The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials
Abstract
:1. Introduction
1.1. Issues in the Design of Metal Oxide Sensors
1.2. Gas Sensing Principles of Metal Oxide Sensors
1.2.1. Oxygen Vacancy Formation and Oxygen Chemisorption
1.2.2. The Ionosorption Model of Sensor Response
1.2.3. The Oxygen Vacancy Model of Sensor Response
1.3. The Concerns on Sensor Materilals from Heterogeneous Catalysis
1.4. The Comparison of Energetic Parameters of n-Type Metal Oxide Semiconductors
2. Types of Active Sites at the MOS Surface

2.1. Acid/Base Sites
2.2. Oxidizing Sites
- physisorbed O2 molecules held at the surface via van der Waals attraction, the physisoption dominates at temperature below −70 °C [90];
- chemisorbed O2 molecules covalently bound with surface cations via a local redistribution of electron density, the chemisorbed oxygen was found to dominate at the surface of tin oxide at temperature below 200 °C [91];
- ionosorbed species O2−, O−, and O2− are formed by molecular and dissociative oxygen adsorption and acceptation of delocalized electrons from the bulk of MOS (Equation (2)) [5].
2.3. Electron Donor Sites
3. Active Sites Concentrations at the Surface of Nanocrystalline n-Type MOS
3.1. Acid Sites
3.2. Donor Sites (Oxygen Vacancies)
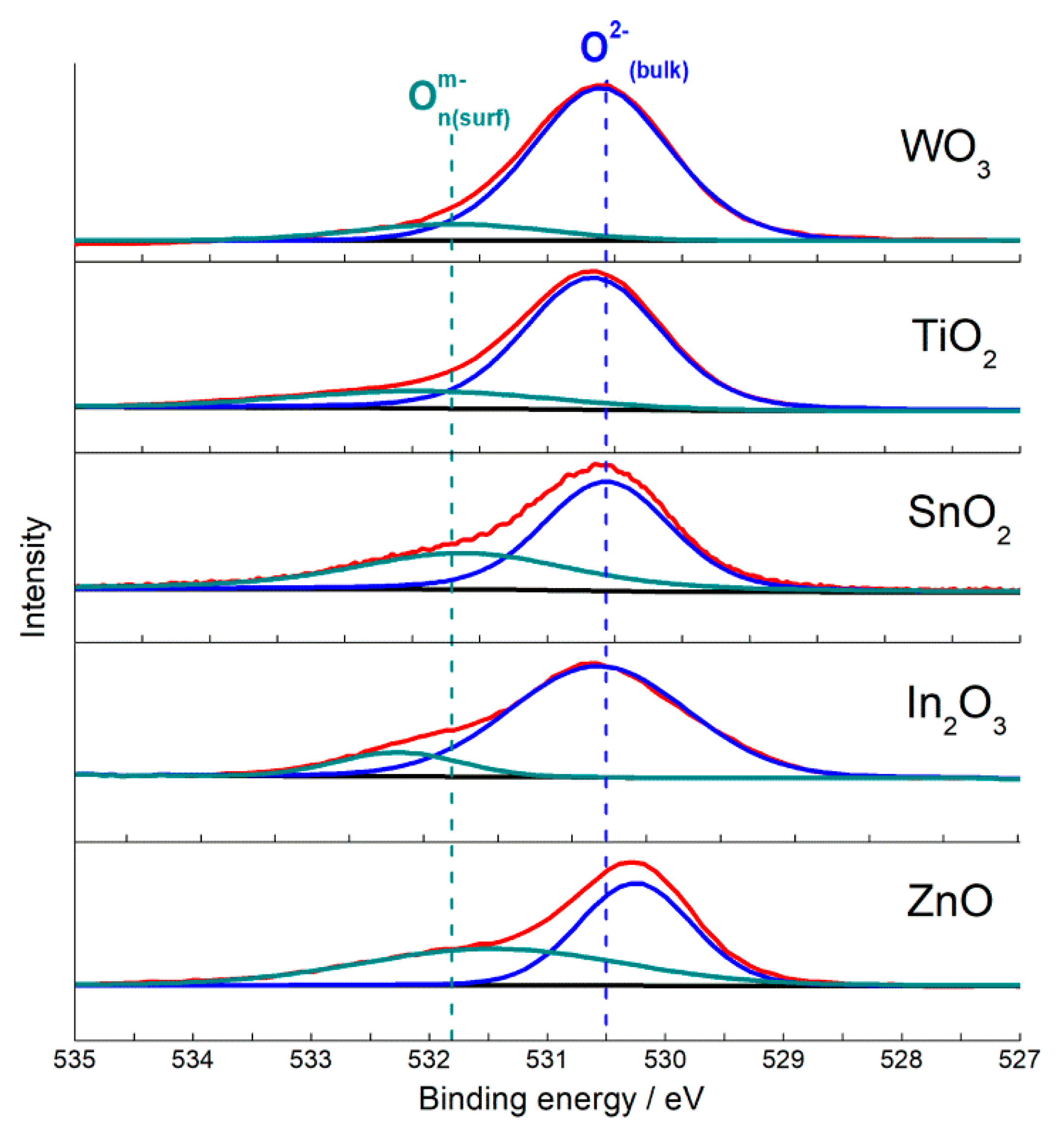
3.3. Oxidizing Sites (Chemisorbed Oxygen)
4. From Simple to Mixed-Metal Oxides: Metal-Oxygen Bond Energy and Active Sites
4.1. Crystal Structure and Metal-Oxygen Bonding
4.2. Concentration of Active Sites
5. Impact of Active Sites on Gas Sensitivity of Nanocrystalline n-Type MOS
5.1. Sensitivity to Ammonia and Surface Acidity

5.2. Sensitivity to CO and VOCs Impacted by Oxidizing Sites and Acid Sites


5.3. Sensitivity to NO2 Determined by Donor Sites

5.4. Effect of Cations on Sensitivity and Selectivity of Mixed-Metal Oxides
6. Crucial Role of Catalytic Clusters in Sensitivity and Selectivity of Functionalized n-Type MOS
6.1. Role of PdOx in Room-Temperature Sensitivity to CO
Pd-CO(ads) + OH(surf) = Pd(surf) + CO2(g) + H+(surf) + e
6.2. Impact of RuO2 on Sensitivity and Selectivity to NH3
Ru4+-NO(ads) + 1/n Onm−(surf) = NO2(g) + m/n e−
6.3. Au-Promoted Oxidizing Sites and Sensitivity to VOCs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A. Techniques for Determination of Active Sites
Appendix A.1. Determination of Acid/Base Sites
| Acid/Base Site | Probe Molecule | Adsorbate | Vibration | Wavenumber, cm−1 | Ref. |
|---|---|---|---|---|---|
| Mn+ cation (Lewis acid) | NH3(g) | NH3(ads) | ν (N-H) | 3260–3380 | [152,155] |
| δas (NH3) | 1600–1630 | ||||
| δs (NH3) | 1150–1300 | ||||
| OH-group (Brønsted acid) | NH4+ | ν (N-H) | 3170–3230 | [152,155,156] | |
| δas (NH4) | 1480–1400 | ||||
| δs (NH4) | 1680–1670 | ||||
| Mn+-O2− (Lewis acid-base pair) | NH2− + OH− | δ (NH2) | 1490–1580 | [152] | |
| ν (O-H) | 3600–3700 | ||||
| O2− anion (Lewis base) | CO2(g) | CO32− bidentate | ν (C=O) | 1530–1670 | [153,157] |
| νas (COO) | 1220–1290 | ||||
| νs (COO) | 980–1030 | ||||
| CO32− bridging | ν (C=O) | 1640–1730 | |||
| νas (COO) | 1280–1285 | ||||
| νs (COO) | 1000–1020 | ||||
| CO32− monodentate | νas (CO3) | 1450–1530 | |||
| νs (CO3) | 1300–1370 | ||||
| ν (C-O) | 1040–1080 | ||||
| OH-group (Lewis base) | HCO3− | νas (COO) | 1600–1625 | [153] | |
| νs (COO) | 1415–1440 | ||||
| δ (C-OH) | 1180–1250 |
Appendix A.2. Determination of Oxidizing Sites
Appendix A.3. Determination of Electron Donor Sites
Appendix A.4. In Situ and In Operando Investigation Techniques
References
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Shankar, P.; Rayappan, J.B.B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases—A review. ScienceJet 2015, 126, 4. [Google Scholar]
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohl, D. Function and applications of gas sensors. J. Phys. D Appl. Phys. 2001, 34, R125–R149. [Google Scholar] [CrossRef]
- Park, C.O.; Akbar, S.A. Ceramics for chemical sensing. J. Mater. Sci. 2003, 38, 4611–4637. [Google Scholar] [CrossRef]
- Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, I.; Hosono, K.; Murayama, N.; Shin, W.; Izu, N. Organically hybridized SnO2 gas sensors. Sens. Actuators B Chem. 2005, 108, 143–147. [Google Scholar] [CrossRef]
- Woo, H.-S.; Na, C.W.; Lee, J.-H. Design of Highly Selective Gas Sensors via Physicochemical Modification of Oxide Nanowires: Overview. Sensors 2016, 16, 1531. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current Understanding of the Fundamental Mechanisms of Doped and Loaded Semiconducting Metal-Oxide-Based Gas Sensing Materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Coles, G.S.V.; Bond, S.E.; Williams, G. Metal Stannates and their Role as Potential Gas-sensing Elements. J. Mater. Chem. 1994, 4, 23–27. [Google Scholar] [CrossRef]
- Balamurugan, C.; Song, S.-J.; Kim, H.-S. Enhancing Gas Response Characteristics of Mixed Metal Oxide Gas Sensors. J. Korean Ceram. Soc. 2018, 55, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Cheng, J.P.; Wang, J.; Li, Q.Q.; Liu, H.G.; Li, Y. A review of recent developments in tin dioxide composites for gas sensing application. J. Ind. Eng. Chem. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Recent trends in the design of chemical sensors based on graphene-metal oxide nanocomposites for the analysis of toxic species and biomolecules. Trends Anal. Chem. 2019, 120, 115660. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nanoheterostructures: A review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Pronin, I.A.; Ham, M.H.; Cho, B.K. Metal Oxides for Application in Conductometric Gas Sensors: How to Choose? Solid State Phenom. 2017, 266, 187–195. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Receptor Function and Response of Semiconductor Gas Sensor. Hindawi J. Sens. 2009, 2009, 875704. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with oxygen, water, and hydrogen. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen Ionosorption and Spectroscopic Evidence for Adsorbed Oxygen. Chem. Phys. Chem. 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Makeeva, E.A.; Badalyan, S.M.; Zhukova, A.A.; Gaskov, A.M. Nanocrystalline SnO2 and In2O3 as materials for gas sensors: The relationship between microstructure and oxygen chemisorption. Thin Solid Films 2009, 518, 1283–1288. [Google Scholar] [CrossRef]
- Vorobyeva, N.A.; Marikutsa, A.V.; Rumyantseva, M.N.; Kozlovskii, V.F.; Filatova, D.G.; Gaskov, A.M. Effect of Ga and In doping on acid centers and oxygen chemisorption on the surface of nanocrystalline ZnO. Inorg. Mater. 2016, 52, 578–583. [Google Scholar] [CrossRef]
- Yang, L.; Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Effect of WO3 particle size on the type and concentration of surface oxygen. Mendeleev Commun. 2020, 30, 126–128. [Google Scholar] [CrossRef]
- Gurlo, A.; Riedel, R. In Situ and Operando Spectroscopy for Assessing Mechanisms of Gas Sensing. Angew. Chem. Int. Ed. 2007, 46, 3826–3848. [Google Scholar] [CrossRef]
- Zhang, Y.; Kolmakov, A.; Lilach, Y.; Moskovits, M. Electronic Control of Chemistry and Catalysis at the Surface of an Individual Tin Oxide Nanowire. J. Phys. Chem. B 2005, 109, 1923–1929. [Google Scholar] [CrossRef]
- Shao, F.; Hoffmann, M.W.G.; Prades, J.D.; Morante, J.R.; Lopez, N.; Hernandez-Ramirez, F. Interaction Mechanisms of Ammonia and Tin Oxide: A Combined Analysis Using Single Nanowire Devices and DFT Calculations. J. Phys. Chem. C 2013, 117, 3520–3526. [Google Scholar] [CrossRef]
- Stankova, M.; Vilanova, X.; Llobet, E.; Calderer, J.; Bittencourt, C.; Pireaux, J.J.; Correig, X. Influence of the annealing and operating temperatures on the gas-sensing properties of rf sputtered WO3 thin-film sensors. Sens. Actuators B Chem. 2005, 105, 271–277. [Google Scholar] [CrossRef]
- Madler, L.; Sahm, T.; Gurlo, A.; Grunwaldt, J.-D.; Barsan, N.; Weimar, U.; Pratsinis, S.E. Sensing low concentrations of CO using flame-spray-made Pt/SnO2 nanoparticles. J. Nanopart. Res. 2006, 8, 783–796. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.K.; Ghosh, R.; Santra, S.; Guhab, P.K.; Pradhan, D. Hierarchical nanostructured WO3–SnO2 for selective sensing of volatile organic compounds. Nanoscale 2015, 7, 12460. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, Z. Comparison of the enhanced gas sensing properties of tin dioxide samples doped with different catalytic transition elements. J. Colloid Interface Sci. 2015, 448, 265–274. [Google Scholar] [CrossRef]
- Pan, Y.; Shen, X.; Yao, L.; Bentalib, A.; Peng, Z. Active Sites in Heterogeneous Catalytic Reaction on Metal and Metal Oxide: Theory and Practice. Catalysts 2018, 8, 478. [Google Scholar] [CrossRef] [Green Version]
- Bond, G.C.; Louis, C.; Thompson, D.T. Catalysis by Gold; Imperial College Press: London, UK, 2006. [Google Scholar]
- Wang, D.; Zhen, Y.; Xue, G.; Fu, F.; Liu, X.; Li, D. Synthesis of mesoporous Bi2WO6 architectures and their gas sensitivity to ethanol. J. Mater. Chem. C 2013, 1, 4153–4162. [Google Scholar] [CrossRef]
- Liu, W.; Qu, Y.; Li, H.; Ji, F.; Dong, H.; Wu, M.; Chen, H.; Lin, Z. Nanostructure Bi2WO6: Surfactant-assisted hydrothermal synthesis for high sensitive and selective sensing of H2S. Sens. Actuators B Chem. 2019, 294, 224–230. [Google Scholar] [CrossRef]
- Bunpang, K.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Effects of reduced graphene oxide loading on gas-sensing characteristics of flame-made Bi2WO6 nanoparticles. Appl. Surf. Sci. 2019, 496, 143613. [Google Scholar] [CrossRef]
- Sergent, N.; Epifani, M.; Comini, E.; Faglia, G.; Pagnier, T. Interactions of nanocrystalline tin oxide powder with NO2: A Raman spectroscopic study. Sens. Actuators B Chem. 2007, 126, 1–5. [Google Scholar] [CrossRef]
- Yang, L.; Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.; Khmelevsky, N.; Gaskov, A. Quasi Similar Routes of NO2 and NO Sensing by Nanocrystalline WO3: Evidence by In Situ DRIFT Spectroscopy. Sensors 2019, 19, 3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, W.; Barsan, N.; Weimar, U. Sensing of hydrocarbons and CO in low oxygen conditions with tin dioxide sensors: Possible conversion paths. Sens. Actuators B Chem. 2004, 103, 362–368. [Google Scholar] [CrossRef]
- Huebner, M.; Pavelko, R.G.; Barsan, N.; Weimar, U. Influence of oxygen backgrounds on hydrogen sensing with SnO2 nanomaterials. Sens. Actuators B Chem. 2011, 154, 264–269. [Google Scholar] [CrossRef]
- Safonova, O.; Bezverkhy, I.; Fabrichnyi, P.; Rumyantseva, M.; Gaskov, A. Mechanism of sensing CO in nitrogen by nanocrystalline SnO2 and SnO2(Pd) studied by Mossbauer spectroscopy and conductance measurements. J. Mater. Chem. 2002, 12, 1174–1178. [Google Scholar] [CrossRef]
- Huebner, M.; Simion, C.E.; Haensch, A.; Barsan, N.; Weimar, U. CO sensing mechanism with WO3 based gas sensors. Sens. Actuators B Chem. 2010, 151, 103–106. [Google Scholar] [CrossRef]
- Staerz, A.; Berthold, C.; Russ, T.; Wicker, S.; Weimar, U.; Barsan, N. The oxidizing effect of humidity on WO3 based sensors. Sens. Actuators B Chem. 2016, 237, 54–58. [Google Scholar] [CrossRef]
- Mueller, S.A.; Degler, D.; Feldmann, C.; Terk, M.; Moos, R.; Fink, K.; Studt, F.; Gerthsen, D.; Barsan, N.; Grunwaldt, J.-D. Exploiting Synergies in Catalysis and Gas Sensing using Noble Metal-Loaded Oxide Composites. ChemCatChem 2018, 10, 864–880. [Google Scholar] [CrossRef]
- Degler, D.; Mueller, S.A.; Doronkin, D.E.; Wang, D.; Grunwaldt, J.-D.; Weimar, U.; Barsan, N. Platinum loaded tin dioxide: A model system for unravelling the interplay between heterogeneous catalysis and gas sensing. J. Mater. Chem. A 2018, 6, 2034–2046. [Google Scholar] [CrossRef]
- Jinkawa, T.; Sakai, G.; Tamaki, J.; Miura, N.; Yamazoe, N. Relationship between ethanol gas sensitivity and surface catalytic property of tin oxide sensors modified with acidic or basic oxides. J. Mol. Catal. A Chem. 2000, 155, 193–200. [Google Scholar] [CrossRef]
- Zaera, F. Nanostructured materials for applications in heterogeneous catalysis. Chem. Soc. Rev. 2013, 42, 2746–2762. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Matar, S.F.; Campet, G.; Subramanian, M.A. Electronic properties of oxides: Chemical and theoretical approaches. Prog. Solid State Chem. 2011, 39, 70–95. [Google Scholar] [CrossRef]
- Idriss, H.; Barteau, M.A. Active sites on oxides: From single crystals to catalysts. J. Adv. Catal. Sci. Technol. 2000, 45, 261–331. [Google Scholar]
- Dimitrov, V.; Komatsu, T. Correlation among electronegativity, cation polarizability, optical basicity and single bond strength of simple oxides. J. Solid State Chem. 2012, 196, 574–578. [Google Scholar] [CrossRef]
- Lany, S. Semiconducting transition metal oxides. J. Phys. Condens. Matter. 2015, 27, 283203. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.D.; Ginley, D.S. Handbook of Transparent Conductors; Ginley, D.S., Ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Proffit, D.E.; Ma, Q.; Buchholz, D.B.; Chang, R.P.H.; Bedzyk, M.J.; Mason, T.O. Structural and Physical Property Studies of Amorphous Zn–In–Sn–O Thin Films. J. Am. Ceram. Soc. 2012, 95, 3657–3664. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Burdett, J.K.; Hughbanks, T.; Miller, G.J.; Richardson, J.W.; Smith, J.V. Structural-Electronic Relationships in Inorganic Solids: Powder Neutron Diffraction Studies of the Rutile and Anatase Polymorphs of Titanium Dioxide at 15 and 295 K. J. Am. Chem. Soc. 1987, 109, 3639–3646. [Google Scholar] [CrossRef]
- Marikutsa, A.; Yang, L.; Kuznetsov, A.N.; Rumyantseva, M.; Gaskov, A. Effect of W‒O bonding on gas sensitivity of nanocrystalline Bi2WO6 and WO3. J. Alloy. Compd. 2020, 856, 158159. [Google Scholar] [CrossRef]
- Zaera, F. The Surface Chemistry of Heterogeneous Catalysis: Mechanisms, Selectivity, and Active Sites. Chem. Rec. 2005, 5, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.; Wachs, I.E. Isopropanol oxidation by pure metal oxide catalysts: Number of active surface sites and turnover frequencies. Appl. Catal. A Gen. 2002, 237, 121–137. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Mishakov, I.V.; Medvedev, D.A.; Noskov, A.S. Characterization of active sites of Pd/Al2O3 model catalysts with low Pd content by luminescence, EPR and ethane hydrogenolysis. Appl. Catal. B Environ. 2011, 103, 397–403. [Google Scholar] [CrossRef]
- Zotov, R.A.; Molchanov, V.V.; Volodin, A.M.; Bedilo, A.F. Characterization of the active sites on the surface of Al2O3 ethanol dehydration catalysts by EPR using spin probes. J. Catal. 2011, 278, 71–77. [Google Scholar] [CrossRef]
- Ross-Medgaarden, E.I.; Wachs, I.E.; Knowles, W.V.; Burrows, A.; Kiely, C.J.; Wong, M.S. Tuning the Electronic and Molecular Structures of Catalytic Active Sites with Titania Nanoligands. J. Am. Chem. Soc. 2009, 131, 680–687. [Google Scholar] [CrossRef]
- Morrison, S.R. The Chemical Physics of Surfaces, 1st ed.; Springer: New York, NY, USA, 1990. [Google Scholar]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; Wiley: Chichester, UK, 2003. [Google Scholar]
- Bukhtiyarov, V.I.; Slinko, M.G. Metallic nanosystems in catalysis. Russ. Chem. Rev. 2001, 70, 147–159. [Google Scholar] [CrossRef]
- Chen, M.; Goodman, D.W. Catalytically Active Gold: From Nanoparticles to Ultrathin Films. Acc. Chem. Res. 2006, 39, 739–746. [Google Scholar] [CrossRef]
- Castellarin-Cudia, C.; Surnev, S.; Schneider, G.; Podlucky, R.; Ramsey, M.G.; Netzer, F.P. Strain-induced formation of arrays of catalytically active sites at the metal–oxide interface. Surf. Sci. 2004, 554, L120–L126. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Konstantinova, E.A.; Grishina, D.A.; Deygen, D.M. CO and NH3 sensor properties and paramagnetic centers of nanocrystalline SnO2 modified by Pd and Ru. Thin Solid Films 2011, 520, 904–908. [Google Scholar] [CrossRef]
- Marikutsa, A.; Yang, L.; Rumyantseva, M.; Batuk, M.; Hadermann, J.; Gaskov, A. Sensitivity of nanocrystalline tungsten oxide to CO and ammonia gas determined by surface catalysts. Sens. Actuators B Chem. 2018, 277, 336–346. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Gaskov, A.; Batuk, M.; Hadermann, J.; Sarmadian, N.; Saniz, R.; Partoens, B.; Lamoen, D. Effect of Zinc Oxide Modification by Indium Oxide on Microstructure, Adsorbed Surface Species, and Sensitivity to CO. Front. Mater. 2019, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Marikutsa, A.; Rumyantseva, M.; Baranchikov, A.; Gaskov, A. Nanocrystalline BaSnO3 as an Alternative Gas Sensor Material: Surface Reactivity and High Sensitivity to SO2. Materials 2015, 8, 6437–6454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marikutsa, A.; Novikova, A.; Rumyantseva, M.; Khmelevsky, N.; Gaskov, A. Comparison of Au-functionalized semiconductor metal oxides in sensitivity to VOC. Sens. Actuators B Chem. 2021, 326, 128980. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Konstantinova, E.A.; Shatalova, T.B.; Gaskov, A.M. Active Sites on Nanocrystalline Tin Dioxide Surface: Effect of Palladium and Ruthenium Oxides Clusters. J. Phys. Chem. C 2014, 118, 21541–21549. [Google Scholar] [CrossRef]
- Abee, M.W.; Cox, D.F. NH3 chemisorption on stoichiometric and oxygen deficient SnO2 (110) surfaces. Surf. Sci. 2002, 520, 65–77. [Google Scholar] [CrossRef]
- Bordes-Richard, E.; Courtine, P. Optical basicity: A scale of acidity/basicity of solids and its application to oxidation catalysis. In Metal Oxides: Chemistry and Application; Fierro, J.L.S., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 319–352. [Google Scholar]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter. 2003, 15, 813–839. [Google Scholar] [CrossRef]
- Pavelko, R.G.; Daly, H.; Hardacre, C.; Vasiliev, A.A.; Llobet, E. Interaction of water, hydrogen and their mixtures with SnO2 based materials: The role of surface hydroxyl groups in detection mechanisms. Phys. Chem. Chem. Phys. 2010, 12, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Marikutsa, A.V.; Vorobyeva, N.A.; Rumyantseva, M.N.; Gaskov, A.M. Active sites on the surface of nanocrystalline semiconductor oxides ZnO and SnO2 and gas sensitivity. Rus. Chem. Bull. 2017, 66, 1728–1764. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, L.; Xu, X.; Liu, Y.; Liu, Y.; Fu, X.; Gao, Z.; Qian, Z.; Xu, J.; Fang, X.; et al. Regulating SnO2 surface by metal oxides possessing redox or acidic properties: The importance of active O2−/O22− and acid sites for toluene deep oxidation. Appl. Catal. Ageneral 2020, 605, 117755. [Google Scholar] [CrossRef]
- Roberts, M.W. The Role of Short-lived Oxygen Transients and Precursor States in the Mechanisms of Surface Reactions; A Different View of Surface Catalysis. Chem. Soc. Rev. 1996, 6, 437–445. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Yashina, L.V.; Gaskov, A.M. Role of surface hydroxyl groups in promoting room temperature CO sensing by Pd-modified nanocrystalline SnO2. J. Solid State Chem. 2010, 183, 2389–2399. [Google Scholar] [CrossRef]
- Haber, J. Selectivity in Heterogeneous Catalytic Oxidation of Hydrocarbons. In Heterogeneous Hydrocarbon Oxidation; Warren, B., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1996; pp. 20–34. [Google Scholar]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem. 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Habgood, M.; Harrison, N. An ab initio study of oxygen adsorption on tin dioxide. Surf. Sci. 2008, 602, 1072–1079. [Google Scholar] [CrossRef]
- Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Basics of Oxygen and SnO2 Interaction; Work Function Change and Conductivity Measurements. Sens. Actuators B Chem. 2006, 118, 78–83. [Google Scholar] [CrossRef]
- Kung, M.C.; Davis, R.J.; Kung, H.H. Understanding Au-Catalyzed Low-Temperature CO Oxidation. J. Phys. Chem. C 2007, 111, 11767–11775. [Google Scholar] [CrossRef]
- Wachs, I.E. Molecular Structures of Surface Metal Oxide Species: Nature of Catalytic Active Sites in Mixed Metal Oxides. In Metal Oxides: Chemistry and Application; Fierro, J.L.S., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 1–26. [Google Scholar]
- Forsh, E.A.; Abakumov, A.M.; Zaytsev, V.B.; Konstantinova, E.A.; Forsh, P.A.; Rumyantseva, M.N.; Gaskov, A.M.; Kashkarov, P.K. Optical and photoelectrical properties of nanocrystalline indium oxide with small grains. Thin Solid Films 2015, 595, 25–31. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Frolov, D.; Morozov, I.; Boltalin, A.; Fedorova, A.; Petukhov, I.; Yashina, L.; Konstantinova, E.; Sadovskaya, E.; et al. Role of PdOx and RuOy Clusters in Oxygen Exchange between Nanocrystalline Tin Dioxide and the Gas Phase. J. Phys. Chem. C 2013, 117, 23858–23867. [Google Scholar] [CrossRef]
- Konstantinova, E.A.; Zaitsev, V.B.; Kytina, E.V.; Marikutsa, A.V. Photoaccumulating Nanoheterostructures Based on Titanium Dioxide. Semiconductors 2021, 55, 219–227. [Google Scholar] [CrossRef]
- Naberezhnyi, D.; Rumyantseva, M.; Filatova, D.; Batuk, M.; Hadermann, J.; Baranchikov, A.; Khmelevsky, N.; Aksenenko, A.; Konstantinova, E.; Gaskov, A. Effects of Ag Additive in Low Temperature CO Detection with In2O3 Based Gas Sensors. Nanomaterials 2018, 8, 801. [Google Scholar] [CrossRef] [Green Version]
- Marikutsa, A.; Sukhanova, A.; Rumyantseva, M.; Gaskov, A. Acidic and catalytic co-functionalization for tuning the sensitivity of sulfated tin oxide modified by ruthenium oxide to ammonia gas. Sens. Actuators B Chem. 2018, 255, 3523–3532. [Google Scholar] [CrossRef]
- Wrledt, H.A. The O-W (Oxygen-Tungsten) System. Bull. Alloy Phase Diagr. 1989, 10, 368–384. [Google Scholar] [CrossRef]
- Ivanovskaya, M.I.; Branitskiy, G.A.; Orlik, D.R.; Malchenko, S.I.; Vrublevskiy, A.I. Nature of paramagnetic centers in tin dioxide. Rus. J. Inorg. Chem. 1992, 37, 1147–1152. [Google Scholar]
- Le, N.T. Influence of the Synthesis Conditions of Nanocrystalline Titanium Dioxide on the Nature and Parameters of Spin Centers. Ph.D. Thesis, Moscow State University, Moscow, Russian, 2017. [Google Scholar]
- Vorobyeva, N.; Rumyantseva, M.; Filatova, D.; Konstantinova, E.; Grishina, D.; Abakumov, A.; Turner, S.; Gaskov, A. Nanocrystalline ZnO(Ga): Paramagnetic centers, surface acidity and gas sensor properties. Sens. Actuators B Chem. 2013, 182, 555–564. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yoda, Y.; Yamazoe, N.; Seiyama, T. Study of Metal Oxide Catalysts by Temperature Programmed Desorption. 4. Oxygen Adsorption on Various Metal Oxides. J. Phys. Chem. 1978, 82, 2564–2570. [Google Scholar] [CrossRef]
- Konstantinova, E.A.; Pentegov, I.S.; Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Kashkarov, P.K. EPR study of nanocrystalline tin dioxide. Phys. Status Solidi C 2011, 8, 1957–1960. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhai, Z.; Jin, G.; Jiang, Q.; Zhao, Y.; Luo, C.; Quan, L. Evaluation of depletion layer width and gas-sensing properties of antimony-doped tin oxide thin film sensors. Sens. Actuators B Chem. 2015, 220, 1354–1360. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Micali, G.; Rizzo, G.; Pinna, N.; Niederberger, M.; Ba, J. Effect of the chemical composition on the sensing properties of In2O3–SnO2 nanoparticles synthesized by a non-aqueous method. Sens. Actuators B Chem. 2008, 130, 222–230. [Google Scholar] [CrossRef]
- Guidi, V.; Carotta, M.C.; Ferroni, M.; Martinelli, G.; Paglialonga, L.; Comini, E.; Sberveglieri, G. Preparation of nanosized titania thick and thin films as gas-sensors. Sens. Actuators B Chem. 1999, 57, 197–200. [Google Scholar] [CrossRef]
- Larramona, G.; Gutierrez, C.; Pereira, I.; Rosa Nunes, M.; da Costa, F.M.A. Characterization of the mixed perovskite BaSn1-xSbxO3 by electrolyte electroreflectance, diffuse reflectance, and X-ray photoelectron spectroscopy. J. Chem. Soc. Faraday Trans. 1 1989, 85, 907–916. [Google Scholar] [CrossRef]
- Punginsang, M.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Ultrafine Bi2WO6 nanoparticles prepared by flame spray pyrolysis for selective acetone gas-sensing. Mater. Sci. Semicond. Process. 2019, 90, 263–275. [Google Scholar] [CrossRef]
- Gonzalez-Borrero, P.P.; Sato, F.; Medina, A.N.; Baesso, M.L.; Bento, A.C.; Baldissera, G.; Persson, C.; Niklasson, G.A.; Granqvist, C.G.; Ferreira da Silva, A. Optical band-gap determination of nanostructured WO3 film. Appl. Phys. Lett. 2010, 96, 061909. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Eng, H.W.; Woodward, P.M. Probing the Electronic Structures of Ternary Perovskite and Pyrochlore Oxides Containing Sn4+ or Sb5+. Inorg. Chem. 2004, 43, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.; Henriques, J.M.; Azevedo, D.L.; Caetano, E.W.S.; Freire, V.N.; Fulco, U.L.; Albuquerque, E.L. Structural and optoelectronic properties, and infrared spectrum of cubic BaSnO3 from first principles calculations. J. Appl. Phys. 2012, 112, 043703. [Google Scholar] [CrossRef]
- Scanlon, D.O. Defect engineering of BaSnO3 for high-performance transparent conducting oxide applications. Phys. Rev. B 2013, 87, 161201(R). [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.-D.; Cho, B.K.; Brinzari, V. Grain Size Effects in Sensor Response of Nanostructured SnO2- and In2O3-Based Conductometric Thin Film Gas Sensor. Crit. Rev. Solid State Mater. Sci. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Morazzoni, F.; Scotti, R.; Origoni, L.; Arienzo, M.; Jimenez, I.; Cornet, A.; Morante, J.R. Mechanism of NH3 interaction with transition metal-added nanosized WO3 for gas sensing: In situ electron paramagnetic resonance study. Catal. Today 2007, 126, 169–176. [Google Scholar] [CrossRef]
- Garshev, A.V.; Ivanov, V.K.; Krotova, A.A.; Filatova, D.G.; Konstantinova, E.A.; Naberezhnyi, D.O.; Khmelevsky, N.O.; Marikutsa, A.V.; Kots, P.A.; Smirnov, A.V.; et al. Enhancement of Lewis Acidity of Cr-Doped Nanocrystalline SnO2: Effect on Surface NH3 Oxidation and Sensory Detection Pattern. ChemPhysChem 2019, 20, 1985–1996. [Google Scholar] [CrossRef]
- Marikutsa, A.; Krivetskiy, V.; Yashina, L.; Rumyantseva, M.; Konstantinova, E.; Ponzoni, A.; Comini, E.; Abakumov, A.; Gaskov, A. Catalytic impact of RuOx clusters to high ammonia sensitivity of tin dioxide. Sens. Actuators B Chem. 2012, 175, 186–193. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Specific Interaction of PdOx- and RuOy-Modified Tin Dioxide with CO and NH3 Gases: Kelvin Probe and DRIFT Studies. J. Phys. Chem. C 2015, 119, 24342–24350. [Google Scholar] [CrossRef]
- Koziej, D.; Thomas, K.; Barsan, N.; Thibault-Starzyk, F.; Weimar, U. Influence of annealing temperature on the CO sensing mechanism for tin dioxide based sensors-Operando studies. Catal. Today 2007, 126, 211–218. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Selectivity of Catalytically Modified Tin Dioxide to CO and NH3 Gas Mixtures. Chemosensors 2015, 3, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Vaishanv, V.S.; Patel, P.D.; Patel, N.G. Indium Tin Oxide Thin-Film Sensor for Detection of Volatile Organic Compounds (VOCs). Mater. Manuf. Process. 2006, 21, 257–261. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Thevenet, F.; Rousseau, A.; Bianchi, D. NO2 adsorption mechanism on TiO2: An in-situ transmission infrared spectroscopy study. Appl. Catal. B Environ. 2016, 198, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Ilin, A.; Martyshov, M.; Forsh, E.; Forsh, P.; Rumyantseva, M.; Abakumov, A.; Gaskov, A.; Kashkarov, P. UV effect on NO2 sensing properties of nanocrystalline In2O3. Sens. Actuators B Chem. 2016, 231, 491–496. [Google Scholar] [CrossRef]
- Tanaka, I.; Oba, F.; Tatsumi, K.; Kunisu, M.; Nakano, M.; Adachi, H. Theoretical Formation Energy of Oxygen-Vacancies in Oxides. Mater. Trans. 2002, 43, 1426–1429. [Google Scholar] [CrossRef] [Green Version]
- Hellman, A.; Baerends, E.J.; Biczysko, M.; Bligaard, T.; Christensen, C.H.; Clary, D.C.; Dahl, S.; van Harrevelt, R.; Honkala, K.; Jonsson, H.; et al. Predicting Catalysis: Understanding Ammonia Synthesis from First-Principles Calculations. J. Phys. Chem. B 2006, 110, 17719–17735. [Google Scholar] [CrossRef]
- Haruta, M. Catalysis of gold nanoparticles deposited on metal oxides. CatTech 2002, 6, 102–115. [Google Scholar] [CrossRef]
- Santra, A.K.; Goodman, D.W. Oxide-supported metal clusters: Models for heterogeneous catalysts. J. Phys. Condens. Matter 2002, 14, R31–R62. [Google Scholar]
- Grabow, L.C.; Hvolbek, B.; Norskov, J.K. Understanding Trends in Catalytic Activity: The Effect of Adsorbate-Adsorbate Interactions for CO Oxidation Over Transition Metals. Top. Catal. 2010, 53, 298–310. [Google Scholar] [CrossRef]
- Cabot, A.; Arbiol, J.; Morante, J.R.; Weimar, U.; Barsan, N.; Gopel, W. Analysis of the noble metal catalytic additives introduced by impregnation of as obtained SnO2 sol–gel nanocrystals for gas sensors. Sens. Actuators B Chem. 2000, 70, 87–100. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Boris, Y.; Ivanov, M.; Schwank, J.; Morante, J. Influence of surface Pd doping on gas sensing characteristics of SnO2 thin films deposited by spray pyrolysis. Thin Solid Film. 2003, 436, 119–126. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Jacobi, K.; Scholne, W.-D.; Ertl, G. Catalytic Oxidation of Ammonia on RuO2(110) Surfaces: Mechanism and Selectivity. J. Phys. Chem. B 2005, 109, 7883–7893. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Bligaard, T.; Abild-Pedersen, F.; Norskov, J.K. Using scaling relations to understand trends in the catalytic activity of transition metals. J. Phys. Condens. Matter 2008, 20, 064239. [Google Scholar] [CrossRef]
- Krivetskiy, V.V.; Rumyantseva, M.N.; Gaskov, A.M. Chemical modification of nanocrystalline tin dioxide for selective gas sensors. Russ. Chem. Rev. 2013, 82, 917–941. [Google Scholar] [CrossRef]
- Wagh, M.S.; Jain, G.H.; Patil, D.R.; Patil, S.A.; Patil, L.A. Modified zinc oxide thick film resistors as NH3 gas sensor. Sens. Actuators B Chem. 2006, 115, 128–133. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Guglielminotti, E. IR study of TiO2-based gas-sensor materials: Effect of ruthenium on the oxidation of NH3, (CH3)3N and NO. Sens. Actuators B Chem. 1994, 21, 27–31. [Google Scholar] [CrossRef]
- Scire, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Carabineiro, S.; Chen, X.; Martynyuk, O.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.; Orfao, J.; Pereira, M.; Figueiredo, J. Gold supported on metal oxides for volatile organic compounds total oxidation. Catal. Today 2015, 244, 103–114. [Google Scholar] [CrossRef]
- Yao, M.-S.; Cao, L.-A.; Hou, G.-L.; Cai, M.-L.; Xiu, J.-W.; Fang, C.-H.; Yuan, F.-L.; Chen, Y.-F. Gold–tin co-sensitized ZnO layered porous nanocrystals: Enhanced responses and antihumidity. RSC Adv. 2017, 7, 20273. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.-H.; Yuasa, M.; Kida, T.; Kanmura, Y.; Huh, J.-S.; Yamazoe, N.; Shimanoe, K. Gas sensor using noble metal-loaded TiO2 nanotubes for detection of large-sized volatile organic compounds. J. Ceram. Soc. Jpn. 2011, 119, 884–889. [Google Scholar] [CrossRef] [Green Version]
- Gardner, S.D.; Hoflund, G.B. Catalytic behavior of noble metal/reducible oxide materials for low-temperature CO oxidation. 1. Comparison of catalyst performance. Langmuir 1991, 7, 2135–2139. [Google Scholar] [CrossRef]
- Gao, F.; Wood, T.E.; Goodman, D.W. The Effects of Water on CO Oxidation over TiO2 Supported Au Catalysts. Catal. Lett. 2010, 134, 9–12. [Google Scholar] [CrossRef]
- Okazaki, K.; Ichikawa, S.; Maeda, Y.; Haruta, M.; Kohyama, M. Electronic structures of Au supported on TiO2. Appl. Catal. A Gen. 2005, 291, 45–54. [Google Scholar] [CrossRef]
- Fujita, T.; Horikawa, M.; Takei, T.; Murayama, T.; Haruta, M. Correlation between catalytic activity of supported gold catalysts for carbon monoxide oxidation and metal–oxygen binding energy of the support metal oxides. Chin. J. Catal. 2016, 37, 1651–1655. [Google Scholar] [CrossRef]
- Okumura, M.; Kitagawa, Y.; Haruta, M.; Yamaguchi, K. The interaction of neutral and charged Au clusters with O2, CO and H2. Appl. Catal. A Gen. 2005, 291, 37–44. [Google Scholar] [CrossRef]
- Gaggiotti, G.; Galdikas, A.; Kacilius, S.; Mattogno, G.; Setkus, A. Surface chemistry of tin oxide based gas sensors. J. Appl. Phys. 1994, 76, 4467–4471. [Google Scholar] [CrossRef]
- Shali, N.B.; Sugunan, S. Influence of transition metals on the surface acidic properties of titania prepared by sol–gel route. Mater. Res. Bull. 2007, 42, 1777–1783. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.; Wang, Y.; Loeffler, E.; Woell, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7099. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, V.; Gomez, R.; Moscosa-Santillan, M.; Amouroux, J. Synthesis, characterization, and catalytic activity in the n-heptane conversion over Pt/In–Al2O3 sol–gel prepared catalysts. J. Sol-Gel Sci. Technol. 2007, 42, 165–171. [Google Scholar] [CrossRef]
- Tanabe, K. Solid Acids and Bases: Their Catalytic Properties; Kodansha Ltd.: Tokyo, Japan, 1970. [Google Scholar]
- Khalaf, H.A.; Mansour, S.E.; El-Madani, E.A. The influence of sulfate contents on the surface properties of sulfate-modified tin(IV) oxide catalysts. J. Assoc. Arab Univ. Basic Appl. Sci. 2011, 10, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Hadjiivanov, K.I. Identification of Neutral and Charged NxOy Surface Species by IR Spectroscopy. Catal. Rev. Sci. Eng. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar]
- Trombetta, M.; Ramis, G.; Busca, G.; Montanari, B.; Vaccari, A. Ammonia Adsorption and Oxidation on Cu/Mg/Al Mixed Oxide Catalysts Prepared via Hydrotalcite-Type Precursors. Langmuir 1997, 13, 4628–4637. [Google Scholar] [CrossRef]
- Morimoto, T.; Yanal, H.; Nagao, M. Infrared Spectra of Ammonia Adsorbed on Zinc Oxide. J. Phys. Chem. 1976, 80, 471–475. [Google Scholar] [CrossRef]
- Belokopytov, Y.V.; Kholyavenko, K.M.; Gerei, S.V. An Infrared Study of the Surface Properties of Metal Oxides 2. The Interaction of Ammonia with the Surface of Fe2O3, ZnO, MoO3, and V2O5. J. Catal. 1979, 60, 1–7. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; Wiley: Hoboken, NJ, USA, 1986. [Google Scholar]
- De Jong, A.M.; Niemantsverdriet, J.W. Thermal desorption analysis: Comparative test of ten commonly applied procedures. Surf. Sci. 1990, 233, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Rumyantseva, M.; Kovalenko, V.; Gaskov, A.; Makshina, E.; Yuschenko, V.; Ivanova, I.; Ponzoni, A.; Faglia, G.; Comini, E. Nanocomposites SnO2/Fe2O3: Sensor and catalytic properties. Sens. Actuators B Chem. 2006, 118, 208–214. [Google Scholar] [CrossRef]
- Redhead, P.A. Thermal desorption of gases. Vacuum 1962, 12, 203–211. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Gaskov, A.M. Chemical modification of nanocrystalline metal oxides: Effect of the real structure and surface chemistry on the sensor properties. Rus. Chem. Bull. Int. Ed. 2008, 57, 1106–1125. [Google Scholar] [CrossRef]
- Hunger, B.; Hoffmann, J.; Heitzsch, O.; Hunger, M. Temperature Programmed Desorption (TPD) of Ammonia from Hzsm-5 Zeolites. J. Therm. 1990, 36, 1379–1391. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Tabata, K.; Suzuki, E. Density functional theory calculations for the interaction of oxygen with reduced M/SnO2(110) (M = Pd, Pt) surfaces. Surf. Sci. 2003, 526, 149–158. [Google Scholar] [CrossRef]
- Tabata, K.; Kawabe, T.; Yamaguchi, Y.; Nagasawa, Y. Chemisorbed oxygen species over the (110) face of SnO2. Catal. Surv. Asia 2003, 7, 251–259. [Google Scholar] [CrossRef]
- Panov, G.I.; Uriarte, A.K.; Rodkin, M.A.; Sobolev, V.I. Generation of active oxygen species on solid surfaces. Opportunity for novel oxidation technologies over zeolites. Catal. Today 1998, 41, 365–385. [Google Scholar] [CrossRef]
- Boulova, M.; Gaskov, A.; Lucazeau, G. Tungsten oxide reactivity to CH4, CO, and NO2 molecules studied by Raman spectroscopy. Sens. Actuators B Chem. 2001, 81, 99–106. [Google Scholar] [CrossRef]
- Moulijn, J.A.; van Leeuwen, P.W.N.M.; van Santen, R.A. (Eds.) Temperature programmed reduction and sulphiding. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1993; Volume 79, pp. 401–417. [Google Scholar]
- Slavinskaya, E.M.; Gulyaeva, R.V.; Zadesenets, A.V.; Stonkusa, O.A.; Zaikovskiia, V.I.; Shubin, Y.V.; Korenev, S.V.; Boronin, A.I. Low-temperature CO oxidation by Pd/CeO2 catalysts synthesized using the coprecipitation method. Appl. Catal. B Environ. 2015, 166–167, 91–103. [Google Scholar] [CrossRef]
- Hurst, N.W.; Gentry, S.J.; Jones, A.; McNicol, B.D. Temperature Programmed Reduction. Catal. Rev.-Sci. Eng. 1982, 24, 233–309. [Google Scholar] [CrossRef]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B Chem. 2019, 301, 126845. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Geng, X.; Wu, K.; Debliquy, M. Metal oxide semiconductors with highly concentrated oxygen vacancies for gas sensing materials: A review. Sens. Actuators A Phys. 2020, 309, 112026. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, F.; Prades, J.D.; Tarancon, A.; Barth, S.; Casals, O.; Jimenez-Diaz, R.; Pellicer, E.; Rodriguez, J.; Morante, J.R.; Juli, M.A.; et al. Insight into the Role of Oxygen Diffusion in the Sensing Mechanisms of SnO2 Nanowires. Adv. Funct. Mater. 2008, 18, 2990–2994. [Google Scholar] [CrossRef]
- Maier, J.; Gopel, W. Investigation of the bulk defect chemistry of polycrystalline tin(IV) oxide. J. Solid State Chem. 1988, 72, 293–302. [Google Scholar] [CrossRef]
- Mizusaki, J.; Koinuma, H.; Shimoyama, J.I.; Kawasaki, M.; Fueki, K. High temperature gravimetric study on nonstoichometry and oxygen adsorption of SnO2. J. Solid State Chem. 1990, 8, 443–450. [Google Scholar] [CrossRef]
- Li-Zi, Y.; Zhi-Tong, S.; Chan-Zheng, W. A thermodynamic study of tin oxides by coulometric titration. J. Solid State Chem. 1994, 113, 221–224. [Google Scholar] [CrossRef]
- Setvin, M.; Wagner, M.; Schmid, M.; Parkinson, G.S.; Diebold, U. Surface point defects on bulk oxides: Atomically-resolved scanning probe microscopy. Chem. Soc. Rev. 2017, 46, 1772–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batzill, M. Surface Science Studies of Gas Sensing Materials: SnO2. Sensors 2006, 6, 1345–1366. [Google Scholar] [CrossRef] [Green Version]
- Rumyantseva, M.N.; Gaskov, A.M.; Rosman, N.; Pagnier, T.; Morante, J.R. Raman Surface Vibration Modes in Nanocrystalline SnO2: Correlation with Gas Sensor Performances. Chem. Mater. 2005, 17, 893–901. [Google Scholar] [CrossRef]
- Smith, J.M.; Vehse, W.E. ESR of electron irradiated ZnO: Confirmation of the F+ center. Phys. Lett. A 1970, 31, 147–148. [Google Scholar] [CrossRef]
- Kappers, L.A.; Gilliama, O.R.; Evans, S.M.; Halliburton, L.E.; Giles, N.C. EPR and optical study of oxygen and zinc vacancies in electron-irradiated ZnO. Nucl. Instrum. Meth. B 2008, 266, 2953–2957. [Google Scholar] [CrossRef]
- Canevali, C.; Chiodini, N.; Morazzoni, F.; Scotti, R. Electron paramagnetic resonance characterization of rutheniumdispersed tin oxide obtained by sol-gel and impregnation methods. J. Mater. Chem. 2000, 10, 773–778. [Google Scholar] [CrossRef]
- Konstantinova, E.; Weidmann, J.; Dittrich, T. Influence of Adsorbed Water and Oxygen on the Photoluminescence and EPR of Por-TiO2 (Anatase). J. Porous Mater. 2000, 7, 389–392. [Google Scholar] [CrossRef]
- Hamlyn, R.C.E.; Mahapatra, M.; Grinter, D.C.; Xu, F.; Luo, S.; Palomino, R.M.; Kattel, S.; Waluyo, I.; Liu, P.; Stacchiola, D.J.; et al. Imaging the ordering of a weakly adsorbed two-dimensional condensate: Ambient pressure microscopy and spectroscopy of CO2 molecules on rutile TiO2(110). Phys. Chem. Chem. Phys. 2018, 20, 13122–13126. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Koziej, D.; Hubner, M.; Barsan, N.; Weimar, U.; Sikorazc, M.; Grunwaldt, J.-D. Operando X-ray absorption spectroscopy studies on Pd-SnO2 based sensors. Phys. Chem. Chem. Phys. 2009, 11, 8620–8625. [Google Scholar] [CrossRef] [PubMed]
- Oprea, A.; Barsan, N.; Weimar, U. Work function changes in gas sensitive materials: Fundamentals and applications. Sens. Actuators B Chem. 2009, 142, 470–493. [Google Scholar] [CrossRef]




















| MOS | r, Å [59] | χ, 1 P.u. [54] | ICP 2 [54] | EM-O3, eV [55] | dM-O, Å | −1/x·ΔfH [55], eV | Eg [54], eV |
|---|---|---|---|---|---|---|---|
| ZnO | 0.74 | 1.65 | 0.45 | 10.4 | 1.96–1.98 [57] | 3.6 | 3.4 |
| In2O3 | 0.79 | 1.78 | 0.53 | 12.4 | 2.18 [60] | 3.2 | 2.8 |
| SnO2 | 0.69 | 1.96 | 0.61 | 15.5 | 2.02 [61] | 6.0 | 3.6 |
| TiO2 | 0.61 | 2.01 | 0.52 | 20.7 | 1.95 (rutile) 1.96 (anatase) [62] | 9.8 | 3.4 |
| WO3 | 0.58 | 2.36 | 0.49 | 36.6 | 1.74–2.17 [63] | 8.7 | 2.8 |
| MOS | Tanneal, °C | dXRD, nm | SSA, m2/g | Active Sites Concentration, 10−6 mole/m2 | ||||
|---|---|---|---|---|---|---|---|---|
| Acid Sites 1 | Oxidizing Sites | Donor Sites VO− 3 | ||||||
| Weak (Broensted) | Strong (Lewis) | Total n (H2,TPR) 2 | O2− 3 | |||||
| ZnO | 300 | 11–13 | 45 | 0.4 ± 0.1 | 1.8 ± 0.4 | 6.6 ± 0.5 | 4.4 × 10−5 | 8 × 10−6 |
| In2O3 | 300 | 7–8 | 100 | n.d. 4 | n.d. 4 | 5.8 ± 0.4 | 1.3 × 10−2 | - |
| 500 | 16–19 | 35 | 0.5 ± 0.1 | 1.7 ± 0.4 | 4.1 ± 0.8 | 1.5 × 10−2 | ||
| SnO2 | 300 | 3–5 | 95 | 0.6 ± 0.1 | 2.4 ± 0.5 | 23.6 ± 0.8 | 1.4 × 10−5 | 3.5 × 10−4 |
| 500 | 10–12 | 25 | 0.5 ± 0.2 | 1.5 ± 0.4 | 14.0 ± 1.2 | |||
| 700 | 16–20 | 10 | 0.5 ± 0.2 | 1.2 ± 0.5 | 12.6 ± 2.6 | |||
| BaSnO3 | 500 | 20–22 | 8 | 0.4 ± 0.2 | 0.7 ± 0.3 | 12.2 ± 2.8 | 2.5 × 10−4 | - |
| TiO2 | 700 | 27–30 (rutile), 38–46 (anatase) | 5 | 3.3 ± 0.8 | 8.2 ± 1.9 | 20.8 ± 3.1 | 6 × 10−6 | 6.8 × 10−4 |
| WO3 | 300 | 7–9 | 35 | 6.1 ± 0.2 | 21.7 ± 0.9 | 5.4 ± 0.6 | - | 3.1 × 10−3 |
| 450 | 19–22 | 9 | 5.1 ± 0.9 | 15.6 ± 1.2 | - | |||
| 600 | 23–25 | 4 | 3.1 ± 0.6 | 8.9 ± 2.0 | - | |||
| Bi2WO6 | 300 | 14–15 | 9 | 0.8 ± 0.3 | 1.7 ± 0.6 | 16.4 ± 2.0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials. Sensors 2021, 21, 2554. https://doi.org/10.3390/s21072554
Marikutsa A, Rumyantseva M, Konstantinova EA, Gaskov A. The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials. Sensors. 2021; 21(7):2554. https://doi.org/10.3390/s21072554
Chicago/Turabian StyleMarikutsa, Artem, Marina Rumyantseva, Elizaveta A. Konstantinova, and Alexander Gaskov. 2021. "The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials" Sensors 21, no. 7: 2554. https://doi.org/10.3390/s21072554






