vinikoor, L.C.; Zolnoun, D. A systematic review of the literature on female sexual dysfunction prevalence and predictors. Annu. Rev. Sex Res. 2004, 15, 40–172. [Google Scholar] [PubMed]
- Hayes, R.D.; Bennett, C.M.; Dennerstein, L.; Taffe, J.R.; Fairley, C.K. Are aspects of study design associated with the reported prevalence of female sexual difficulties? Fertil. Steril. 2008, 90, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Stones, R.W.; Selfe, S.A.; Fransman, S.; Horn, S.A. Psychosocial and economic impact of chronic pelvic pain. Bailliere’s Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Thavorn, K.; Shen, M.; Goddard, Y.; Yong, P.; MacRae, G.S.; Nishi, C.; Matar, A.; Allaire, C. Hospital-associated Costs of Chronic Pelvic Pain in Canada: A Population-based Descriptive Study. J. Obstet. Gynaecol. Canada 2017, 39, 174–180. [Google Scholar] [CrossRef]
- Oshinowo, A.; Ionescu, A.; Anim, T.E.; Lamvu, G. Dyspareunia and Vulvodynia. In Pelvic Pain Management; Valovska, A.T., Ed.; Oxford University Press: New York, NY, USA, 2016; pp. 44–57. [Google Scholar]
- Armour, M.; Sinclair, J.; Ng, C.H.M.; Hyman, M.S.; Lawson, K.; Smith, C.A.; Abbott, J. Endometriosis and chronic pelvic pain have similar impact on women, but time to diagnosis is decreasing: An Australian survey. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Fleshman, J.; Palkó, A.; Sand, P.K. Pelvic Floor Disorders: Imaging and Multidisciplinary Approach to Management; Santoro, G.A., Wieczorek, A.P., Bartram, C.I., Eds.; Springer: Milano, Italy, 2010; ISBN 9788847015418. [Google Scholar]
- McLean, L.; Brooks, K. What Does Electromyography Tell Us About Dyspareunia? Sex. Med. Rev. 2017, 5, 282–294. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Mateus-Vasconcelos, E.C.L.; da Silva, T.D.; de Oliveira Brito, L.G.; de Oliveira, H.F. Functional assessment of the pelvic floor muscles by electromyography: Is there a normalization in data analysis? A systematic review. Fisioter. Pesqui. 2018, 25, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Konrad, P. The ABC of EMG—A Practical Introduction to Kinesiological Electromyography; Version 1.0.; Noraxon Inc.: Scottsdale, AZ, USA, 2005; ISBN 0977162214. [Google Scholar]
- White, G.; Jantos, M.; Glazer, H. Establishing the diagnosis of vulvar vestibulitis. J. Reprod. Med. Obstet. Gynecol. 1997, 42, 157–160. [Google Scholar]
- Glazer, H.I.; Jantos, M.; Hartmann, E.H.; Swencionis, C. Electromyographic comparisons of the pelvic floor in women with dysesthetic vulvodynia and asymptomatic women. J. Reprod. Med. Obstet. Gynecol. 1998, 43, 959–962. [Google Scholar]
- Gentilcore-Saulnier, E.; McLean, L.; Goldfinger, C.; Pukall, C.F.; Chamberlain, S. Pelvic floor muscle assessment outcomes in women with and without provoked vestibulodynia and the impact of a physical therapy program. J. Sex. Med. 2010, 7, 1003–1022. [Google Scholar] [CrossRef] [PubMed]
- Engman, M.; Lindehammar, H.; Wijma, B. Surface electromyography diagnostics in women with partial vaginismus with or without vulvar vestibulitis and in asymptomatic women. J. Psychosom. Obstet. Gynecol. 2004, 25, 281–294. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef] [Green Version]
- González-Izal, M.; Malanda, A.; Gorostiaga, E.; Izquierdo, M. Electromyographic models to assess muscle fatigue. J. Electromyogr. Kinesiol. 2012, 22, 501–512. [Google Scholar] [CrossRef]
- Rampichini, S.; Vieira, T.M.; Castiglioni, P.; Merati, G. Complexity analysis of surface electromyography for assessing the myoelectric manifestation of muscle fatigue: A review. Entropy 2020, 22, 529. [Google Scholar] [CrossRef]
- Sung, P.S.; Zurcher, U.; Kaufman, M. Comparison of spectral and entropic measures for surface electromyography time series: A pilot study. J. Rehabil. Res. Dev. 2007, 44, 599–609. [Google Scholar] [CrossRef]
- Pereira, L.C.; Botelho, S.; Marques, J.; Adami, D.B.; Alves, F.K.; Palma, P.; Riccetto, C. Electromyographic pelvic floor activity: Is there impact during the female life cycle? Neurourol. Urodyn. 2016, 35, 230–234. [Google Scholar] [CrossRef]
- Bocardi, D.A.S.; Pereira-Baldon, V.S.; Ferreira, C.H.J.; Avila, M.A.; Beleza, A.C.S.; Driusso, P. Pelvic floor muscle function and EMG in nulliparous women of different ages: A cross-sectional study. Climacteric 2018, 21, 462–466. [Google Scholar] [CrossRef]
- Castro-Pardiñas, M.A.; Torres-Lacomba, M.; Navarro-Brazález, B. Muscle function of the pelvic floor in healthy and puerperal women and with pelvic floor dysfunction. Actas Urol. Esp. 2017, 41, 249–257. [Google Scholar] [CrossRef]
- Petricelli, C.D.; Resende, A.P.M.; Elito Júnior, J.; Araujo Júnior, E.; Alexandre, S.M.; Zanetti, M.R.D.; Nakamura, M.U. Distensibility and strength of the pelvic floor muscles of women in the third trimester of pregnancy. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Alves, F.K.; Riccetto, C.; Adami, D.B.V.; Marques, J.; Pereira, L.C.; Palma, P.; Botelho, S. A pelvic floor muscle training program in postmenopausal women: A randomized controlled trial. Maturitas 2015, 81, 300–305. [Google Scholar] [CrossRef]
- Pereira-Baldon, V.S.; Avila, M.A.; Dalarmi, C.B.; de Oliveira, A.B.; Driusso, P. Effects of different regimens for pelvic floor muscle training in young continent women: Randomized controlled clinical trial. J. Electromyogr. Kinesiol. 2019, 44, 31–35. [Google Scholar] [CrossRef]
- Sapsford, R.R.; Richardson, C.A.; Maher, C.F.; Hodges, P.W. Pelvic Floor Muscle Activity in Different Sitting Postures in Continent and Incontinent Women. Arch. Phys. Med. Rehabil. 2008, 89, 1741–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halski, T.; Ptaszkowski, K.; Słupska, L.; Dymarek, R.; Paprocka-Borowicz, M. Relationship between lower limb position and pelvic floor muscle surface electromyography activity in menopausal women: A prospective observational study. Clin. Interv. Aging 2017, 12, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K. Investigation of electromyographic activity of pelvic floor muscles in different body positions to prevent urinary incontinence. Med. Sci. Monit. 2019, 25, 9357–9363. [Google Scholar] [CrossRef]
- Micussi, M.T.; Freitas, R.P.; Angelo, P.H.; Soares, E.M.; Lemos, T.M.; Maranhão, T.M. Is there a difference in the electromyographic activity of the pelvic floor muscles across the phases of the menstrual cycle? J. Phys. Ther. Sci. 2015, 27, 2233–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, C.J.; Donald Gilmore, L.; Kuznetsov, M.; Roy, S.H. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Albaladejo-Belmonte, M.; Tarazona, M.; Nohales, F.J.; Alberola-Rubio, J.; Garcia-Casado, J. Influence of voluntary contractions on the basal sEMG activity of the pelvic floor muscles. In Proceedings of the Libro de Actas del XXXVIII Congreso Anual de la Sociedad Española de Ingeniería Biomédica, Valladolid, Spain, 25–27 November 2020; pp. 240–243. [Google Scholar]
- Merletti, R.; Farina, D. Surface Electromyography: Physiology, Engineering and Applications; Merletti, R., Farina, D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016; ISBN 9781119082934. [Google Scholar]
- Phinyomark, A.; Thongpanja, S.; Hu, H.; Phukpattaranont, P.; Limsakul, C. The Usefulness of Mean and Median Frequencies in Electromyography Analysis. In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; Naik, G.R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 195–220. [Google Scholar]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojić, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Tengku Zawawi, T.N.S.; Abdullah, A.R.; Jopri, M.H.; Sutikno, T.; Saad, N.M.; Sudirman, R. A review of electromyography signal analysis techniques for musculoskeletal disorders. Indones. J. Electr. Eng. Comput. Sci. 2018, 11, 1136–1146. [Google Scholar] [CrossRef]
- Arabadzhiev, T.I.; Dimitrov, G.V.; Dimitrova, N.A. Simulation analysis of the performance of a novel high sensitive spectral index for quantifying M-wave changes during fatigue. J. Electromyogr. Kinesiol. 2005, 15, 149–158. [Google Scholar] [CrossRef]
- González-Izal, M.; Malanda, A.; Navarro-Amézqueta, I.; Gorostiaga, E.M.; Mallor, F.; Ibañez, J.; Izquierdo, M. EMG spectral indices and muscle power fatigue during dynamic contractions. J. Electromyogr. Kinesiol. 2010, 20, 233–240. [Google Scholar] [CrossRef]
- Dimitrov, G.V.; Arabadzhiev, T.I.; Mileva, K.N.; Bowtell, J.L.; Crichton, N.; Dimitrova, N.A. Muscle fatigue during dynamic contractions assessed by new spectral indices. Med. Sci. Sports Exerc. 2006, 38, 1971–1979. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039-49. [Google Scholar] [CrossRef] [Green Version]
- Lake, D.E.; Richman, J.S.; Pamela Griffin, M.; Randall Moorman, J. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R789–R797. [Google Scholar] [CrossRef] [Green Version]
- Al-Angari, H.M.; Sahakian, A.V. Use of sample entropy approach to study heart rate variability in obstructive sleep apnea syndrome. IEEE Trans. Biomed. Eng. 2007, 54, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Jie, X.; Cao, R.; Li, L. Emotion recognition based on the sample entropy of EEG. Biomed. Mater. Eng. 2014, 24, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Duchene, J.; Goubel, F. Surface electromyogram during voluntary contraction: Processing tools and relation to physiological events. Crit. Rev. Biomed. Eng. 1993, 21, 313–397. [Google Scholar]
- Dias, N.; Zhang, C.; Spitznagle, T.; Lai, H.H.; Zhang, Y. High-Density Surface Electromyography Assessment of Pelvic Floor Dysfunction in Women with Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2020, 204, 1275–1283. [Google Scholar] [CrossRef]
- Knight, S.; Luft, J.; Nakagawa, S.; Katzman, W.B. Comparisons of pelvic floor muscle performance, anxiety, quality of life and life stress in women with dry overactive bladder compared with asymptomatic women. BJU Int. 2012, 109, 1685–1689. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore-Saulnier, E.; Auchincloss, C.; McLean, L. Electromyography. In The Overactive Pelvic Floor; Padoa, A., Rosenbaum, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–203. [Google Scholar]
- Ashton-Miller, J.A.; DeLancey, J.O.L. Functional anatomy of the female pelvic floor. Ann. N. Y. Acad. Sci. 2007, 1101, 266–296. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.B.; Hosker, G.L.; Warrell, D.W. The role of partial denervation of the pelvic floor in the aetiology of genitourinary prolapse and stress incontinence of urine. A neurophysiological study. BJOG 1989, 96, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, A.; Malvasi, A.; Rahimi, S.; Negro, R.; Vergara, D.; Martignago, R.; Pellegrino, M.; Cavallotti, C. Age-related pelvic floor modifications and prolapse risk factors in postmenopausal women. Menopause 2010, 17, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Kim, H.J. Electrophysiological characteristics according to activity level of myofascial trigger points. J. Phys. Ther. Sci. 2015, 27, 2841–2843. [Google Scholar] [CrossRef]
- Komemushi, Y.; Komemushi, A.; Morimoto, K.; Yoneda, Y.; Yoshimura, R.; Tanaka, T.; Katou, T.; Nakatani, T. Quantitative evaluation of age-related changes to pelvic floor muscles in magnetic resonance images from 369 patients. Geriatr. Gerontol. Int. 2019, 19, 834–837. [Google Scholar] [CrossRef]
- Narita, K.; Yamada, Y.; Yamada, M.; Yokoyama, Y.; Nakahara, T.; Jinzaki, M. Pelvic floor morphology in the standing position using upright computed tomography: Age and sex differences. Int. Urogynecol. J. 2020, 31, 2387–2393. [Google Scholar] [CrossRef]
- Swenson, C.W.; Masteling, M.; DeLancey, J.O.; Nandikanti, L.; Schmidt, P.; Chen, L. Aging effects on pelvic floor support: A pilot study comparing young versus older nulliparous women. Int. Urogynecol. J. 2020, 31, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Alperin, M.; Cook, M.; Tuttle, L.J.; Esparza, M.C.; Lieber, R.L. Impact of vaginal parity and aging on the architectural design of pelvic floor muscles. Am. J. Obstet. Gynecol. 2016, 215, 312.e1–312.e9. [Google Scholar] [CrossRef] [Green Version]
- Routzong, M.R.; Rostaminia, G.; Moalli, P.A.; Abramowitch, S.D. Pelvic floor shape variations during pregnancy and after vaginal delivery. Comput. Methods Programs Biomed. 2020, 194, 105516. [Google Scholar] [CrossRef] [PubMed]
- Quartly, E.; Hallam, T.; Kilbreath, S.; Refshauge, K. Strength and endurance of the pelvic floor muscles in continent women: An observational study. Physiotherapy 2010, 96, 311–316. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef] [Green Version]
- Dimpfl, T.; Jaeger, C.; Mueller-Felber, W.; Anthuber, C.; Hirsch, A.; Brandmaier, R.; Schuessler, B. Myogenic changes of the levator ani muscle in premenopausal women: The impact of vaginal delivery and age. Neurourol. Urodyn. 1998, 17, 197–205. [Google Scholar] [CrossRef]
- Cashaback, J.G.A.; Cluff, T.; Potvin, J.R. Muscle fatigue and contraction intensity modulates the complexity of surface electromyography. J. Electromyogr. Kinesiol. 2013, 23, 78–83. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Differential changes with age in multiscale entropy of electromyography signals from leg muscles during treadmill walking. PLoS ONE 2016, 11, e0162034. [Google Scholar] [CrossRef]
- Enck, P.; Vodušek, D.B. Electromyography of pelvic floor muscles. J. Electromyogr. Kinesiol. 2006, 16, 568–577. [Google Scholar] [CrossRef]
- Peng, Y.; He, J.; Khavari, R.; Boone, T.B.; Zhang, Y. Functional mapping of the pelvic floor and sphincter muscles from high-density surface EMG recordings. Int. Urogynecol. J. 2016, 27, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Dias, N.; Li, X.; Zhang, C.; Zhang, Y. Innervation asymmetry of the external anal sphincter in aging characterized from high-density intra-rectal surface EMG recordings. Neurourol. Urodyn. 2018, 37, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Wallner, C.; van Wissen, J.; Maas, C.P.; Dabhoiwala, N.F.; DeRuiter, M.C.; Lamers, W.H. The Contribution of the Levator Ani Nerve and the Pudendal Nerve to the Innervation of the Levator Ani Muscles; a Study in Human Fetuses. Eur. Urol. 2008, 54, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Začesta, V.; Rezeberga, D.; Plaudis, H.; Drusany-Staric, K.; Cescon, C. Could the correct side of mediolateral episiotomy be determined according to anal sphincter EMG? Int. Urogynecol. J. 2018, 29, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Sörnmo, L.; Laguna, P. Electrocardiogram (ECG) Signal Processing. In Wiley Encyclopedia of Biomedical Engineering; Akay, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Aljuraifani, R.; Stafford, R.E.; Hall, L.M.; Hodges, P.W. Activity of Deep and Superficial Pelvic Floor Muscles in Women in Response to Different Verbal Instructions: A Preliminary Investigation Using a Novel Electromyography Electrode. J. Sex. Med. 2019, 16, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Jundt, K.; Kiening, M.; Fischer, P.; Bergauer, F.; Rauch, E.; Janni, W.; Peschers, U.; Dimpfl, T. Is the histomorphological concept of the female pelvic floor and its changes due to age and vaginal delivery correct? Neurourol. Urodyn. 2005, 24, 44–50. [Google Scholar] [CrossRef]
- Kaya, S.; Hermans, L.; Willems, T.; Roussel, N.; Meeus, M. Central sensitization in urogynecological chronic pelvic pain: A systematic literature review. Pain Physician 2013, 16, 291–308. [Google Scholar] [CrossRef]

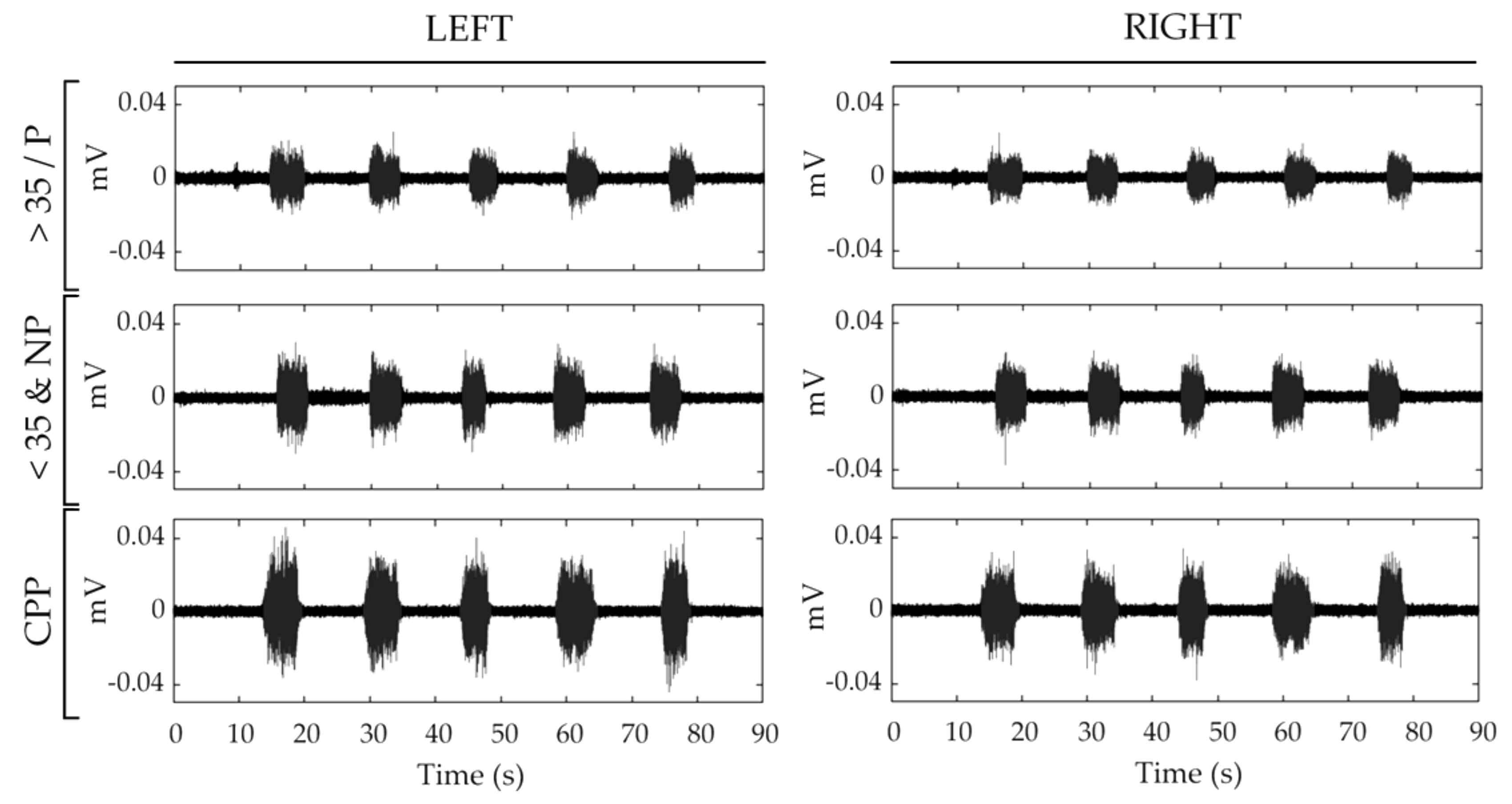
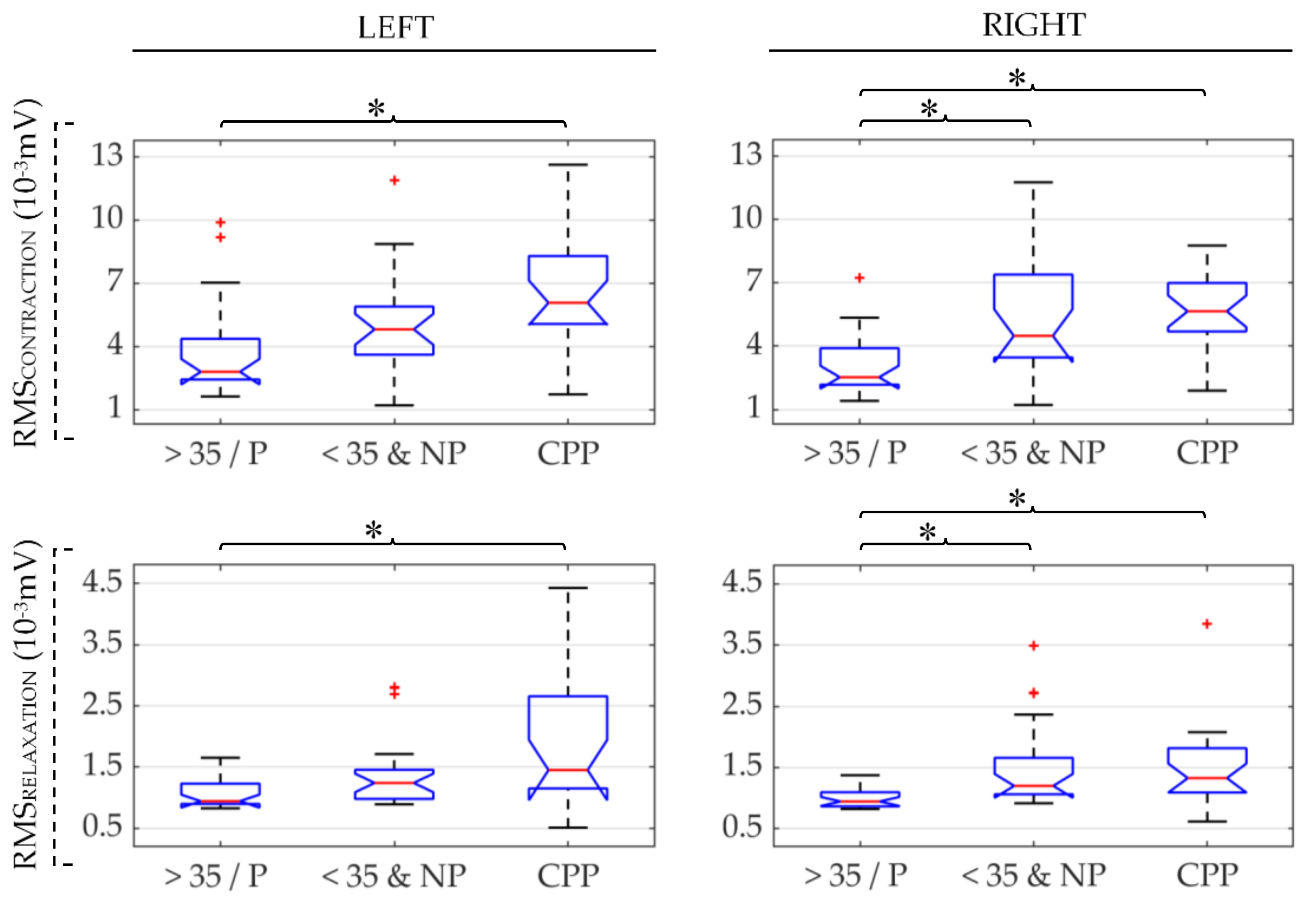
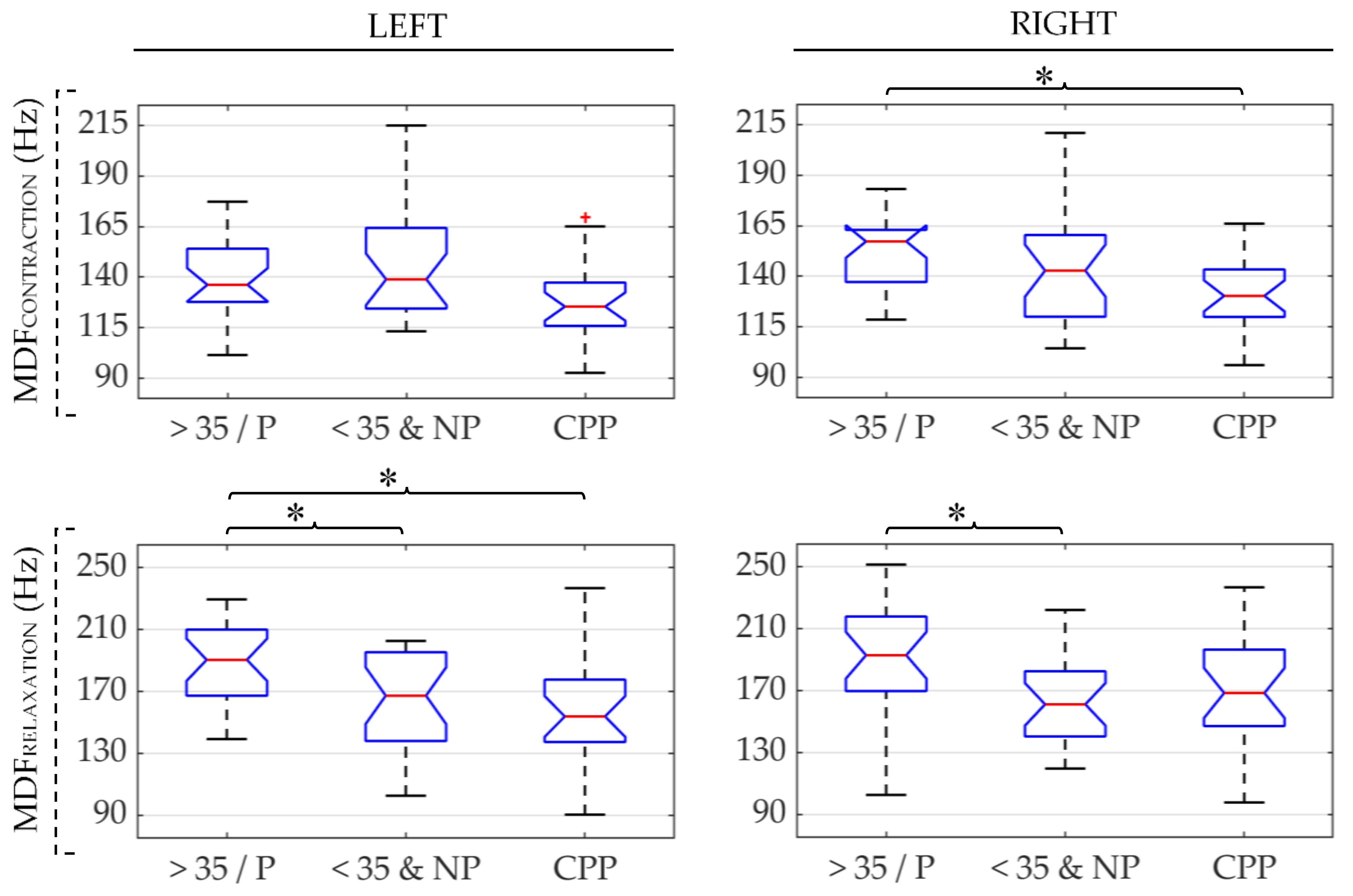
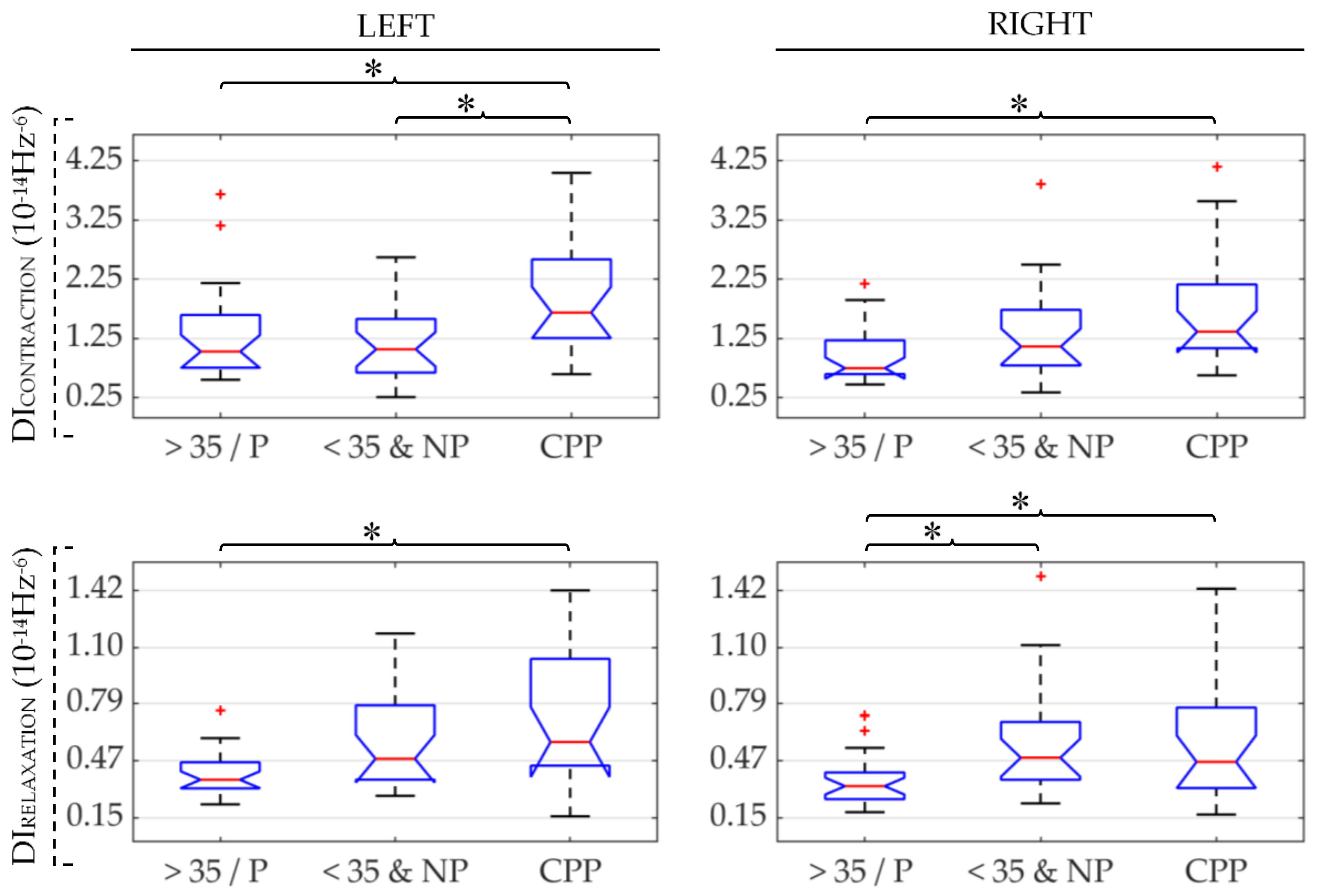

| CPP | >35/P | <35&NP | ||
|---|---|---|---|---|
| Age | mean ± SD (y) | 43.8 ± 8.8 * | 40.9 ± 7.2 ◊ | 28.1 ±3.2 *◊ |
| Weight | mean ± SD (kg) | 65.8 ± 11.2 * | 61.8 ± 9.3 | 57.1 ± 7.3 * |
| Height | mean ± SD (cm) | 162.6 ± 5.4 | 162.0 ± 3.8 ◊ | 165.8 ± 6.2 ◊ |
| No. deliveries | mean ± SD | 1.5 ± 0.7 * | 1.5 ± 0.8 ◊ | 0.0 ± 0.0 *◊ |
| Menopause | N (%) | 8 (33.3%) * | 3 (12.5%) | 0 (0.0%) * |
| Laterality of pain | N (%) | 4 (16.6%) Right 10 (41.7%) Left 10 (41.7%) Bilateral |
| CPP vs. >35/P | CPP vs. <35&NP | 35/P vs. <35&NP | ||||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| Contraction | RMS ↑ | RMS ↑ | RMS ↓ | |||
| MDF ↓ | ||||||
| DI ↑ | DI ↑ | DI ↑ | ||||
| SampEn ↓ | SampEn ↓ | |||||
| Relaxation | RMS ↑ | RMS ↑ | RMS ↓ | |||
| MDF ↓ | MDF ↑ | MDF ↑ | ||||
| DI ↑ | DI ↑ | DI ↓ | ||||
| SampEn ↓ | SampEn ↓ | SampEn ↑ | ||||
| Contractions and relaxations | CC ↓ | CC ↓ | CC ↓ | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albaladejo-Belmonte, M.; Tarazona-Motes, M.; Nohales-Alfonso, F.J.; De-Arriba, M.; Alberola-Rubio, J.; Garcia-Casado, J. Characterization of Pelvic Floor Activity in Healthy Subjects and with Chronic Pelvic Pain: Diagnostic Potential of Surface Electromyography. Sensors 2021, 21, 2225. https://doi.org/10.3390/s21062225
Albaladejo-Belmonte M, Tarazona-Motes M, Nohales-Alfonso FJ, De-Arriba M, Alberola-Rubio J, Garcia-Casado J. Characterization of Pelvic Floor Activity in Healthy Subjects and with Chronic Pelvic Pain: Diagnostic Potential of Surface Electromyography. Sensors. 2021; 21(6):2225. https://doi.org/10.3390/s21062225
Chicago/Turabian StyleAlbaladejo-Belmonte, Monica, Marta Tarazona-Motes, Francisco J. Nohales-Alfonso, Maria De-Arriba, Jose Alberola-Rubio, and Javier Garcia-Casado. 2021. "Characterization of Pelvic Floor Activity in Healthy Subjects and with Chronic Pelvic Pain: Diagnostic Potential of Surface Electromyography" Sensors 21, no. 6: 2225. https://doi.org/10.3390/s21062225
APA StyleAlbaladejo-Belmonte, M., Tarazona-Motes, M., Nohales-Alfonso, F. J., De-Arriba, M., Alberola-Rubio, J., & Garcia-Casado, J. (2021). Characterization of Pelvic Floor Activity in Healthy Subjects and with Chronic Pelvic Pain: Diagnostic Potential of Surface Electromyography. Sensors, 21(6), 2225. https://doi.org/10.3390/s21062225








