Research of Fluorescent Properties of a New Type of Phosphor with Mn2+-Doped Ca2SiO4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Experimental Setup
3. Results and Discussion
3.1. Structure Properties
3.2. Fluorescence Spectrum and Lifetime
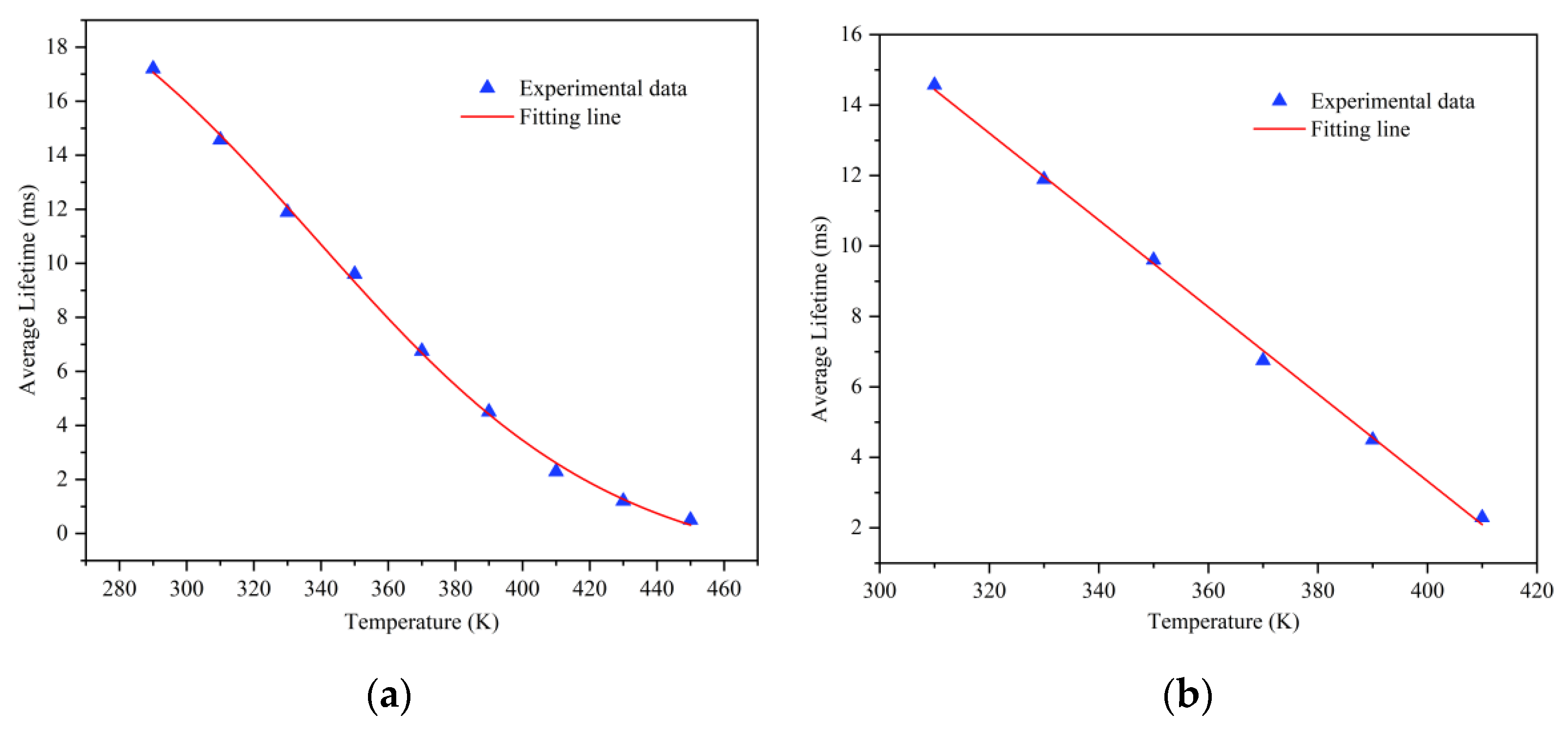
3.3. The Relationship between Spectrum and Lifetime with Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Weaver, J.B. Bioimaging: Hot nanoparticles light up cancer. Nat. Nanotechnol. 2010, 5, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Michalski, L.; Eckersdorf, K.; Kucharski, J.; McGhee, J. Temperature Measurement; Wiley: Chichester, UK, 2001. [Google Scholar]
- Maekawa, S. Physics of Transition Metal Oxides; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Beheim, G. Integrated Optics Microstructure Sensors; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Zhang, H.; Ye, J.; Wang, X.; Zhao, S.; Lei, R.; Huang, L.; Xu, S. Highly reliable all-fiber temperature sensor based on the fluoresence intensity ratio (FIR) technique in Er3+/Yb3+ co-doped NaYF4 phosphors. J. Mater. Chem. C. 2019, 7, 15269–15275. [Google Scholar] [CrossRef]
- Zhao, F.; Tang, Z.; Cui, H.Y. On-line monitoring system of electrical equipment based on fluorescent optical fiber temperature measurement. Electr. Meas. Instrum. 2015, 52, 85–89. [Google Scholar]
- Wang, F.; Zhu, H.; Li, Y.P. Research on temperature sensing of dislocation fiber interference laser spectroscopy combined with BP neural network. Spectrosc. Spectr. Anal. 2016, 36, 3732–3736. [Google Scholar]
- Wang, D.D. Research on Fiber Grating Temperature Measurement System Based on F-P Filter Demodulation. Master’s Thesis, Beijing Industry University, Beijing, China, 2011. [Google Scholar]
- Hu, K.; Fu, H.N.; Luo, X.X. Multi-channel self-calibrating gallium arsenide absorption optical fiber temperature monitoring system. Chin. J. Lasers 2015, 42, 114–122. [Google Scholar]
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef]
- Liu, J.X.; Lang, Y.X.; Wei, D.F. Research on calibration and temperature compensation method of cable distributed optical fiber temperature measuring device. J. Electr. Eng. 2020, 15, 121–127. [Google Scholar]
- Suo, H.; Guo, C.; Zheng, J.; Zhou, B.; Ma, C.; Zhao, X.; Li, T.; Guo, P.; Goldys, E.M. Sensitivity modulation of upconverting thermometry through engineering phonon energy of a matrix. ACS Appl. Mater. Interfaces 2016, 8, 30312–30319. [Google Scholar] [CrossRef]
- Boruc, Z.; Kaczkan, M.; Fetlinski, B.; Turczynski, S.; Malinowski, M. Blue emissions in Dy3+ doped Y4Al2O9 crystals for temperature sensing. Opt. Lett. 2012, 37, 5214–5216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bu, Y.; Yan, X.; Cai, P.; Wang, J.; Qin, L.; Vu, T.; Seo, H.J. Detecting the origin of luminescence in Er3+-doped hexagonal Na1.5Gd1.5F6 phosphors. Opt. Lett. 2016, 41, 5314–5317. [Google Scholar] [CrossRef]
- Back, M.; Trave, E.; Ueda, J.; Tanabe, S. Ratiometric optical thermometer based on dual near-infrared emission in Cr3+-doped bismuth-based gallate host. Chem. Mater. 2016, 28, 8347–8356. [Google Scholar] [CrossRef]
- Suo, Y. Optical Fiber Temperature Measurement System Based on Fluoresence Intesity. Master’s Thesis, Zhejiang University, Hangzhou, China, 2017. [Google Scholar]
- Collins, S.F.; Baxter, G.W.; Wade, S.A.; Sun, T.; Grattan, K.T.; Zhang, Z.Y.; Palmer, A.A. Comparison of fluorescence-based temperature sensor schemes: Theoretical analysis and experimental validation. J. Appl. Phys. 1998, 84, 4649–4654. [Google Scholar] [CrossRef]
- Jiang, B.; Chi, F.; Zhao, L.; Wei, X.; Chen, Y.; Yin, M. Luminescence properties of a new green emitting long afterglow phosphor Ca14Zn6Ga10O35:Mn2+,Ge4+. J. Lumin. 2019, 206, 234–239. [Google Scholar] [CrossRef]
- Shoaib, M.; Rooh, G.; Chanthima, N.; Kim, H.J.; Rajaramakrishna, R.; Kothan, S.; Kaewkhao, J.; Siengsanoh, K. The physical, optical, photo and radioluminescence studies of Dy3+ doped Zinc Barium Gadolinium phosphate glasses. Glass Phys. Chem. 2021, 46, 474–486. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, G.; Li, X.; Jiang, S.; Wei, X.; Chen, Y.; Yin, M.; Duan, C. Strategy for thermometry via Tm3+-doped NaYF4 core-shell nanoparticles. Opt. Lett. 2014, 39, 6687–6690. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadeethi, Y.; Sayyed, M.I.; Raffah, B.M.; Bekyarova, E.; Abid, A.A.; Rammah, Y.S. Synthesis, physical, optical properties, and gamma-ray absorbing competency or capability of PbO–B2O3–CaO glasses reinforced with Nd3+/Er3+ ions. Eur. Phys. J. Plus 2021, 136, 1–9. [Google Scholar] [CrossRef]
- Asada, S.; Okada, G.; Kato, T.; Nakamura, F.; Kawano, N.; Kawaguchi, N.; Yanagida, T. Eu-doped Ca2SiO4 as a new radio-photoluminescence phosphor. Chem. Lett. 2017, 42, 1102. [Google Scholar] [CrossRef]
- Zheng, B.F.; Yang, X.Y.; Yan, C.G. Raman spectra and fluorescence spectra of Tb doped aluminosilicate. Spectrosc. Spectr. Anal. 2019, 39, 2371–2376. [Google Scholar]
- Lojpur, V.; Nikolić, M.G.; Jovanović, D.; Medić, M.; Antić, Ž.; Dramićanin, M.D. Luminescence thermometry with Zn2SiO4:Mn2+ powder. Appl. Phys. Lett. 2013, 103, 141912. [Google Scholar] [CrossRef]
- Huang, F.; Chen, D.Q. Synthesis of Mn2+:Zn2SiO4–Eu3+:Gd2O3 nanocomposites for highly sensitive optical thermometry through the synergistic luminescence from lanthanide-transition metal ions. J. Mater. Chem. C 2017, 5, 5176–5182. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Li, X.; Guo, H.; Liu, S.; Li, X. Cr3+-doped Bi2Ga4O9-Bi2Al4O9 solid-solution phosphors: Crystal-field modulation and lifetime-based temperature sensing. Opt. Lett. 2017, 42, 4950–4953. [Google Scholar] [CrossRef]
- Wu, Y.X. Study on Cr3+:MgAl2O4 Optical Fiber Fluoresence Lifetme Temperature Sensor; Zhejiang University: Hangzhou, China, 2011. [Google Scholar]
- Chi, F.; Jiang, B.; Zhao, Z.; Chen, Y.; Wei, X.; Duan, C.; Yin, M.; Xu, W. Multimodal temperature sensing using Zn2GeO4:Mn2+ phosphor as highly sensitive luminescent thermometer. Sens. Actuators B Chem. 2019, 296, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, C.; Liu, X.; Li, G.; Luo, Y.; Quan, Z.; Xiang, H.; Lin, J. Tunable photoluminescent and cathodoluminescent properties of ZnO and ZnO:Zn phosphors. J. Phys. Chem. B 2006, 110, 9469–9476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, C.; Liu, X.; Li, G.; Luo, Y.; Quan, Z.; Xiang, H.; Lin, J. Fe3+ red phosphors based on lithiumaluminates and an aluminum lithium oxyfluoride prepared from LiF as the Li Source. J. Lumin. 2017, 182, 53–58. [Google Scholar]
- Duan, X.L.; Yuan, D.R.; Wang, L.H.; Yu, F.P.; Cheng, X.F.; Liu, Z.Q.; Yan, S.S. Synthesis and optical properties of Co2+ doped ZnGa2O4 nanocrystals. J. Cryst. Growth 2006, 296, 234–238. [Google Scholar] [CrossRef]
- Ni, J. Synthesis and Luminescent Properties of SiAlON Phosphors Doped by Transition Metal Mn. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2018. [Google Scholar]
- Chi, F.; Wei, X.; Jiang, B.; Chen, Y.; Duan, C.; Yin, M. Luminescence properties and the thermalquenching mechanism of Mn2+ doped Zn2GeO4 long persistent phosphors. Dalton Trans. 2018, 47, 1303–1311. [Google Scholar] [CrossRef]
- Zhang, Y. Synthesis and Research on Photoluminescence of Rare-Earth Ions Doped Silicate Phosphors. Master’s Thesis, University of Science and Technology of China, Hefei, China, 2019. [Google Scholar]
- Sato, Y.; Kato, H.; Kobayashi, M.; Masaki, T.; Yoon, D.H.; Kakihana, M. Tailoring of deep-red luminesence in Ca2SiO4:Eu2+. Angew. Chem. Int. Ed. Engl. 2014, 53, 7756. [Google Scholar] [CrossRef]
- Kalaji, A.; Mikami, M.; Cheetham, A.K. Ce3+-activated γ-Ca2SiO4 and other olivine-type ABXO4 phosphors for solid-state lighting. Chem. Mater. 2014, 26, 3966–3975. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, Y.; Xu, W.; Wang, K.; Liu, Z.; Hong, M. Photoluminescence properties and thermal stability of Eu2+ and Mn2+ co-doped BaSi3O4N2 phosphors. J. Alloy. Compd. 2015, 653, 148–155. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Wei, X.; Duan, C.; Yin, M. A new mechanism for temperature sensing based on the thermal population of 7F2 state in Eu3+. Sens. Actuators B Chem. 2016, 231, 641–645. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, X.; Zhao, L.; Chen, Y.; Yin, M. Investigation of SrB4O7:Sm2+ as a multimode temperature sensor with high sensitivity. ACS Appl. Mater. Inter. 2016, 8, 34546–34551. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liu, Z.; Lv, C.; Qin, W. Temperature Sensor Based on the UV Upconversion Luminescence of Gd3+ in Yb3+-Tm3+-Gd3+ Codoped NaLuF4 Microcrystals. J. Mater. Chem. C. 2013, 1, 5502–5507. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, V.K.; Kumar, V.; Kumar, V.; Swart, H.C. Upconversion based temperature sensing ability of Er3+–Yb3+ codoped SrWO4: An optical heating phosphor. Sens. Actuator B Chem. 2015, 209, 352–358. [Google Scholar] [CrossRef]
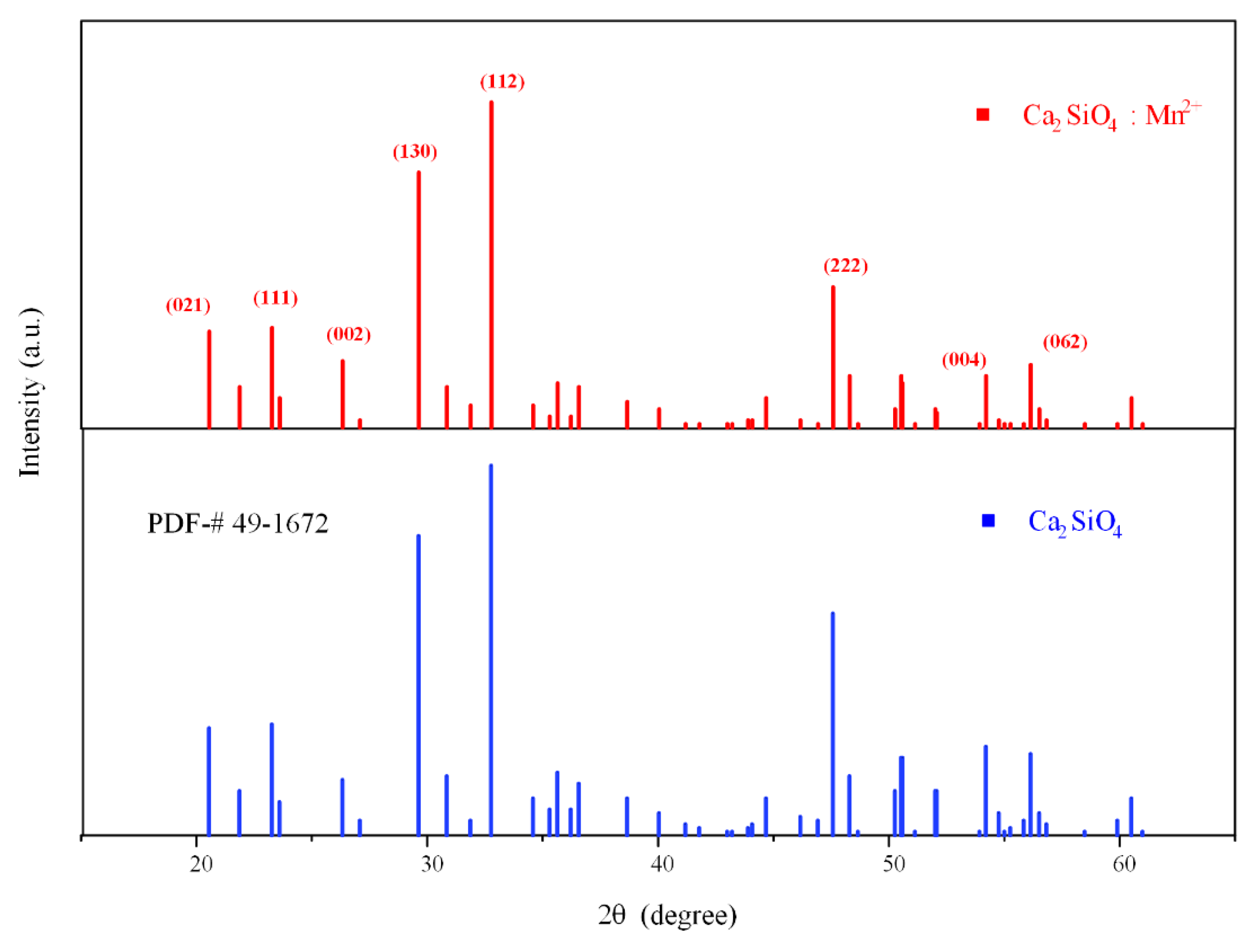

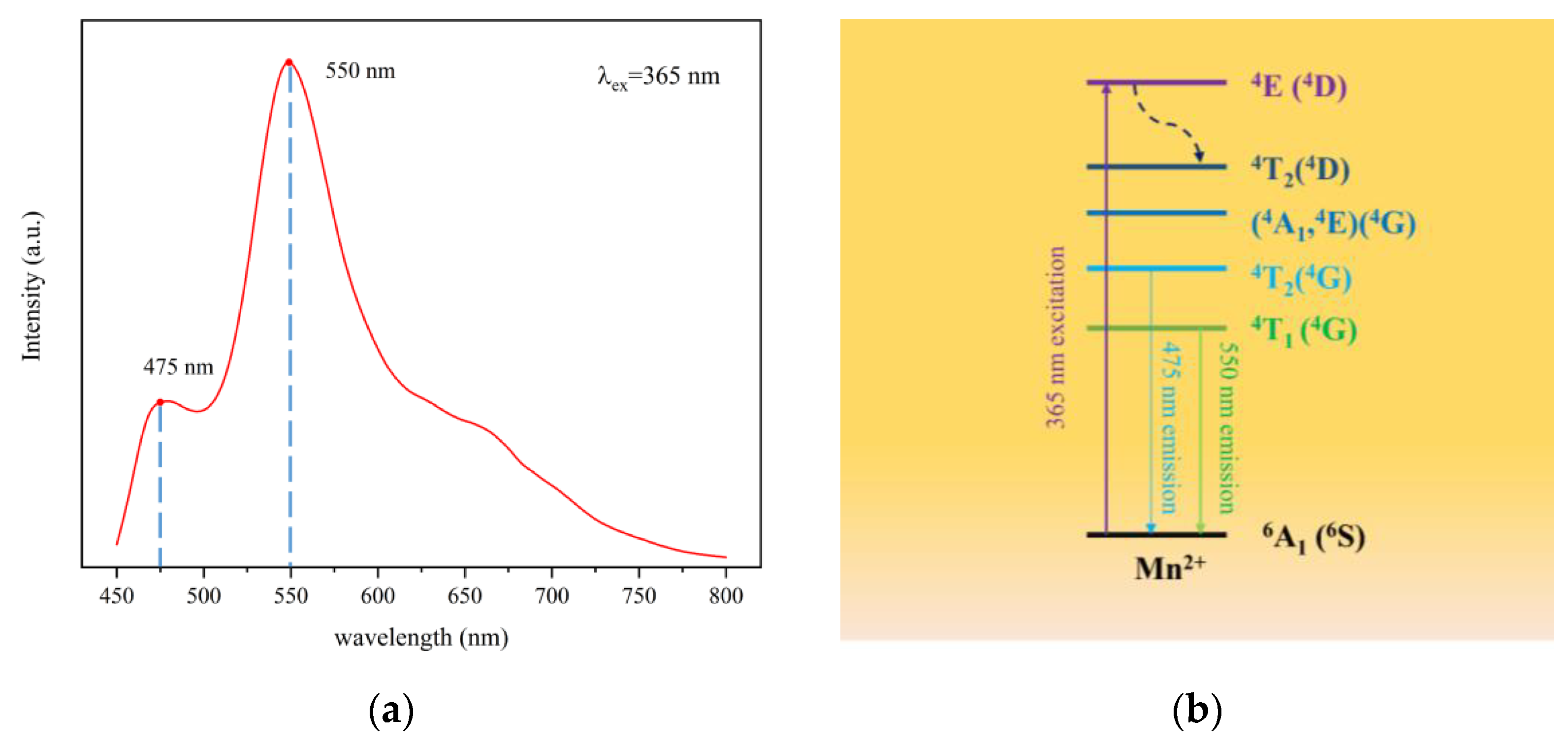
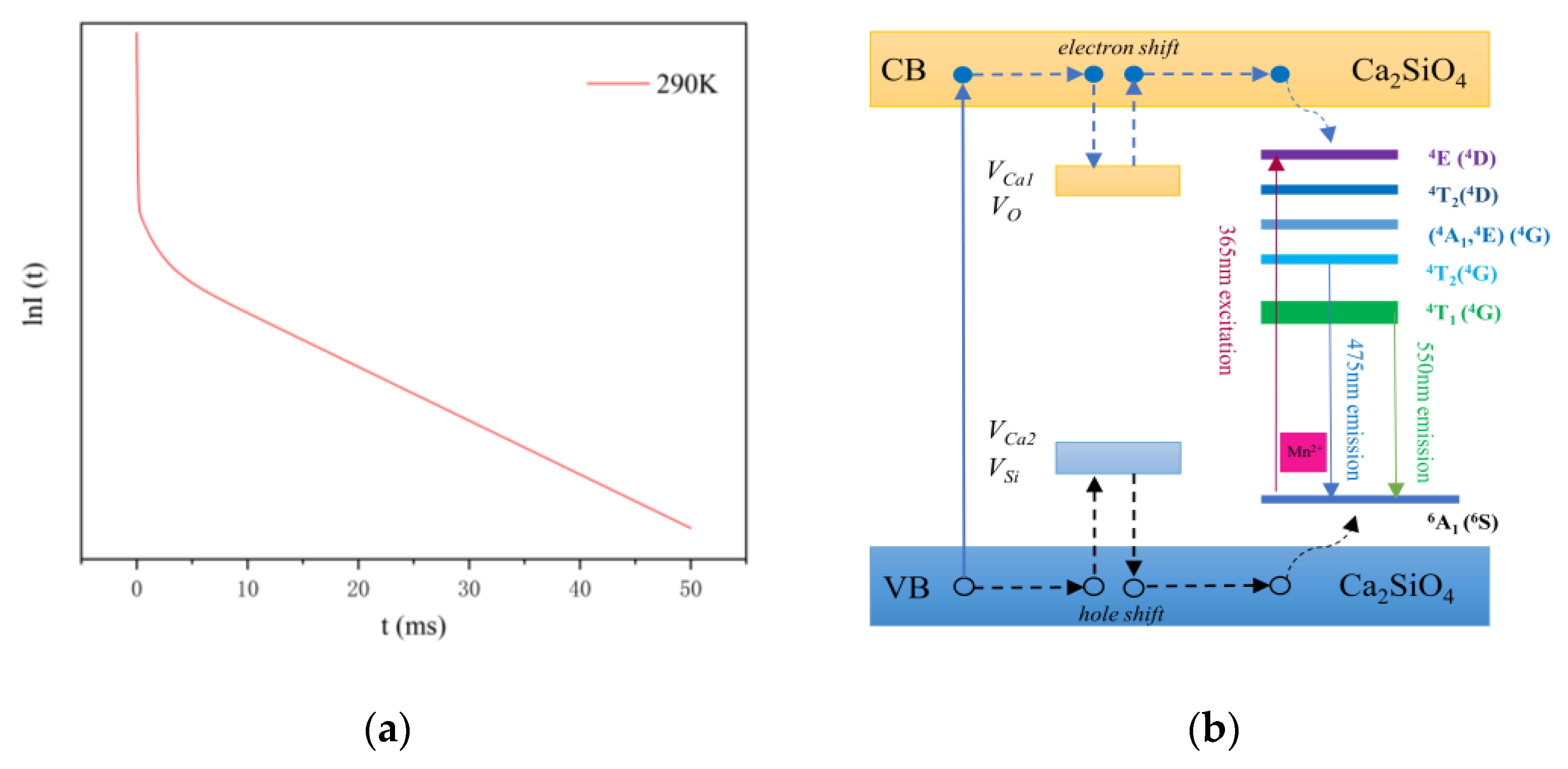

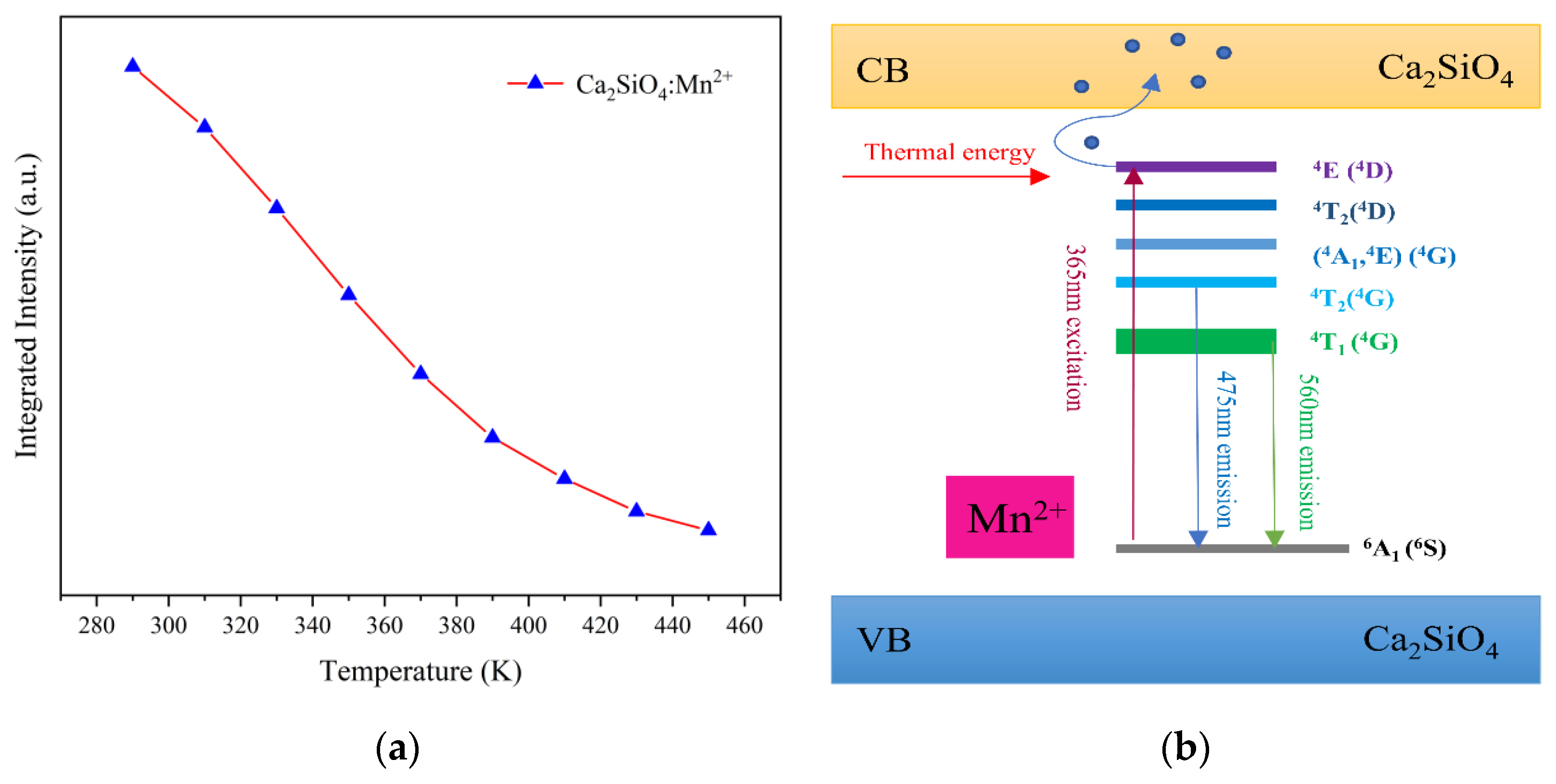


| Sample | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|
| CSO | 5.076 | 11.214 | 6.758 | 384.7 |
| CSO:Mn2+ | 5.032 | 11.173 | 6.701 | 376.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Zhang, W.; Lü, F.; Sui, Y.; Wang, J.; Xu, Z. Research of Fluorescent Properties of a New Type of Phosphor with Mn2+-Doped Ca2SiO4. Sensors 2021, 21, 2788. https://doi.org/10.3390/s21082788
Fan X, Zhang W, Lü F, Sui Y, Wang J, Xu Z. Research of Fluorescent Properties of a New Type of Phosphor with Mn2+-Doped Ca2SiO4. Sensors. 2021; 21(8):2788. https://doi.org/10.3390/s21082788
Chicago/Turabian StyleFan, Xiaozhou, Wenqi Zhang, Fangcheng Lü, Yueyi Sui, Jiaxue Wang, and Ziqiang Xu. 2021. "Research of Fluorescent Properties of a New Type of Phosphor with Mn2+-Doped Ca2SiO4" Sensors 21, no. 8: 2788. https://doi.org/10.3390/s21082788
APA StyleFan, X., Zhang, W., Lü, F., Sui, Y., Wang, J., & Xu, Z. (2021). Research of Fluorescent Properties of a New Type of Phosphor with Mn2+-Doped Ca2SiO4. Sensors, 21(8), 2788. https://doi.org/10.3390/s21082788





