Hybrid Multisite Silicon Neural Probe with Integrated Flexible Connector for Interchangeable Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microfabrication of the Neural Probe
2.2. Neural Probe Packaging
2.3. Electrical Impedance Analysis
2.4. In Vivo Electrophysiological Recordings and Analysis
2.5. Histology
3. Results and Discussion
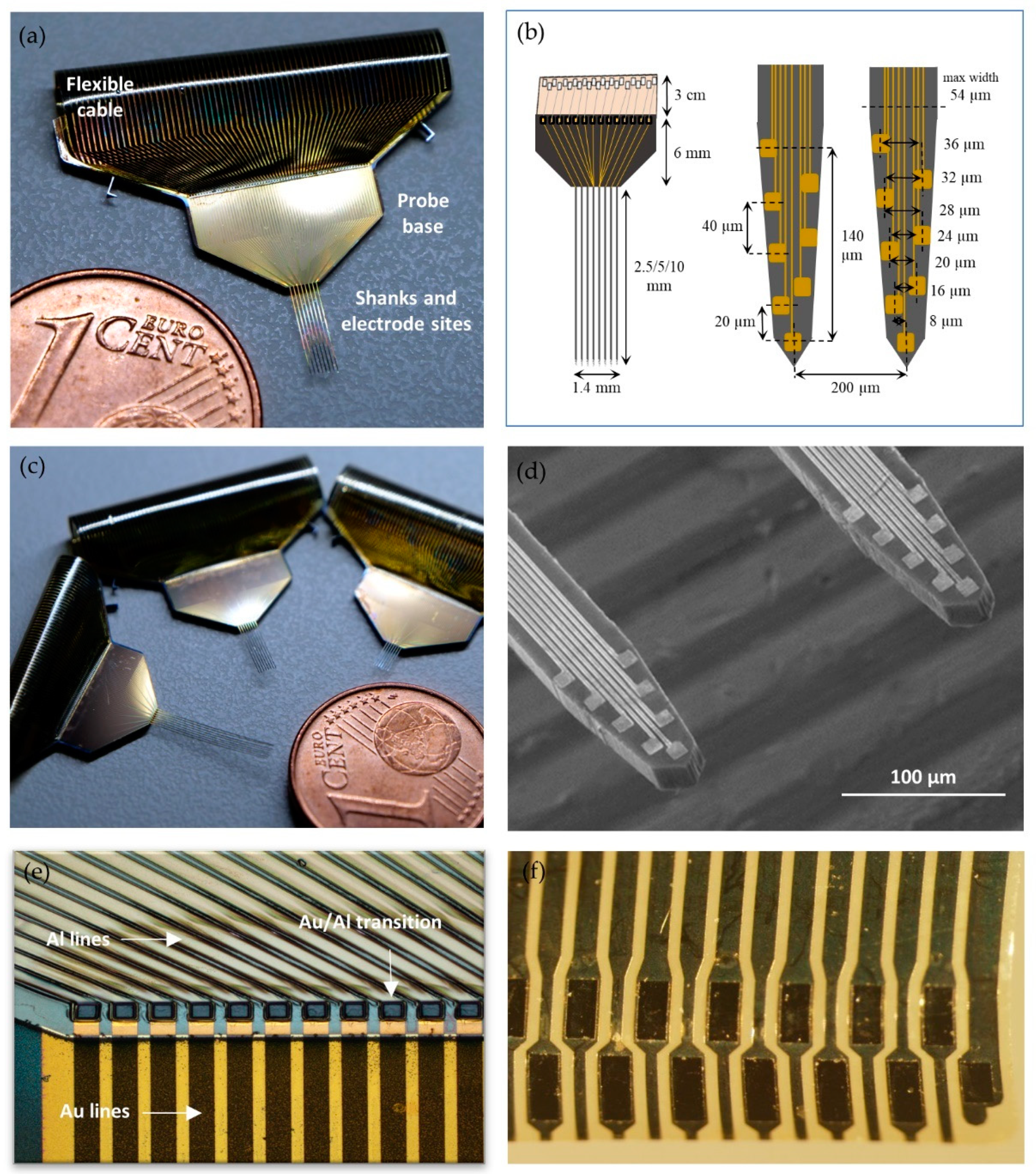
3.1. Design and Fabrication of Multishank Silicon Probe
3.2. Fabrication and Monolithic Integration of Flexible Polyimide Cable
3.3. Electrical Impedance of Neural Probe
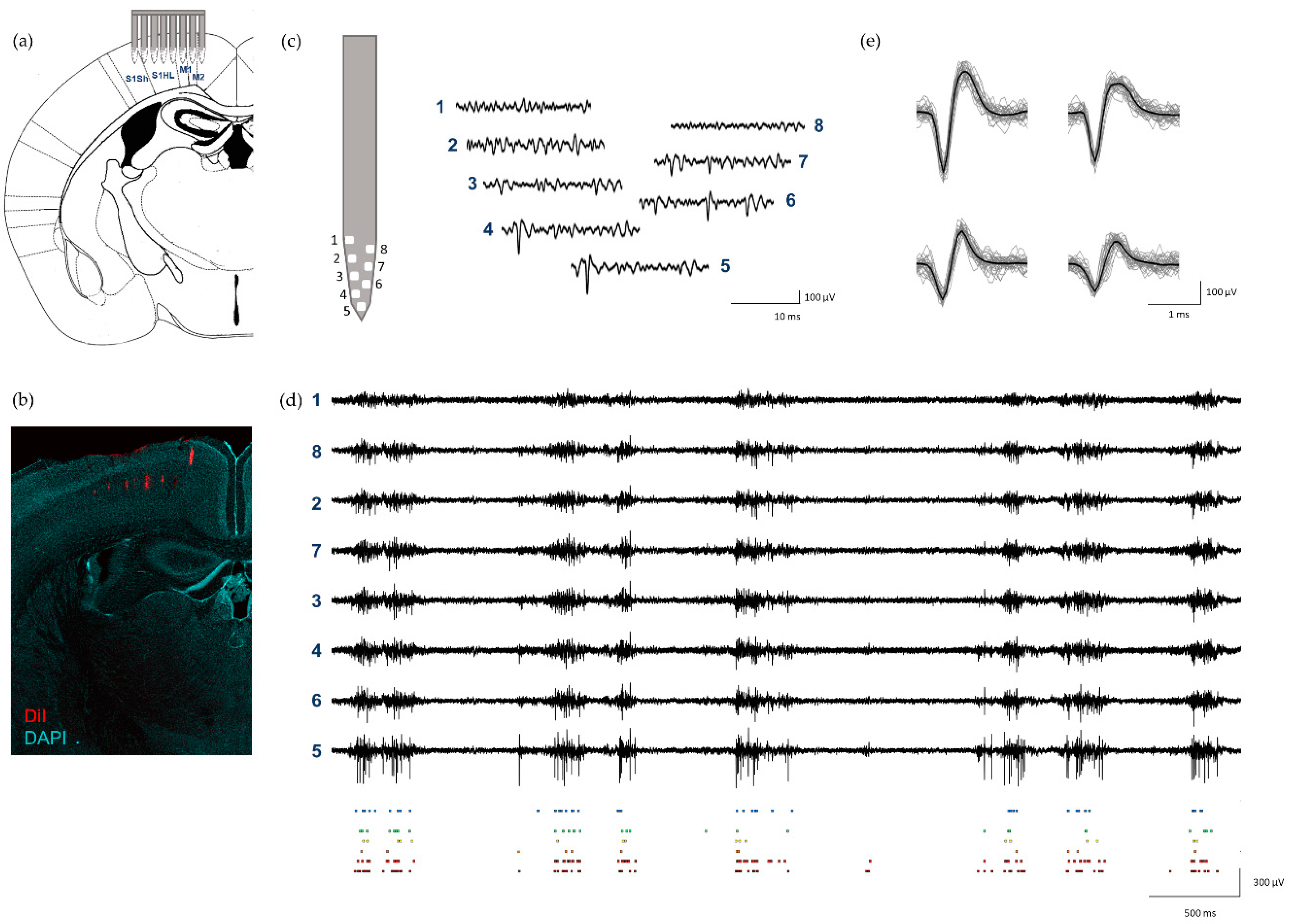
3.4. In Vivo Electrophysiology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Statement
Appendix A
Appendix A.1. Neural Probe Structural Characterization

Appendix A.2. Structural and Electrical Assessment of Flexible Connector Reinsertion Cycles

References
- Seymour, J.P.; Wu, F.; Wise, K.D.; Yoon, E. State-of-the-art mems and microsystem tools for brain research. Microsyst. Nanoeng. 2017, 3, 16066. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, K.; Lee, E.; An, T.; Choi, W.; Lim, G.; Shin, J.H. Recent progress on microelectrodes in neural interfaces. Materials 2018, 11, 1995. [Google Scholar] [CrossRef] [Green Version]
- Angotzi, G.N.; Boi, F.; Lecomte, A.; Miele, E.; Malerba, M.; Zucca, S.; Casile, A.; Berdondini, L. SiNAPS: An implantable active pixel sensor CMOS-probe for simultaneous large-scale neural recordings. Biosens. Bioelectron. 2019, 126, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydin, C.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Blanche, T.J.; Harrison, R.R.; Lester, H.A.; Masmanidis, S.C. Multiplexed, high density electrophysiology with nanofabricated neural probes. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, U.G.; Folkers, A.; Mosch, F.; Malina, T.; Menne, K.M.L.; Biella, G.; Fagerstedt, P.; De Schutter, E.; Jensen, W.; Yoshida, K.; et al. A novel high channel-count system for acute multisite neuronal recordings. IEEE Trans. Biomed. Eng. 2006, 53, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Scholvin, J.; Kinney, J.P.; Bernstein, J.G.; Moore-Koochlacs, C.; Kopell, N.; Fonstad, C.G.; Boyden, E.S. Close-packed silicon microelectrodes for scalable spatially oversampled neural recording. IEEE Trans. Biomed. Eng. 2016, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruther, P.; Paul, O. New approaches for CMOS-based devices for large-scale neural recording. Curr. Opin. Neurobiol. 2015, 32, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csicsvari, J.; Henze, D.A.; Jamieson, B.; Harris, K.D.; Sirota, A.; Bartho, P.; Wise, K.D.; Buzsaki, G. Massively parallel recording of unit and local field potentials with silicon-based electrodes. J. Neurophysiol. 2003, 90, 1314–1323. [Google Scholar] [CrossRef]
- Shobe, J.L.; Claar, L.D.; Parhami, S.; Bakhurin, K.I.; Masmanidis, S.C. Brain activity mapping at multiple scales with silicon microprobes containing 1,024 electrodes. J. Neurophysiol. 2015, 114, 2043–2052. [Google Scholar] [CrossRef] [Green Version]
- Juavinett, A.L.; Bekheet, G.; Churchland, A.K. Chronically implanted neuropixels probes enable high-yield recordings in freely moving mice. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, M.A.; Nicolelis, M.A.L. Brain-machine interfaces: From basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 2017, 97, 767–837. [Google Scholar] [CrossRef]
- Schiavone, G.; Lacour, S.P. Conformable bioelectronic interfaces: Mapping the road ahead. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weltman, A.; Yoo, J.; Meng, E. Flexible, penetrating brain probes enabled by advances in polymer microfabrication. Micromachines 2016, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiolo, L.; Polese, D.; Convertino, A. The rise of flexible electronics in neuroscience, from materials selection to in vitro and in vivo applications. Adv. Phys. X 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Araki, T.; Bongartz, L.M.; Kaiju, T.; Takemoto, A.; Tsuruta, S.; Uemura, T.; Sekitani, T. Flexible neural interfaces for brain implants-The pursuit of thinness and high density. Flex. Print. Electron. 2020, 5. [Google Scholar] [CrossRef]
- Felix, S.H.; Shah, K.G.; Tolosa, V.M.; Sheth, H.J.; Tooker, A.C.; Delima, T.L.; Jadhav, S.P.; Frank, L.M.; Pannu, S.S. Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. J. Vis. Exp. 2013, 79. [Google Scholar] [CrossRef] [Green Version]
- Na, K.; Sperry, Z.J.; Lu, J.; Voroslakos, M.; Parizi, S.S.; Bruns, T.M.; Yoon, E.; Seymour, J.P. Novel diamond shuttle to deliver flexible neural probe with reduced tissue compression. Microsyst. Nanoeng. 2020, 6, 37. [Google Scholar] [CrossRef]
- Chung, J.E.; Joo, H.R.; Fan, J.L.; Liu, D.F.; Bernett, A.H.; Chen, S.; Geaghan-Breiner, C.; Karlsson, M.P.; Karlsson, M.; Lee, K.Y.; et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 2019, 101, 21–31. [Google Scholar] [CrossRef]
- Scholten, K.; Larson, C.E.; Xu, H.; Song, D.; Meng, E. A 512-channel multi-layer polymer-based neural probe array. J. Microelectromech. Syst. 2020, 29, 1054–1058. [Google Scholar] [CrossRef]
- Sim, K.; Rao, Z.; Li, Y.; Yang, D.; Yu, C. Curvy surface conformal ultra-thin transfer printed Si optoelectronic penetrating microprobe arrays. NPJ Flex. Electron. 2018, 2. [Google Scholar] [CrossRef]
- Xu, H.; Hirschberg, A.W.; Scholten, K.; Berger, T.W.; Song, D.; Meng, E. Acute in vivo testing of a conformal polymer microelectrode array for multi-region hippocampal recordings. J. Neural Eng. 2018. [Google Scholar] [CrossRef]
- Yao, Y.; Gulari, M.N.; Casey, B.; Wiler, J.A.; Wise, K.D. Silicon microelectrodes with flexible integrated cables for neural implant applications. In Proceedings of the 3rd International IEEE EMBS Conference on Neural Engineering, Kona, HI, USA, 2–5 May 2007; pp. 398–401. [Google Scholar] [CrossRef]
- Huang, R.; Pang, C.; Tai, Y.C.; Emken, J.; Ustun, C.; Andersen, R.; Burdick, J. Integrated parylene-cabled silicon probes for neural prosthetics. In Proceedings of the IEEE International Conference on Micro Electro. Mechanical Systems (MEMS), Tucson, AZ, USA, 13–17 January 2008; pp. 240–243. [Google Scholar] [CrossRef] [Green Version]
- Kisban, S.; Janssen, P.; Herwik, S.; Stieglitz, T.; Paul, O.; Ruther, P. Hybrid microprobes for chronic implantation in the cerebral cortex. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS’08–Personalized Healthcare through Technology, Vancouver, BC, Canada, 20–25 August 2008; pp. 2016–2019. [Google Scholar] [CrossRef]
- Michon, F.; Aarts, A.; Holzhammer, T.; Ruther, P.; Borghs, G.; McNaughton, B.; Kloosterman, F. Integration of silicon-based neural probes and micro-drive arrays for chronic recording of large populations of neurons in behaving animals. J. Neural Eng. 2016. [Google Scholar] [CrossRef] [PubMed]
- Barz, F.; Ruther, P.; Takeuchi, S.; Paul, O. Mechanically adaptive silicon-based neural probes for chronic high-resolution neural recording. Procedia Eng. 2015. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.G.R.; John, J.K.; Tu, H.; Zheng, Q.; Loeb, J.; Zhang, J.; Xu, Y. A hybrid silicon-parylene neural probe with locally flexible regions. Sens. Actuators B Chem. 2014, 195, 416–422. [Google Scholar] [CrossRef]
- Yang, L.; Lee, K.; Villagracia, J.; Masmanidis, S.C. Open source silicon microprobes for high throughput neural recording. J. Neural Eng. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.M.; Hong, G.; Viveros, R.D.; Zhou, T.; Lieber, C.M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.J.; Kuo, J.T.W.; Hara, S.A.; Lee, C.D.; Yu, L.; Gutierrez, C.A.; Hoang, T.Q.; Pikov, V.; Meng, E. 3D Parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norlin, P.; Kindlundh, M.; Mouroux, A.; Yoshida, K.; Hofmann, U.G. A 32-site neural recording probe fabricated by DRIE of SOI substrates. J. Micro Mech. Micro Eng. 2002, 12, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Schander, A.; Stemmann, H.; Kreiter, A.K.; Lang, W. Silicon-based microfabrication of free-floating neural probes and insertion tool for chronic applications. Micromachines 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Pang, C.; Cham, J.G.; Nenadic, Z.; Musallam, S.; Tai, Y.-C.; Burdick, J.W.; Andersen, R.A. A new multi-site probe array with monolithically integrated parylene flexible cable for neural prostheses. In Proceedings of the 27th Annual International Conference of the IEEE Engineering in Medicine and Biology, Shanghai, China, 1–4 September 2005; pp. 7114–7117. [Google Scholar] [CrossRef] [Green Version]
- Garcia, I.S.; Moreira, E.E.; DIas, R.A.; Gaspar, J.; Alves, F.S.; Rocha, L.A. Sub-micron mems accelerometer with handle-layer patterning for damping enhancement using time transduction. In Proceedings of the 20th International Conference on Solid-State Sensors, Actuators and Microsystems and Eurosensors XXXIII, Transducers 2019 and Eurosensors XXXIII, Berlin, Germany, 23–27 June 2019; pp. 2045–2048. [Google Scholar] [CrossRef]
- Fischer, L.M.; Tenje, M.; Heiskanen, A.R.; Masuda, N.; Castillo, J.; Bentien, A.; Emneus, J.; Jakobsen, M.H.; Boisen, A. Gold cleaning methods for electrochemical detection applications. Microelectron. Eng. 2009, 86, 1282–1285. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 1983; Volume 3. [Google Scholar]
- Siegle, J.H.; López, A.C.; Patel, Y.A.; Abramov, K.; Ohayon, S.; Voigts, J. Open Ephys: An open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. 2017, 14. [Google Scholar] [CrossRef]
- Jun, J.J.; Mitelut, C.; Lai, C.; Gratiy, S.L.; Anastassiou, C.A.; Harris, T.D. Real-time spike sorting platform for high-density extracellular probes with ground-truth validation and drift correction. bioRxiv 2017. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Son, Y.; Chae, U.; Kim, J.; Choi, N.; Lee, H.J.; Woo, J.; Cho, Y.; Yang, S.H.; Lee, C.J.; et al. Multifunctional multi-shank neural probe for investigating and modulating long-range neural circuits in vivo. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Karumbaiah, L.; Saxena, T.; Carlson, D.; Patil, K.; Patkar, R.; Gaupp, E.A.; Betancur, M.; Stanley, G.B.; Carin, L.; Bellamkonda, R.V. Relationship between intracortical electrode design and chronic recording function. Biomaterials 2013, 34, 8061–8074. [Google Scholar] [CrossRef] [PubMed]
- Szarowski, D.H.; Andersen, M.D.; Retterer, S.; Spence, A.J.; Isaacson, M.; Craighead, H.G.; Turner, J.N.; Shain, W. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar] [CrossRef]
- Peled, A.; Pevzner, A.; Peretz Soroka, H.; Patolsky, F. Morphological and chemical stability of silicon nanostructures and their molecular overlayers under physiological conditions: Towards long-term implantable nanoelectronic biosensors. J. Nano Biotechnol. 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.-Y.; Park, W.-T.; Yu, A.; Xue, R.-F.; Tan, K.L.; Yu, D.; Lee, S.-H.; Gan, C.L.; Je, M. A flexible polyimide cable for implantable neural probe arrays. Microsyst. Technol. 2013, 19, 1111–1118. [Google Scholar] [CrossRef]
- Wu, F.; Stark, E.; Im, M.; Cho, I.-J.; Yoon, E.-S.; Buzsaki, G.; Wise, K.D.; Yoon, E. An implantable neural probe with monolithically integrated dielectric waveguide and recording electrodes for optogenetics applications. J. Neural Eng. 2013. [Google Scholar] [CrossRef] [PubMed]
- Abadias, G.; Chason, E.; Keckes, J.; Sebastiani, M.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.E.; Stoessel, C.H.; Martinu, L. Review Article: Stress in thin films and coatings: Current status, challenges, and prospects. J. Vac. Sci. Technol. A 2018. [Google Scholar] [CrossRef] [Green Version]
- Buzsáki, G.; Stark, E.; Berenyi, A.; Khodaholy, D.; Kipke, D.R.; Yoon, E.; Wise, K.D. Tools for probing local circuits: High-density silicon probes combined with optogenetics. Neuron 2015, 86, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Herwik, S.; Kisban, S.; Aarts, A.A.A.; Seidl, K.; Girardeau, G.; Benchenane, K.; Zugaro, M.B.; Wiener, S.I.; Paul, O.; Neves, H.P.; et al. Fabrication technology for silicon-based microprobe arrays used in acute and sub-chronic neural recording. J. Micro Mech. Micro Eng. 2009, 19. [Google Scholar] [CrossRef]
- Ronova, I.A.; Bruma, M.; Schmidt, H.W. Conformational rigidity and dielectric properties of polyimides. Struct. Chem. 2012, 23, 219–226. [Google Scholar] [CrossRef]
- Khanna, V.K. Adhesion-delamination phenomena at the surfaces and interfaces in microelectronics and MEMS structures and packaged devices. J. Phys. D Appl. Phys. 2011, 44. [Google Scholar] [CrossRef]
- Gutierrez, C.A.; Lee, C.; Kim, B.; Meng, E. Epoxy-less packaging methods for electrical contact to parylene-based flat flexible cables. In Proceedings of the 16th International Solid-State Sensors, Actuators and Microsystems Conference, TRANSDUCERS’11, Beijing, China, 5–9 June 2011. [Google Scholar] [CrossRef] [Green Version]
- Neto, J.P.; Baiao, P.; Lopes, G.; Frazao, J.; Nogueira, J.; Fortunato, E.; Barguinha, P.; Kammpff, A.R. Does impedance matter when recording spikes with polytrodes? Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novais, A.; Calaza, C.; Fernandes, J.; Fonseca, H.; Monteiro, P.; Gaspar, J.; Jacinto, L. Hybrid Multisite Silicon Neural Probe with Integrated Flexible Connector for Interchangeable Packaging. Sensors 2021, 21, 2605. https://doi.org/10.3390/s21082605
Novais A, Calaza C, Fernandes J, Fonseca H, Monteiro P, Gaspar J, Jacinto L. Hybrid Multisite Silicon Neural Probe with Integrated Flexible Connector for Interchangeable Packaging. Sensors. 2021; 21(8):2605. https://doi.org/10.3390/s21082605
Chicago/Turabian StyleNovais, Ashley, Carlos Calaza, José Fernandes, Helder Fonseca, Patricia Monteiro, João Gaspar, and Luis Jacinto. 2021. "Hybrid Multisite Silicon Neural Probe with Integrated Flexible Connector for Interchangeable Packaging" Sensors 21, no. 8: 2605. https://doi.org/10.3390/s21082605
APA StyleNovais, A., Calaza, C., Fernandes, J., Fonseca, H., Monteiro, P., Gaspar, J., & Jacinto, L. (2021). Hybrid Multisite Silicon Neural Probe with Integrated Flexible Connector for Interchangeable Packaging. Sensors, 21(8), 2605. https://doi.org/10.3390/s21082605






