Dielectrophoresis Prototypic Polystyrene Particle Synchronization toward Alive Keratinocyte Cells for Rapid Chronic Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
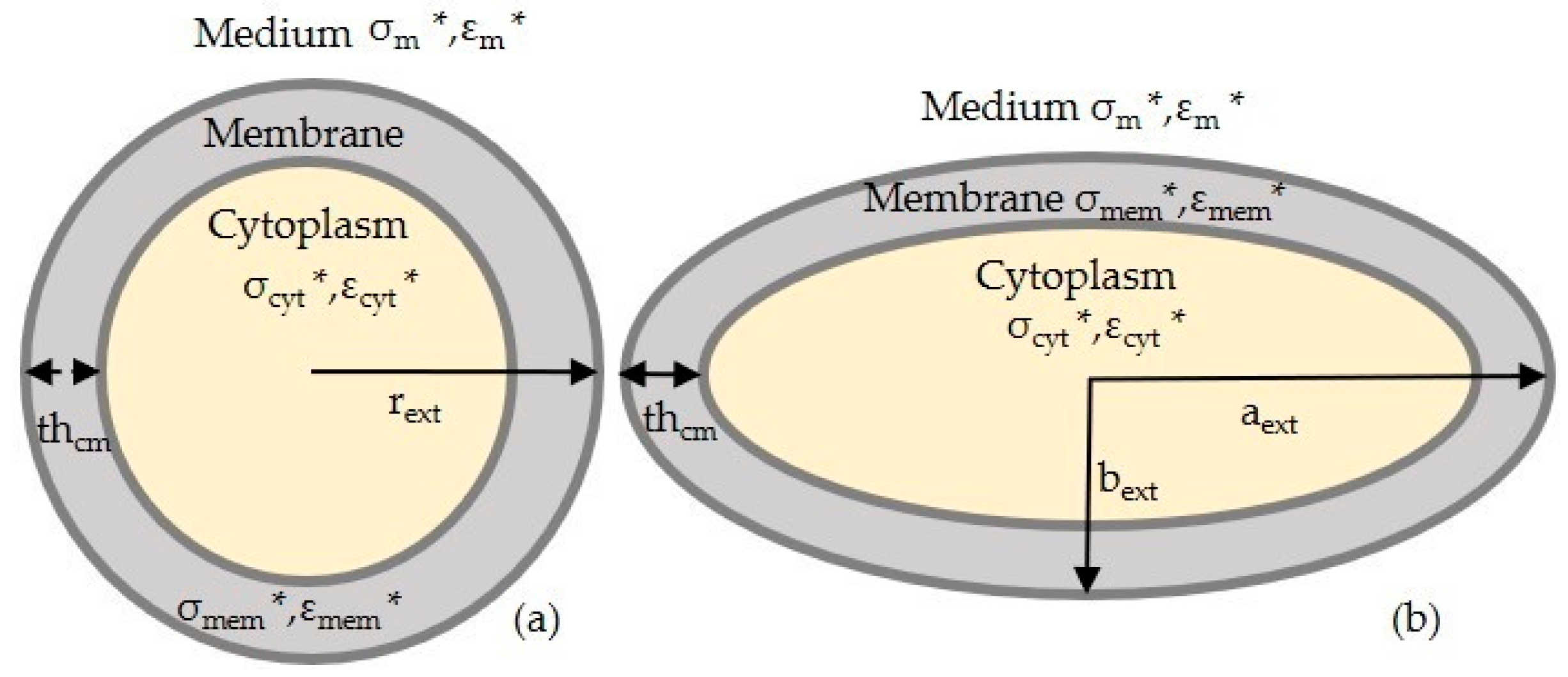
2.1. FDEP Theory
2.2. MyDEP for Numerical Simulations
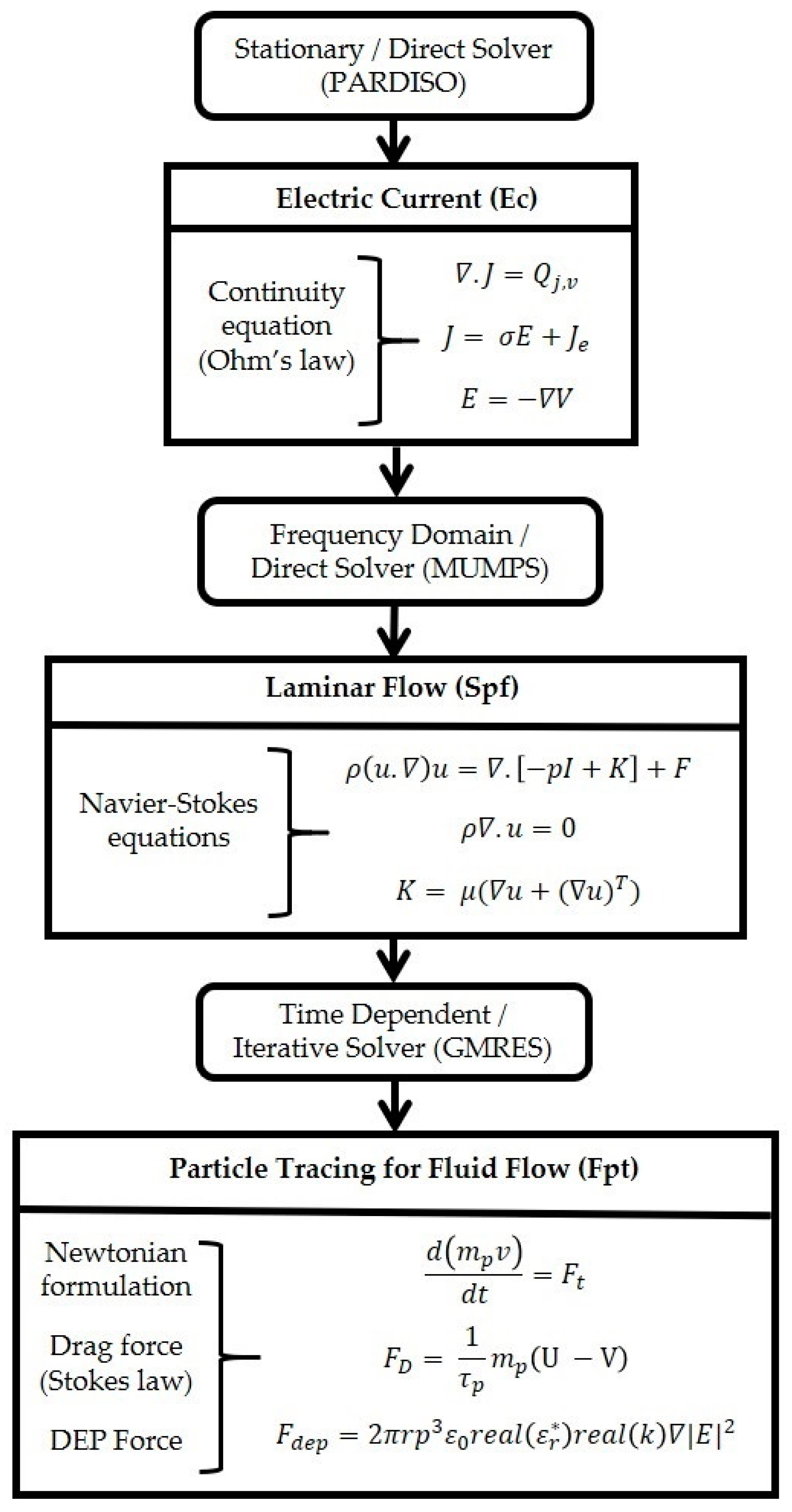
2.3. FEM Simulation
- Study 1, the electrical current and the laminar flow interface are used. Electric current defines the electrical potential and it is conducted in a frequency domain (AC). The laminar flow is interlinked to the stationary study.
- Study 2 involves the particle tracing for fluid flow interface. This interface relies on the results from Study 1. The coupling was achieved by interlinking the frequency domain solution of Study 1 to solve the particle trajectory with FDEP. The coupled equation system of Study 2 was solved using the acquired Study 1 solution; therefore, Study 1 and 2 are interlinked. A time-dependent study was used to support this analysis.
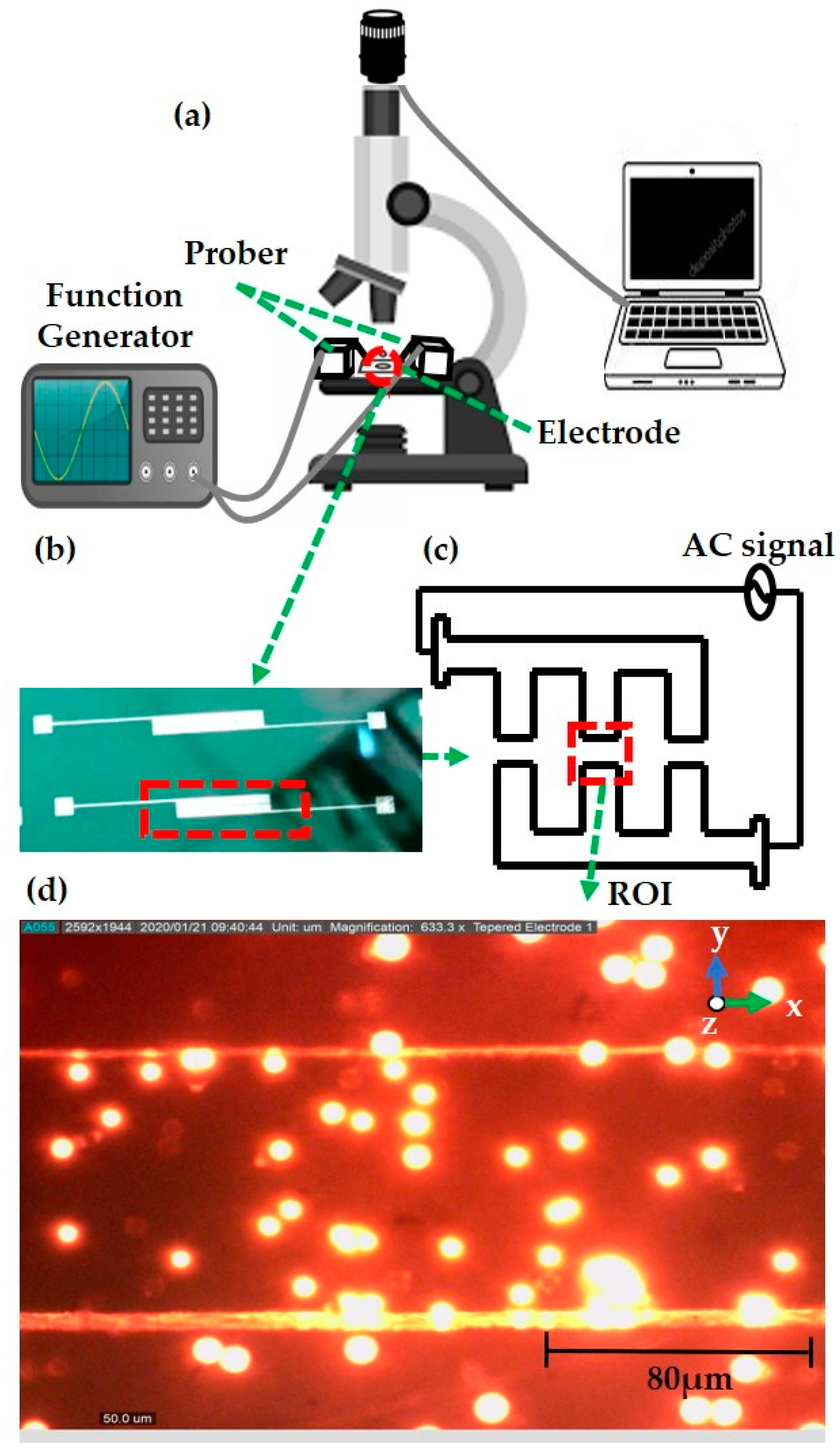
2.4. Fabrication of a Microelectrode
2.5. DEP Experimental Setup
2.6. DIPP-MotionV Motion Analysis
3. Results
3.1. Numerical Analysis by MyDEP
3.1.1. Modelling of Polystyrene Particles
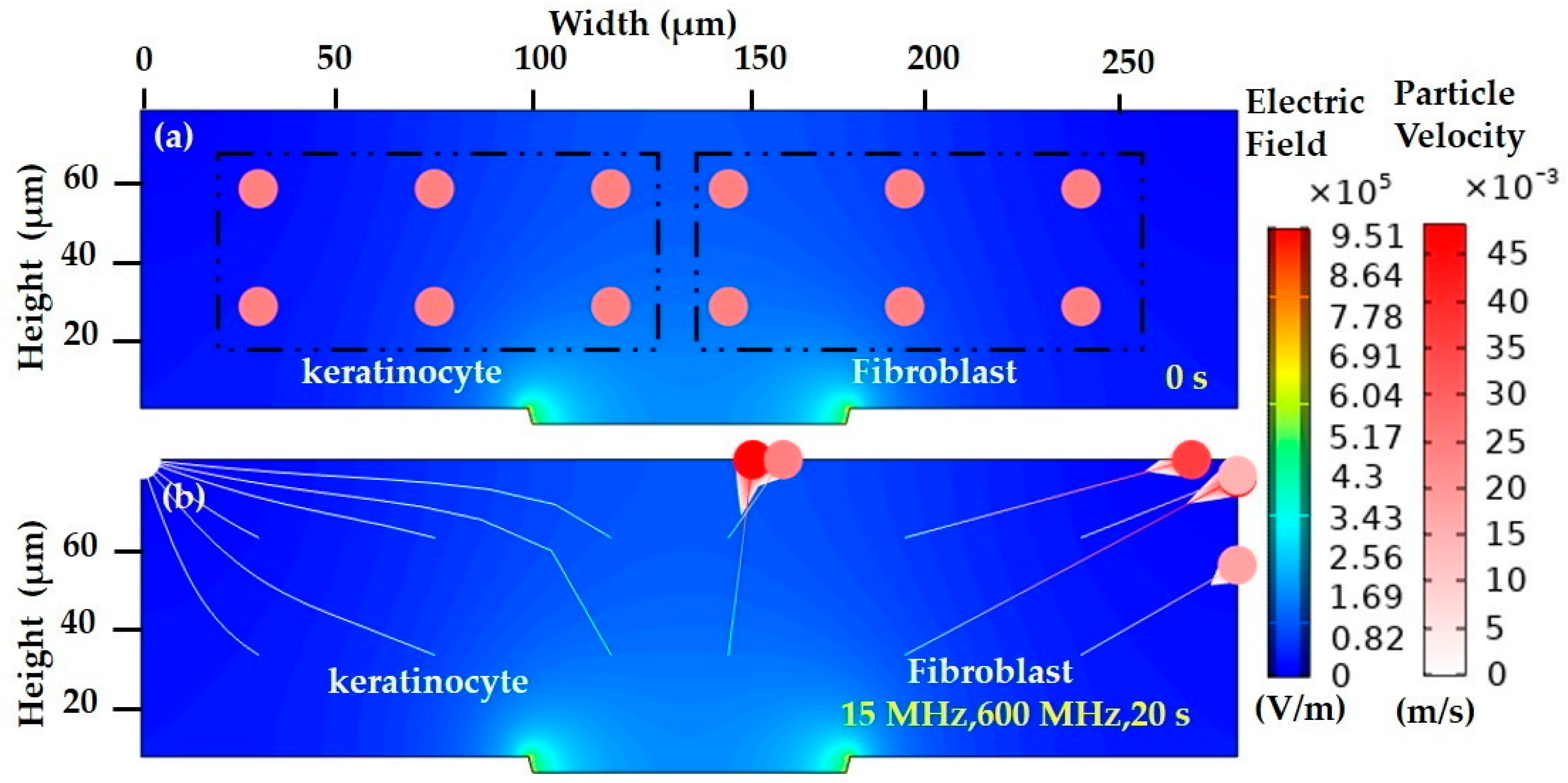
3.1.2. Modelling of Keratinocyte and Fibroblast Cells in a Low Conductive Medium
3.1.3. Modelling of Keratinocyte and Fibroblast Cells in a High Conductive Medium
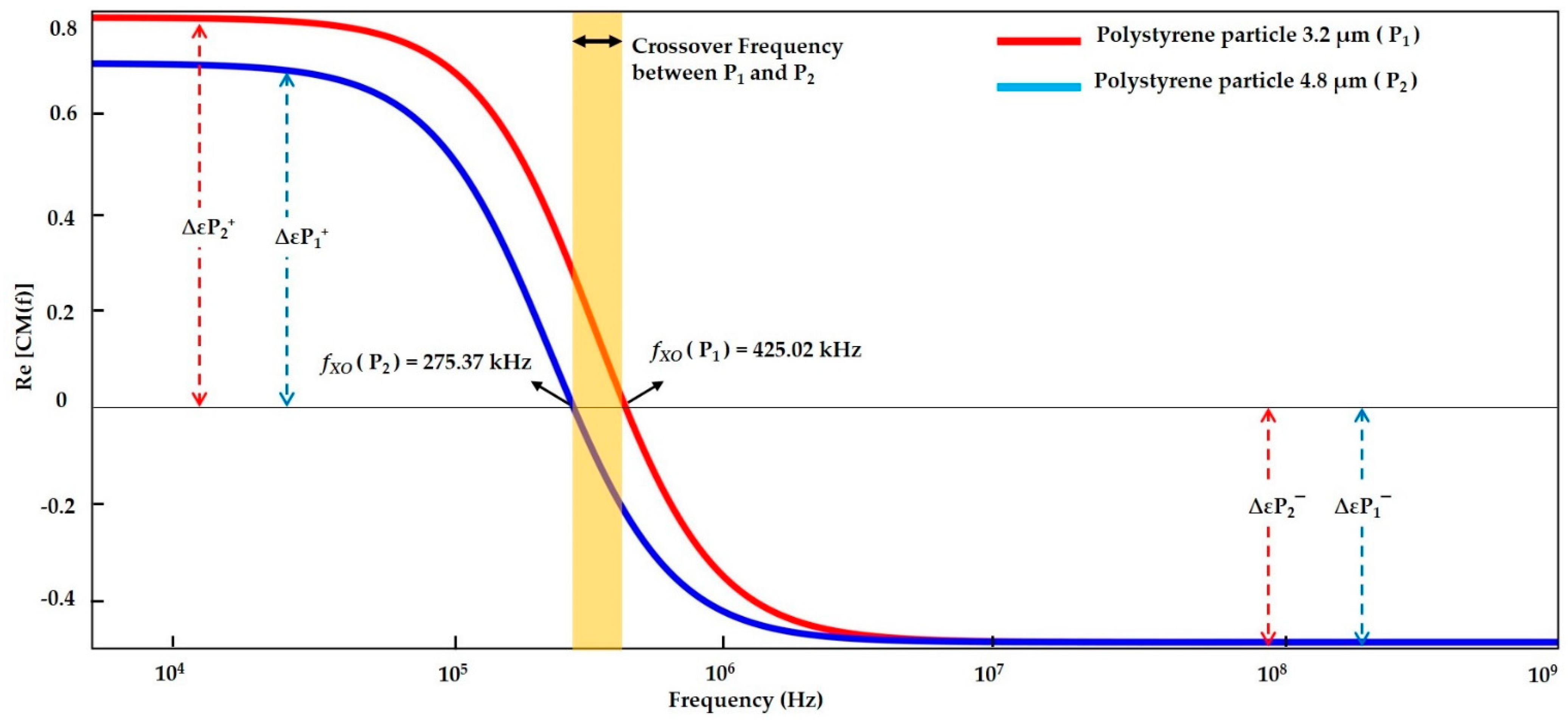
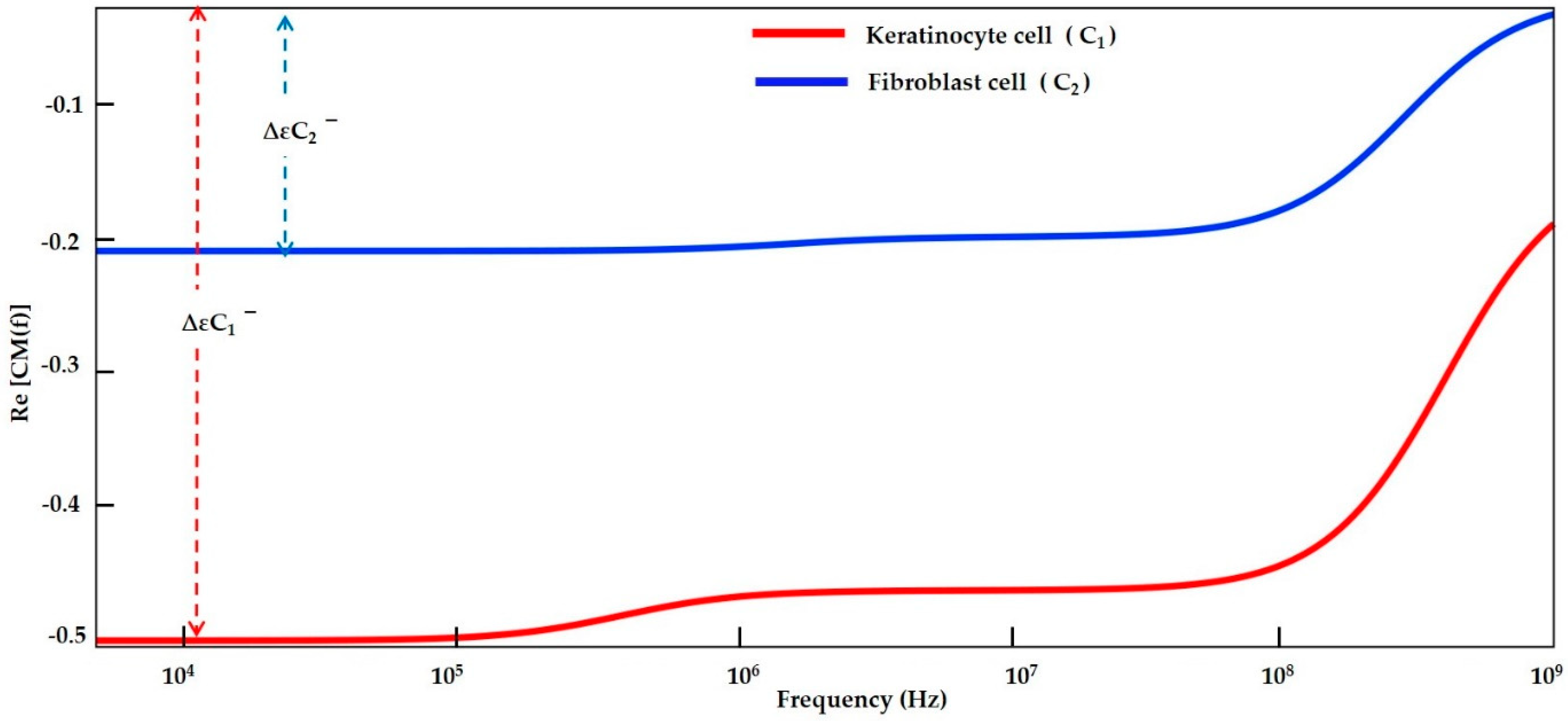
3.1.4. Numerical Result Evaluation of Polystyrene Particles and Biological Skin Cells
3.2. FEM Analysis
3.2.1. Electric Current
3.2.2. FEM of Polystyrene Particles
3.2.3. FEM of Biological Cells in a Low Conductive Medium
3.2.4. FEM of Biological Cells in a Highly Conductive Medium
3.3. DEP Experiment
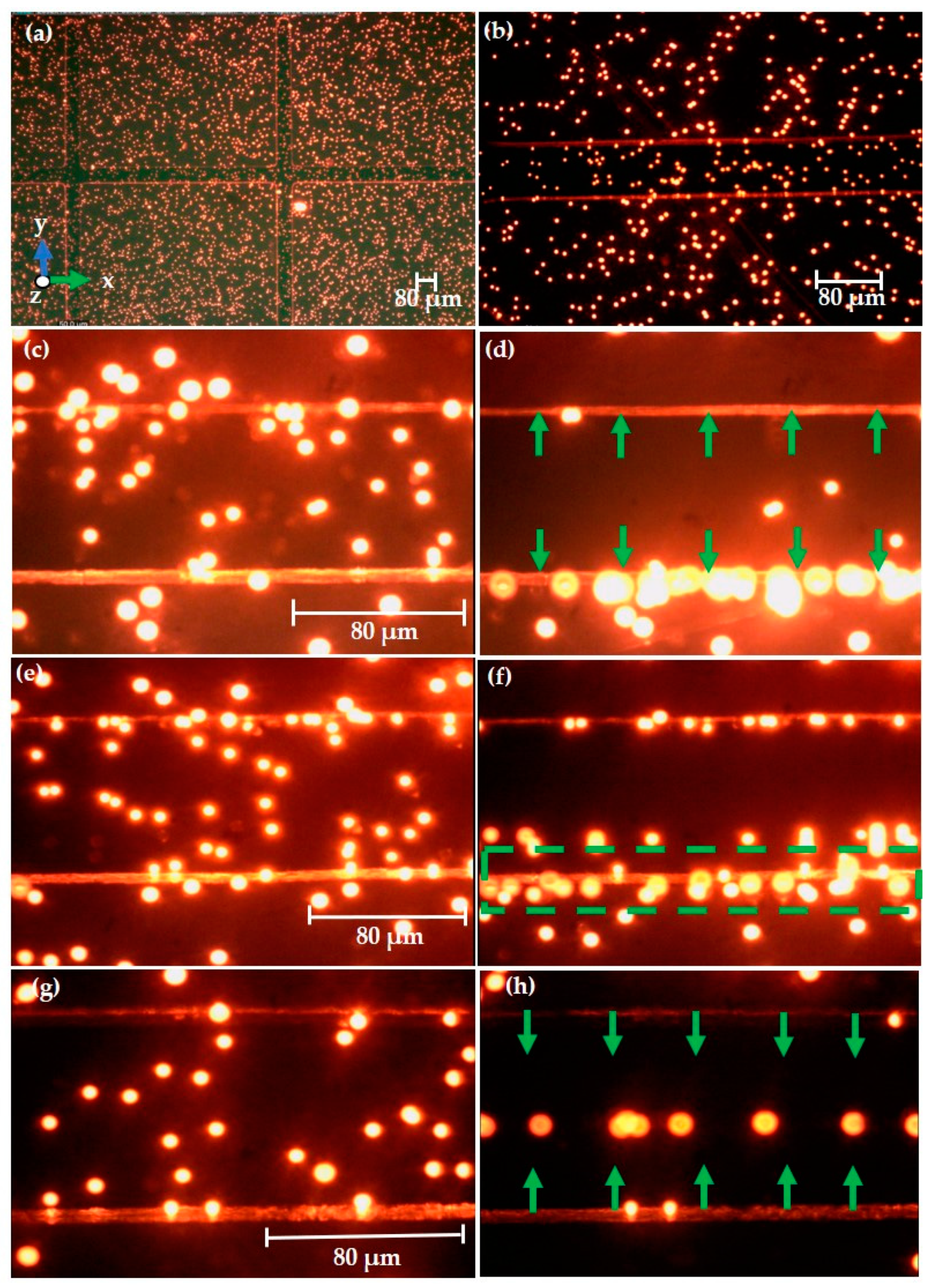
3.3.1. Experimental Observation of Polystyrene Particles
3.3.2. Experimental Observation of Keratinocyte Cells
3.4. DIPP-MotionV Motion Analysis for Keratinocyte Cells
4. Discussion
5. Future Perspective for Wound Healing Application
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunckler, J.; de Mel, A. A current affair: Electrotherapy in wound healing. J. Multidiscip. Healthc. 2017, 10, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Sun, Y.; Geng, Z.; Ma, K.; Sun, X.; Fu, X. Abnormalities in the basement membrane structure promote basal keratinocytes in the epidermis of hypertrophic scars to adopt a proliferative phenotype. Int. J. Mol. Med. 2016, 37, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.S.; Peng, S.W.; Cheng, J.Y. In vitro electrical-stimulated wound-healing chip for studying electric field-assisted wound-healing process. Biomicrofluidics 2012, 6, 034117. [Google Scholar] [CrossRef] [Green Version]
- Thakral, G.; LaFontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4, 22081. [Google Scholar] [CrossRef]
- Ashrafi, M.; Alonso-Rasgado, T.; Baguneid, M.; Bayat, A. The efficacy of electrical stimulation in lower extremity cutaneous wound healing: A systematic review. Exp. Dermatol. 2017, 26, 171–178. [Google Scholar] [CrossRef]
- Ramadan, A.; Elsaidy, M.; Zyada, R. Effect of low-intensity direct current on the healing of chronic wounds: A literature review. J. Wound Care 2008, 17, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Houghton, P.E. Electrical stimulation therapy to promote healing of chronic wounds: A review of reviews. Chronic Wound Care Manag. Res. 2017, 4, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Ud-Din, S.; Bayat, A. Electrical Stimulation and Cutaneous Wound Healing: A Review of Clinical Evidence. Healthcare 2014, 2, 445–467. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yuan, Y.J. A review of polystyrene bead manipulation by dielectrophoresis. RSC Adv. 2019, 9, 4963–4981. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.A.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for Biomedical Sciences Applications: A Review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, W.H.; Zhang, J.; Alici, G.; Wen, W. A review of microfabrication techniques and dielectrophoretic microdevices for particle manipulation and separation. J. Phys. D Appl. Phys. 2014, 47, 063001. [Google Scholar] [CrossRef]
- Pethig, R. Dielectrophoresis: An assessment of its potential to aid the research and practice of drug discovery and delivery. Adv. Drug Deliv. Rev. 2013, 65, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Buyong, M.R.; Kayani, A.A.; Hamzah, A.A.; Majlis, B.Y. Dielectrophoresis manipulation: Versatile lateral and vertical mechanisms. Biosensors 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, C.; Huang, H.; Chen, L.; Li, X.; Ge, Z.; Chen, T.; Yang, Z.; Sun, L. Dielectrophoresis for bioparticle manipulation. Int. J. Mol. Sci. 2014, 15, 18281–18309. [Google Scholar] [CrossRef] [Green Version]
- Buyong, M.R.; Larki, F.; Caille, C.E.; Takamura, Y.; Hamzah, A.A.; Majlis, B.Y. Determination of lateral and vertical dielectrophoresis forces using tapered microelectrode array. Micro Nano Lett. 2018, 13, 143–148. [Google Scholar] [CrossRef]
- Derakhshan, R.; Ramiar, A.; Ghasemi, A. Numerical investigation into continuous separation of particles and cells in a two-component fluid flow using dielectrophoresis. J. Mol. Liq. 2020, 310, 113211. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, Z.; Yuan, Y.J. Study on non-bioparticles and: Staphylococcus aureus by dielectrophoresis. RSC Adv. 2020, 10, 2598–2614. [Google Scholar] [CrossRef]
- Punjiya, M.; Nejad, H.R.; Mathews, J.; Levin, M.; Sonkusale, S. A flow through device for simultaneous dielectrophoretic cell trapping and AC electroporation. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sari, Y.; Hartono; Sutrisna, E.; Saryono. The effect of short duration of electrical stimulation on wound healing in acute wound in a rat model. Wound Med. 2019, 24, 36–44. [Google Scholar] [CrossRef]
- Cottet, J.; Fabregue, O.; Berger, C.; Buret, F.; Renaud, P.; Frénéa-Robin, M. MyDEP: A New Computational Tool for Dielectric Modeling of Particles and Cells. Biophys. J. 2019, 116, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Martínez-López, J.I.; Moncada-Hernández, H.; Baylon-Cardiel, J.L.; Martínez-Chapa, S.O.; Rito-Palomares, M.; Lapizco-Encinas, B.H. Characterization of electrokinetic mobility of microparticles in order to improve dielectrophoretic concentration. Anal. Bioanal. Chem. 2009, 394, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Natu, R.; Larraga-Martinez, M.F.; Martinez-Duarte, R. Enrichment of diluted cell populations from large sample volumes using 3D carbon-electrode dielectrophoresis. Biomicrofluidics 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Kinio, S.; Mills, J.K. Design of electrode topologies for dielectrophoresis through the use of genetic optimization with COMSOL Multiphysics. In Proceedings of the 2015 IEEE International Conference on Mechatronics and Automation, ICMA 2015, Beijing, China, 2–5 August 2015; pp. 1019–1024. [Google Scholar] [CrossRef]
- Tang, S.Y.; Zhang, W.; Baratchi, S.; Nasabi, M.; Kalantar-Zadeh, K.; Khoshmanesh, K. Modifying dielectrophoretic response of nonviable yeast cells by ionic surfactant treatment. Anal. Chem. 2013, 85, 6364–6371. [Google Scholar] [CrossRef]
- Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, A.; Voldman, J. Dielectrophoretic Traps for Single-Particle Patterning. Biophys. J. 2005, 88, 2193–2205. [Google Scholar] [CrossRef] [Green Version]
- Khoshmanesh, K.; Nahavandi, S.; Baratchi, S.; Mitchell, A.; Kalantar-zadeh, K. Dielectrophoretic platforms for bio-microfluidic systems. Biosens. Bioelectron. 2011, 26, 1800–1814. [Google Scholar] [CrossRef]
- Pethig, R. Review—Where Is Dielectrophoresis (DEP) Going? J. Electrochem. Soc. 2017, 164, B3049–B3055. [Google Scholar] [CrossRef]
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2018, 123, 18–32. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Ahmadi Ashtiani, H.R.; Jaffary, F.; Jahangiri, F.; Nikkhah, N.; Mahmoudbeyk, M.; Fard, M.; Ansari, Z.; Zare, S. Dermal Fibroblast Cells: Biology and Function in Skin Regeneration. J. Ski. Stem Cell 2017, 1–5, in press. [Google Scholar] [CrossRef] [Green Version]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef]
- Simeonova, M.; Wachner, D.; Gimsa, J. Cellular absorption of electric field energy: Influence of molecular properties of the cytoplasm. Bioelectrochemistry 2002, 56, 215–218. [Google Scholar] [CrossRef]
- Guo, J.; Kang, Y.; Ai, Y. Radiation dominated acoustophoresis driven by surface acoustic waves. J. Colloid Interface Sci. 2015, 455, 203–211. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Faiz, M.S.; Hamzah, A.A.; Yunas, J.; Majlis, B.Y. A tapered aluminium microelectrode array for improvement of dielectrophoresis-based particle manipulation. Sensors 2015, 15, 10973–10990. [Google Scholar] [CrossRef] [Green Version]
- Buyong, M.R.; Yunas, J.; Hamzah, A.A.; Yeop Majlis, B.; Larki, F.; Abd Aziz, N. Design, fabrication and characterization of dielectrophoretic microelectrode array for particle capture. Microelectron. Int. 2015, 32, 96–102. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Takamura, Y.; Majlis, B.Y. Tapered microelectrode array system for dielectrophoretically filtration: Fabrication, characterization, and simulation study. J. Micro/Nanolithogr. MEMS MOEMS 2017, 16, 044501. [Google Scholar] [CrossRef]
- Khoshmanesh, K.; Zhang, C.; Nahavandi, S.; Tovar-Lopez, F.J.; Baratchi, S.; Mitchell, A.; Kalantar-Zadeh, K. Size based separation of microparticles using a dielectrophoretic activated system. J. Appl. Phys. 2010, 108, 034904. [Google Scholar] [CrossRef] [Green Version]
- Ojo, K.O. Development of Sensors for Detection of Magnesium Metal Corrosion. Ph.D. Dissertation, University of Cincinnati, Cincinnati, OH, USA, 2016. [Google Scholar]
- Huang, Y.; Wang, X.B.; Becker, F.F.; Gascoyne, P.R.C. Introducing dielectrophoresis as a new force field for field-flow fractionation. Biophys. J. 1997, 73, 1118–1129. [Google Scholar] [CrossRef] [Green Version]
- Huclova, S.; Erni, D.; Fröhlich, J. Modelling and validation of dielectric properties of human skin in the MHz region focusing on skin layer morphology and material composition. J. Phys. D Appl. Phys. 2012, 45, 025301. [Google Scholar] [CrossRef]
- Park, S.; Koklu, M.; Beskok, A. Particle trapping in high-conductivity media with electrothermally enhanced negative dielectrophoresis. Anal. Chem. 2009, 81, 2303–2310. [Google Scholar] [CrossRef]
- Abd Samad, M.I.; Buyong, M.R.; Kim, S.S.; Yeop Majlis, B. Dielectrophoresis velocities response on tapered electrode profile: Simulation and experimental. Microelectron. Int. 2019, 36, 45–53. [Google Scholar] [CrossRef]
- Adekanmbi, E.O.; Srivastava, S.K. Dielectrophoretic applications for disease diagnostics using lab-on-a-chip platforms. Lab Chip 2016, 16, 2148–2167. [Google Scholar] [CrossRef]
- Tang, S.Y.; Zhang, W.; Soffe, R.; Nahavandi, S.; Shukla, R.; Khoshmanesh, K. High resolution scanning electron microscopy of cells using dielectrophoresis. PLoS ONE 2014, 9, 3–10. [Google Scholar] [CrossRef]
- Nerguizian, V.; Stiharu, I.; Al-Azzam, N.; Yassine-Diab, B.; Alazzam, A. The effect of dielectrophoresis on living cells: Crossover frequencies and deregulation in gene expression. Analyst 2019, 144, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Meng, F.; Chan, C.L. Cell transport and suspension in high conductivity electrothermal flow with negative dielectrophoresis by immersed boundary-lattice Boltzmann method. Int. J. Heat Mass Transf. 2019, 128, 1229–1244. [Google Scholar] [CrossRef]
- Broche, L.M.; Bhadal, N.; Lewis, M.P.; Porter, S.; Hughes, M.P.; Labeed, F.H. Early detection of oral cancer—Is dielectrophoresis the answer? Oral Oncol. 2007, 43, 199–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethig, R. Limitations of the Clausius-Mossotti function used in dielectrophoresis and electrical impedance studies of biomacromolecules. Electrophoresis 2019, 40, 2575–2583. [Google Scholar] [CrossRef]
- Das, D.; Biswas, K.; Das, S. Dielectrophoresis based microfluidic chip for continuous label-free separation of cells. In Proceedings of the 2015 9th International Conference on Sensing Technology (ICST), Auckland, New Zealand, 8–10 December 2015; pp. 728–732. [Google Scholar] [CrossRef]
- Douglas, T.A.; Alinezhadbalalami, N.; Balani, N.; Schmelz, E.M.; Davalos, R.V. Separation of Macrophages and Fibroblasts Using Contactless Dielectrophoresis and a Novel ImageJ Macro. Bioelectricity 2019, 1, 49–55. [Google Scholar] [CrossRef]
- Ling, S.H.; Lam, Y.C.; Chian, K.S. Continuous cell separation using dielectrophoresis through asymmetric and periodic microelectrode array. Anal. Chem. 2012, 84, 6463–6470. [Google Scholar] [CrossRef]
- Kaler, K.V.; Jones, T.B. Dielectrophoretic spectra of single cells determined by feedback-controlled levitation. Biophys. J. 1990, 57, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Dalili, A.; Montazerian, H.; Sakthivel, K.; Tasnim, N.; Hoorfar, M. Dielectrophoretic manipulation of particles on a microfluidics platform with planar tilted electrodes. Sens. Actuators B Chem. 2020, 129204. [Google Scholar] [CrossRef]
- Huan, Z.; Ma, W.; Xu, M.; Zhong, Z.; Li, X.; Zhu, Z. Cell patterning via optimized dielectrophoretic force within hexagonal electrodes in vitro for skin tissue engineering. Int. J. Adv. Manuf. Technol. 2019, 105, 4899–4907. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Current Type | Kind of EST | Function of Current | Briefing of Techniques | Pros and Cons in Wound Healing |
|---|---|---|---|---|
| Alternating current (AC) | (i) Transcutaneous electrical nerve simulation (TENS) (ii) Dielectrophoresis (DEP) (Present work) | (i) Bidirectional distribution of charged particles and constant movement changes in the direction of the charge flow. (ii) Alternate polarity | (i) Electrode alternates the polarity continuously with each cycle. Thus, no charge production under the electrode is observed. (ii) In medical care, TENS therapy is used to minimize chronic and acute pain. | PROS: AC is more effective than DC in minimizing the wound volume or size. CONS: Low-frequency AC is not broadly used due to the shortage of polarity in the treatment of the wound healing process. |
| Key Features | Keratinocyte Cell | Fibroblast Cell |
|---|---|---|
| Layer | Epidermal layer | Dermal layer |
| Primary task | Provide protective properties | Synthesizes extracellular matrix (ECM) and collagen |
| Structure | Auto-renewing stratified squamous epithelium | Large, smooth and elongated |
| Shape | Cuboid to spherical | Spindle like ellipsoid |
| Cell model | Two-dimensional | Three-dimensional |
| Nucleus | Nucleus-free | Flat and oval |
| Size | 7.96 µm | 49 µm |
| Particle Position | Particles/Cells | Initial Coordinates | |
|---|---|---|---|
| Grid-1 (Electrode left) | 3.2 µm polystyrene particle/keratinocyte cell | qx,0 (30,45,130) µm | qy,0 (30,30,60) µm |
| Grid-2 (Electrode Right) | 4.8 µm polystyrene particle/fibroblast cell | qx,0 (150,45,270) µm | qy,0 (30,30,60) µm |
| Symbol | Values (µm) |
|---|---|
| W1 | 100 |
| W2 | 80 |
| W3 | 100 |
| W4 | 280 |
| A1 | 75° |
| A2 | 15° |
| H1 | 4 |
| H2 | 4 |
| H3 | 80 |
| Conductive Medium | Electrical Conductivity (σp) (S/m) | Relative Permittivity (εp) | Reference |
|---|---|---|---|
| Deionized Water | 2 × 10−4 | 78 | [38] |
| Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 | 2.3 | 80 | [39] |
| Dulbecco’s Modified Eagle Medium | 1.5 | 80 | [40] |
| Particle/Cells | Diameter (µm) | Electrical Conductivity (σp) (S/m) | Relative Permittivity (εp) | Reference |
|---|---|---|---|---|
| 3.2 µm Polystyrene particle | 3.2 | 2.5 × 10−3 | 2.56 | [38] |
| 4.8 µm Polystyrene particle | 4.8 | 1.6 × 10−3 | 2.56 | [38] |
| Epidermis layer (keratinocyte) | 5.97, 5.97, 11.95 | Cytoplasm—0.12 | 50 | [41] |
| Cell membrane—1 × 10−6 | 9.04 | |||
| Dermis layer (fibroblast) | 70, 70, 7 | Cytoplasm—0.75 | 75 | [40] |
| Cell membrane—0.0047 | 53 |
| Response | Input Frequency | Particle Mobility |
|---|---|---|
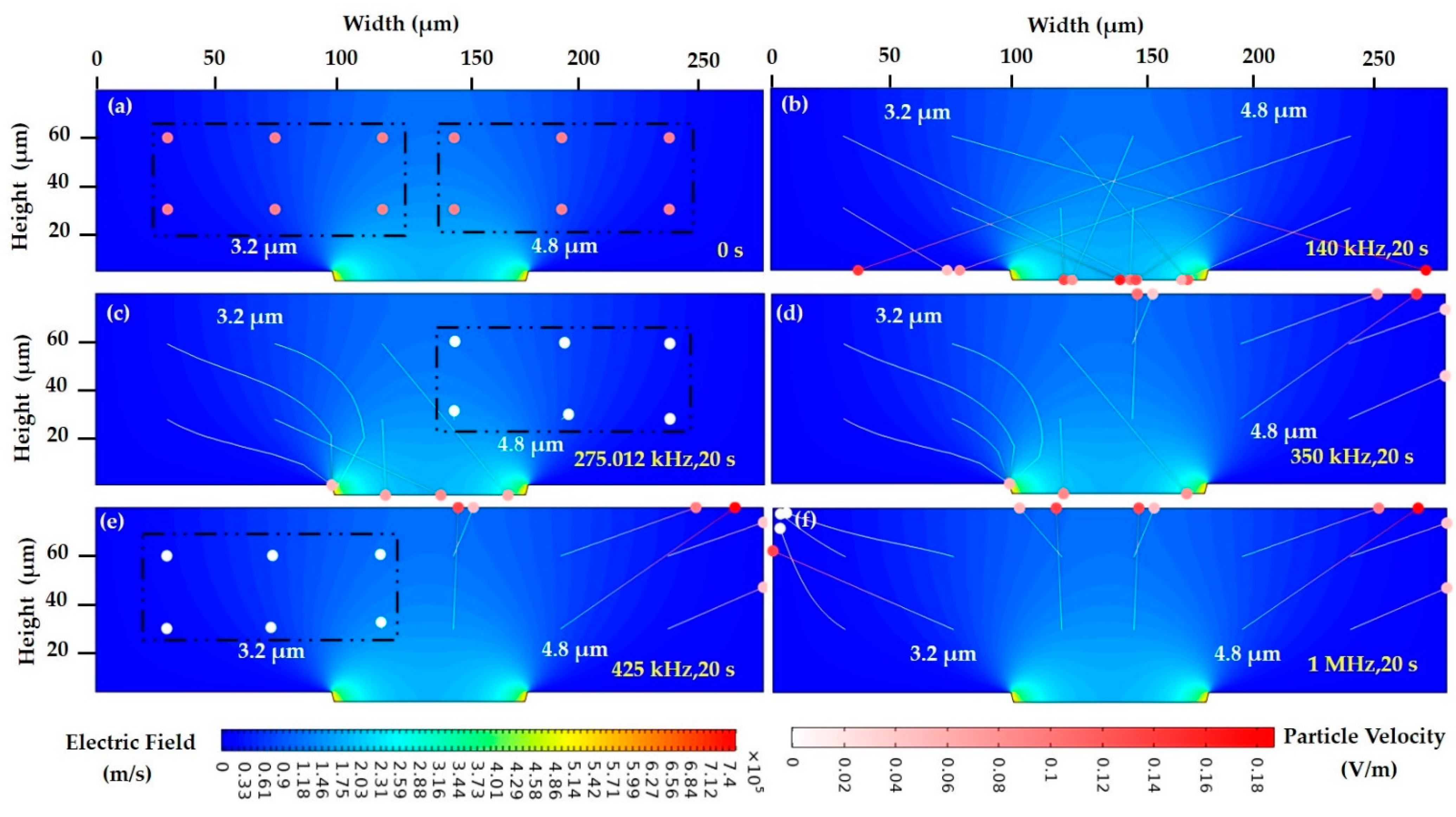
| PDEP (Lateral attraction) | f < 260 kHz | Attracted to the top surface of microelectrode—high field intensity |
| Intermediate frequency (lateral attraction and repulsion) | 260 kHz < f < 420 kHz | Attraction to high intensity—3.2 µm Repulsion to low intensity—4.8 µm |
| NDEP (lateral repulsion) | f > 420 kHz | Repelled in between the microelectrode—low field intensity |
| Frequency | Cells | Speed (µm/s) | Acceleration (µm/s2) | Coordinate Position (µm) |
|---|---|---|---|---|
| 300 kHz | C1 | −5.67382 | 438.2029 | 30.86125 |
| C2 | 23.46077 | −6672.94 | 182.3383 | |
| 800 kHz | C1 | −8.73413 | −107.999 | 592.298 |
| C2 | 11.15337 | −69.2376 | 657.8384 | |
| C3 | −5.78761 | 100.1757 | 699.9772 | |
| C4 | 11.24438 | −32.3256 | 763.6354 | |
| 15 MHz | C1 | 28.33875 | −5.31 × 10−14 | 52.33616 |
| C2 | −22.9694 | −1.84 × 10−7 | 270.3973 |
| Medium | Particles/Cells | MyDEP | Experimental Results | FEM Response | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PDEP | fXO | NDEP | PDEP | fXO | NDEP | PDEP | fXO | NDEP | ||
| DI water- (Low conductive Medium) | Polystyrene particle 3.2 µm | f < 425.02 kHz | 425.02 kHz | f > 425.02 kHz | f < 420 kHz | 420 kHz | f > 420 kHz | f < 425 kHz | 425 kHz | f > 425 kHz |
| Polystyrene particle 4.8 µm | f < 275.37 kHz | 275.37 kHz | f > 275.37 kHz | f < 260 kHz | 260 kHz | f > 260 kHz | f < 275.013 kHz | 275.013 kHz | f > 275.013 kHz | |
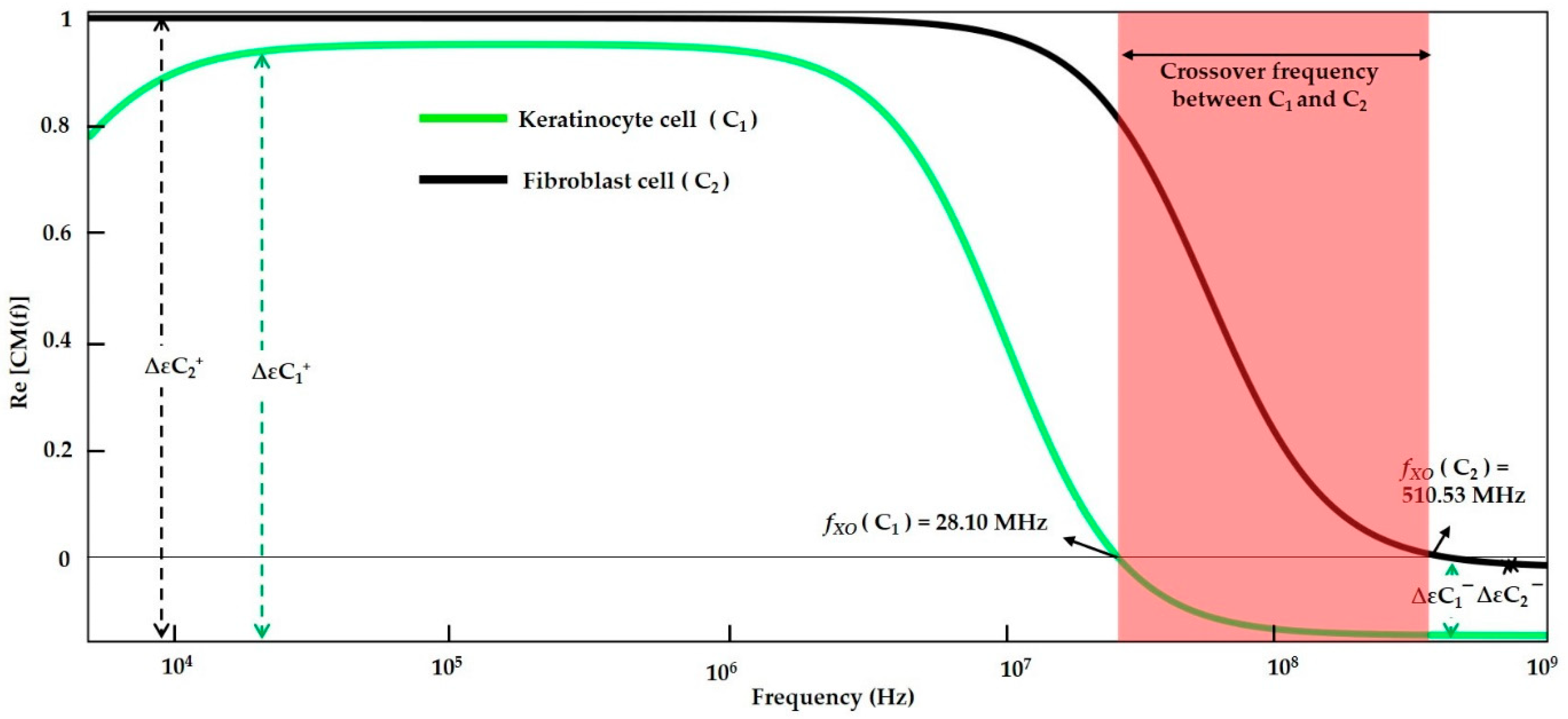
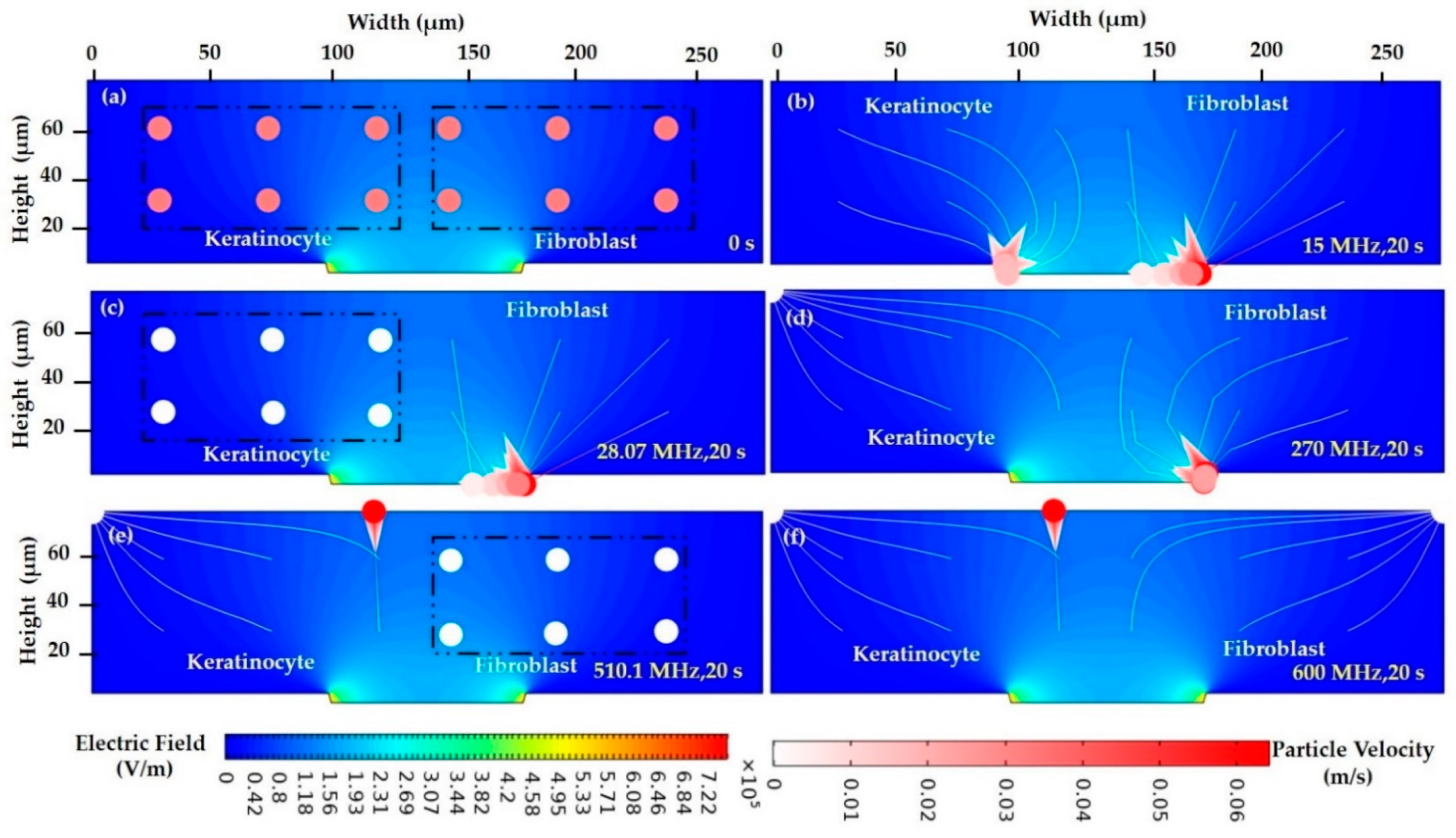
| DI water- (Low conductive Medium) | Keratinocyte cell | f < 28.10 MHz | 28.10 MHz | f > 28.10 MHz | - | f < 28.07 MHz | 28.07 MHz | f > 28.07 MHz | ||
| Fibroblast cells | f < 510.53 MHz | 510.53 MHz | f > 510.53 MHz | f < 510.1 MHz | 510.1 MHz | f > 510.1 MHz | ||||
| DMEM/F12- (High conductive Medium) | Keratinocyte cell | N/A | N/A | All range only NDEP exist | N/A | N/A | All range only NDEP exist | N/A | N/A | All range only NDEP exist |
| DMEM-High conductive Medium | Fibroblast cells | |||||||||
| Target Cells | Input Frequency | Efficiency/Supply Voltage |
|---|---|---|
| Separation of Human normal epithelial cells (HaCaT) from polystyrene beads [50] | f = 100 kHz to 5 MHz, NDEP (beads) f = 700 kHz. fXO(cell) | 10 Vpp, frequency sweep—100 kHz to 5 MHz, Continuous separation at 1 MHz. |
| Separation of Macrophages and Fibroblasts [51] | f = 20 kHz, 90% of fibroblasts trapped, macrophages trapped less than 20%. | At 350 Vrms and 1.25 µL/min |
| Separation of live and dead Swiss mouse fibroblast (NIH-3T3) cells [52] | f = 150 kHz or 300 kHz, separation between the live and dead cells | VSEP = 3.4 VPP, 87.3% efficiency, Throughput~1302 cells/min |
| Characterize Canola plant protoplast and ligament fibroblast cells [53] | f = 1 kHz to 50 MHz (PDEP response for both cells). | Ceff = 0.47 ± 0.03, µF/cm2 (Protoplasts) Ceff = 1.52 ± 0.26 µF/cm2 (fibroblasts) |
| Manipulation of fibroblast (3T3) cells [54] | f = 50 kHz, CMF close to − 0.5 (separation) | AC voltage at 10 Vpp, Frequency sweep—10 MHz and 50 kHz. |
| Characterize Normal keratinocytes (UP) versus oral squamous cell carcinoma cell lines (H357) [48] | f = 5 kHz, collected H357 cells from keratinocyte cell. | Cmem = (11.3 mF/m2) Ccytoplasm = 0.45 (S/m) |
| Manipulation of Human keratinocyte (Present work) | In low conductivity, f = 28.43 MHz (fXO, keratinocyte cell) f = 510.1MHz (fXO, Fibroblast cell) | Ac signal at 10 Vpp, frequency sweep—10 kHz to 800 MHz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deivasigamani, R.; Maidin, N.N.M.; Wee, M.F.M.R.; Mohamed, M.A.; Buyong, M.R. Dielectrophoresis Prototypic Polystyrene Particle Synchronization toward Alive Keratinocyte Cells for Rapid Chronic Wound Healing. Sensors 2021, 21, 3007. https://doi.org/10.3390/s21093007
Deivasigamani R, Maidin NNM, Wee MFMR, Mohamed MA, Buyong MR. Dielectrophoresis Prototypic Polystyrene Particle Synchronization toward Alive Keratinocyte Cells for Rapid Chronic Wound Healing. Sensors. 2021; 21(9):3007. https://doi.org/10.3390/s21093007
Chicago/Turabian StyleDeivasigamani, Revathy, Nur Nasyifa Mohd Maidin, M. F. Mohd Razip Wee, Mohd Ambri Mohamed, and Muhamad Ramdzan Buyong. 2021. "Dielectrophoresis Prototypic Polystyrene Particle Synchronization toward Alive Keratinocyte Cells for Rapid Chronic Wound Healing" Sensors 21, no. 9: 3007. https://doi.org/10.3390/s21093007
APA StyleDeivasigamani, R., Maidin, N. N. M., Wee, M. F. M. R., Mohamed, M. A., & Buyong, M. R. (2021). Dielectrophoresis Prototypic Polystyrene Particle Synchronization toward Alive Keratinocyte Cells for Rapid Chronic Wound Healing. Sensors, 21(9), 3007. https://doi.org/10.3390/s21093007









